Abstract
The effects of ionic liquid BMIMCL (1-buthyl-3-methyl-imidazolium-chloride) on the solution of soy protein isolate (SPI) were first studied. In situ polymerization of acrylonitrile monomer in the presence of SPI was conducted in a BMIMCL/dimethyl sulfoxide (DMSO) mixture solvent to produce SPI-g-PAN. This graft polymer was blended with pure PAN in BMIMCL/DMSO (1:5) and spun into fiber using a wet spinning method. The effects of SPI content and solution temperature on the viscoelasticity of the spinning dope were studied. FTIR, DSC and solution studies were used to confirm the grafting of PAN. Microstructure and mechanical properties of the spun fibers under different draw ratios were investigated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Global fiber consumption in 2009 was about 70.5 million tones, including 44.1 million tons of manmade fibers and 26.4 million tons of natural fibers [1]. The development of eco-friendly textile fibers has become increasingly important because of volatile crude oil price and its unstable supply, rising awareness of environmental degradation (e.g., depletion of natural resources, destruction of ecosystems, extinction of wildlife) and growing demand for eco-friendly products [2]. Soy protein, as a low cost renewable material, have found many important applications including membranes [3], elastomers [4], adhesives [5], composites [6, 7], plastics [8], and fibers [9–13]. Soy protein molecules consist of amino acids connected by covalent peptide bonds. The molecules in their natural form assume a four-level hierarchical structure due to the inter- and intra-molecular interactions originating from disulfide bonds, hydrogen bonds, electrostatic forces, and hydrophobic interactions. Most common organic solvents cannot destruct this structure and therefore cannot dissolve soy protein. A common method to destruct the structure of soy protein is to use combined alkaline/acidic and thermal treatments, which often cause soy protein degradation.
Solution of soy protein and its spinning technique have been studied. Xiao et al. [14] investigated SPI dissolution in a Dimethyl sulfoxide (DSMO)/urea mixture solvent and measured the rheological properties of the solution. They found that urea facilitated the dissolution of SPI in DMSO. The highest SPI solubility of 4.8 % was achieved at 3 M urea at 90 °C. Zhang et al. [15] studied the effects of alkali, urea, and sodium sulfite on the viscosity of the soybean protein solutions in aqueous NaOH and investigated the spinnability of the solutions. They found that the pH value of 11.5 led to the largest extent of soy protein unfolding (highest viscosity). The viscosity decreased rapidly above this pH value due to protein degradation (peptide bond cleavage). Urea, as a strong hydrogen bond breaker, was found to significantly reduce the viscosity of the soy protein solution, whereas sodium sulfite, a disulfide bond breaker, showed relatively small influence on the viscosity. Poor fiber drawability was noticed, which was attributed to protein degradation and the formation of microgels in the solution. Soy protein solutions in aqueous NaOH were also used to produce soy protein nanofibers using electrospinning [16–18]. A co-spinning polymer, e.g., polyvinyl alcohol (PVA), polyethylene oxide (PEO), PAN and zein, was used to improve the spinnability of the solutions and increase the properties of the spun fibers.
Room temperature ionic liquids (ILs) have been intensively studied as green solvents and reusable reaction mediums. They have been used to replace organic solvents in a wide range of reactions because of their advantages including excellent dissolution ability, high thermal, chemical and electrochemical stability, free from the effect of vapor pressure, and ease of recycling. ILs have been used as a solvent to dissolve synthetic polymers [19] and natural polymeric materials such as wood [20], cellulose [21–23], protein [24, 25], and chitin [26]. They have also been used as mediums for polymerization reactions or for polymer dissolution/dispersion, which enables further chemical transformations or material processing [27]. Phillips et al. found that ion liquid 1-Butyl-3-methylimidazolium Chloride (BMIMCl) was highly effective in breaking extensive hydrogen bonding network of silkworm silk and could dissolve the silk protein within a relatively short period of time. The solution was also successfully spun into fibers [28].
In this study, we used DMSO and BMIMCl as the solvent and co-solvent to dissolve SPI. The effects of BMIMCl on the dissolution of SPI in DMSO were investigated. The viscoelasticity of the obtained solutions was studied. SPI was modified by in situ graft polymerization of acrylonitrile monomer. Additional pure polyacrylonitrile (PAN) was blended with the graft polymer solution and the mixture was spun into fibers in a coagulation bath. Morphology, thermal and mechanical properties of the fibers were studied. This research demonstrated a novel method to produce textile fibers from renewable resources.
Experimental
Materials
Ionic liquid BMIMCl (purity > 95 %) from Fluka was used as received. Dimethyl sulfoxide (DMSO) (purity > 99.9 %) was purchased from Mallinckrodt Baker, Inc. Acrylonitrile (AN) monomer (purity > 99.0 %) was purchased from Fluka and was purified before use by treating it with sodium hydroxide solution followed by distillation. 2,2-Azobisisobulyronitrile (AIBN, purity > 98.0 %) was purchased from Aldrich and was purified by recrystallization. Soy protein isolate (006–974) was received from Archer Daniels Midland Company. PAN (Mw = 90,000) was obtained from Shanghai Petrochemical Corp.
Methods
Dissolution of SPI
SPI powder was dried at 80 °C in a vacuum oven for 12 h prior to use. SPI and DMSO were charged into a two-neck flask and the mixture was stirred at 60 °C to achieve a homogeneous dispersion. BMIMCl was then added to the dispersion and the temperature of the dispersion was raised to 75 °C. A clear SPI solution in the mixture solvent was obtained after stirring for ~20 min. The final SPI concentration in the mixture solvent was 7.5 wt%, and the weight ratio of BMIMCl to DMSO was 1:5.
In-Situ Polymerization of Acrylonitrile in the Presence of SPI
AIBN (0.5 wt% based on AN weight) was first added to the SPI solution as the initiator, followed by gradual addition of AN using a dropping funnel to reach an AN:SPI ratio of 1:1 (wt/wt). The reaction occurred at 75 °C under continuous nitrogen flow and stirring. The reaction was stopped after 3 h and the product was transferred to a bottle, which was kept still for 48 h for degassing. Some SPI-g-PAN dry powder was obtained by coagulating part of the product in 50 % ethanol followed by vacuum drying.
Fiber Spinning
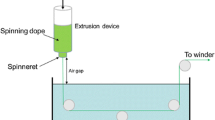
The polymer solution used for fiber spinning (i.e. spinning dope) was prepared by mixing the above reaction product (SPI-g-PAN) with predetermined amount of pure PAN solution (in DMSO). The final polymer concentration in the spinning dope was 15 wt%. SPI content was 10 wt% on the basis of total polymer weight. The procedure of fiber spinning is shown in Fig. 1. The spinning dope was degassed and loaded into the reservoir whose temperature was maintained constant at 70 °C. The dope was extruded into the coagulation bath by compressed CO2. The fiber coagulated in the bath and was drawn by the rotating godets. The fiber was further washed and drawn in the hot water bath (90 °C). The coagulation bath consisted of water and ethanol (1:1) and its temperature was maintained constant at ~4 °C. The diameter of the spinneret orifices was 11 μm.
Characterizations
SPI dissolution in the BMIMCl/DMSO solvent was investigated using a polarized optical microscope (POM, Olympus BX2M) operating at transmission mode. The microscope was equipped with a hot stage (Linklam TMS94) to control sample temperature. Dry SPI powder was added to the solvent to form a suspension. One drop of the suspension was placed between two glass slides whose temperature was maintained constant at 80 °C. Micrographs of the suspension were taken every 2 min by a CCD camera to illustrate the dissolution process.
Rheological properties of the fiber spinning dope, i.e., the mixtures of SPI-g-PAN and neat PAN in BMIMCl/DMSO (1:5) were measured using a 50-mm parallel plate rheometer (TA, Discover HD-2). The dope with different SPI contents (5, 10 and 15 wt% based on total solute weight) were prepared. The total solute weight was fixed at 15 wt% for all the solutions. The gap between the plates was set at 0.5-mm for all tests. A strain sweep test was first performed to determine the linear elastic range of the solutions. Then a frequency sweep test was conducted to examine their viscoelasticity at 70 °C. Temperature sweep tests were also performed to study the effects of temperature on their rheological properties.
Fiber surfaces and fiber cross-sections were examined by a field emission scanning electron microscope (FESEM, FEI Quanta 200F). The cross-section was prepared by cryofracture in liquid nitrogen. All the surfaces were sputter coated with gold prior to examination.
Thermal properties of the fibers were studied using differential scanning calorimetry (DSC, TA 2920). All the samples were tested in sealed aluminum crucibles. The samples were directly scanned from 50 to 350 °C at a heating rate of 10 °C min−1 without erasing their thermal history. The fibers were dried in a vacuum oven overnight prior to use.
FTIR analysis of SPI powder, neat PAN, and PAN/SPI simple mixture and SPI-g-PAN was performed using a Thermo Nicolet Nexus 670 spectrometer. The materials were dried in a vacuum oven, ground with KBr and pressed into discs for the tests. SPI-g-PAN was repeatedly washed with deionized water to remove unreacted AN monomers. The spectra from 400 to 4,000 cm−1 were recorded with a resolution of 2 cm−1 and 32 repetitive scans.
Results and Discussion
Dissolution Behavior of SPI Powder, SPI-g-PAN, and Simple Mixture of SPI and PAN Powder
SPI did not dissolve in DMSO, though swelling of SPI particles was noticed. Despite being a polar solvent, DMSO still lacks the ability to cleave the covalent bonds (i.e., disulfide bonds) that contribute to the folded structure of soy protein molecules. The addition of small amount of BMIMCl (BMIMCl:DMSO weight ratios 0.5:9.5 and 1:9) effectively cleaved those bonds and led to the solution of SPI. However, for the 0.5:9.5 solvent the solution gelled after being at rest for 1 week. The gel could be disrupted by vibration or heat, indicating that the gel was formed due to physical crosslinking. The crosslinking was likely caused by hydrogen bonding between the protein molecular chains [15]. BMIMCl at sufficient high concentrations (e.g. 1:9) could prevent the formation of the hydrogen bonds and therefore prevented the gelation process.
The dissolution process of SPI powder in BMIMCl/DMSO (1:9 w/w) at 80 °C is shown in Fig. 2. The content of SPI was 6 wt%. The powder started swelling as soon as it was added to the hot solvent (Fig. 2a). The surface of the particles was transformed into a transparent gel-like layer due to its interactions with the solvent molecules. The dark cores of the particles were the parts that remained unaffected by the solvent. The dark cores continued to shrink with time until they eventually disappeared in the solvent within 20 min (Fig. 2c–f). No visible solid phase could be found in the solution, indicating that the SPI powder dissolved in the solvent completely.
To demonstrate the effects of PAN grafting on the dissolution of SPI, the dissolution of pure SPI powder, a simple mixture of SPI and PAN, and SPI-g-PAN powder in DMSO and BMIMCl/DMSO mixture solvent was studied. Since DMSO can dissolve PAN but not SPI, the simple mixture of SPI and PAN in DMSO formed a translucent suspension, in which the undissolved SPI powder was evident (Fig. 3a). By contrast, the SPI-g-PAN dissolved in DMSO completely and a clear solution was formed (Fig. 3b). The dissolution of SPI-g-PAN in DMSO could be due to the loss of soy protein hierarchical structure after PAN grafting, which disassociated interconnected soy protein molecules and facilitated their solution. These two different solution results confirmed the change of SPI structure after the grafting. Both SPI and SPI-g-PAN dissolved in BMIMCl/DMSO mixture solvent (Fig. 4).
Rheology
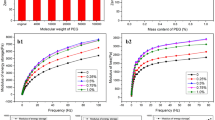
The rheological properties of spinning solutions have profound influences on the spinnability of the solutions and the properties of the spun fibers. Figure 5 shows the effects of SPI content on the storage modulus (G′), loss modulus (G″) and complex viscosity (η*) of the SPI solutions. Pure PAN showed the highest G′, G″ and η* among the tested materials, and the three properties tended to decrease with increasing SPI concentration. This was because the pure PAN had relatively high molecular weight (90 k) compared to SPI (50 k) [29] and the grafted PAN produced by in situ polymerization. Furthermore, the molecular weight of SPI could be reduced during the solution and graft reaction processes. With increasing content of low molecular weight polymers in the solution, the entanglements between polymer chains decreased and therefore the rheological properties of the solution were reduced.
Temperature is an important factor in controlling the rheological properties of a solution. G′, G″ and η* of the SPI solution (10 % SPI) were all found to increase and then decrease with increasing temperature (Fig. 6). The maximum values of G′, G″ and η* occurred simultaneously at 70 °C. Determination of this temperature dependence of the rheological properties of the solution was very important to the selection of fiber spinning temperature. Further study is undergoing to determine the mechanisms behind this temperature dependence of the three properties.
FTIR Spectroscopy
FTIR spectrums of the SPI powder, neat PAN, PAN/SPI simple mixture and SPI-g-PAN are shown in Fig. 7. Soy protein molecules comprise a variety of amino acid residuals whose major characteristic bonds include amide, carboxyl, hydroxyl, phenyl, and disulfide bonds. The absorption at 1,670 cm−1 was the C=O stretch (amide I) of the soy protein. The absorption of the neat (commercial) PAN at 2,250 cm−1 was attributed to the C≡N stretch, the peak at 1,740 cm−1 was due to the C=O stretch of the second monomer in the commercial PAN such as methyl acrylate, vinyl acetate or methyl methacrylate. Because there was no second monomer used in our in situ polymerization of acrylonitrile, the peak of C=O stretch was not found in the spectrum. Both C≡N stretch and amide I C=O stretch occurred on the spectrum of SPI-g-PAN, thus indicating that PAN was indeed grafted onto SPI molecules, as previously shown by the dissolution test results in Figs. 3 and 4.
DSC
DSC thermograms of neat PAN, PAN/SPI blend, SPI-g-PAN are shown in Fig. 8. The neat PAN showed an exothermic peak located at 300 °C when PAN oxidation and cyclization occurred [30, 31]. For the PAN/SPI blend, two exothermic peaks, located at 293 and 234 °C, respectively, could be identified. The lower peak was due to thermal degradation of SPI and the higher one was due to PAN. The degradation temperature of PAN (in the mixture) was 7 °C lower compared to that of the neat PAN. The SPI phase decomposed first in the mixture, which left a porous structure (i.e. PAN phase). The decomposition of the PAN phase was presumably accelerated because the interconnected pores facilitated heat and mass transfer. It was also possible that the decomposition products of SPI could catalyze PAN thermal degradation. Both reasons could lead to the lower degradation temperature of the PAN phase. For SPI-g-PAN, the peaks representing SPI and PAN degradation were shifted down further to 209 °C (SPI) and 274 °C (PAN), respectively. This could be attributed to conformational and molecular weight factors of SPI and PAN, i.e., unfolding of SPI molecules after PAN grafting, SPI molecular weight reduction during dissolution and graft reaction processes, and low molecular weight of the PAN side chains.
Fiber Morphology
The cross sections of the fibers with different drawing ratios are shown in Fig. 9. All fiber exhibited porous microstructure which is typical of wet spun fiber due to solvent diffusion and phase separation during coagulation process. The pore size was reduced by drawing in hot water but did not change substantially with increasing drawing ratio. The pores should collapse and disappear and as a result, the fibers should consolidate when they are drawn to a sufficiently high ratio at a temperature above the glass transition temperature (~100 °C by DSC) of the polymer. The remaining pores in this study were due to insufficient drawing of the fibers in the hot water bath (~95 °C) before their fracture. Indeed, when the fibers were drawn in an oven (110 °C), their drawability was improved at the higher temperature and a higher draw ratio was achieved. As a result, the fibers were fully consolidated (Fig. 9e). The surfaces of the fibers are shown in Fig. 10. The wrinkled skin of the undrawn fiber (Fig. 10a), which was probably caused by the underneath pores, was smoothened after hot drawing.
Tensile Properties
Figure 11 shows the representative stress–strain curves for the spun fibers drawn to different ratios. The result indicates that drawing greatly improved the strength and modulus of the fibers. Yielding and strain hardening (i.e., stress increases with strain) were noted for all three samples. Tensile properties of the fibers under different drawing ratios are summarized in Table 1. The rapid increases of tensile strength and modulus were mainly attributed to reduction in fiber voids and alignment of polymer chains in the fiber axial direction. The reduction in fiber voids lowered the probability of premature fiber failure, thus increasing the elongation of the fibers. The progressive alignment of polymer chains also led to strain hardening when increasing number of chains were aligned in the stress direction.
Conclusions
A novel wet spinning method was developed to produce SPI/PAN fibers in this study. Solution of SPI and its in situ graft reaction with AN in the presence of ionic liquid BMIMCl were investigated. It was found that BMIMCl as a co-solvent greatly assisted the dissolution of soy protein in DMSO. PAN was successfully grafted onto soy protein by in situ AN polymerization. Morphology study showed that fiber voids persisted after hot water drawing due to insufficient draw ratio and low drawing temperature. Under a higher temperature a larger drawing ratio could be achieved and the fiber was consolidated completely. Fiber strength was found to increase with increasing draw ratio. Because the ionic liquid can also dissolve non-proteineous ingredients in soybean, use of the ionic liquid as a solvent has the potential to bring added benefits of maximizing the use of soybean in fiber spinning.
References
http://www.oerlikontextile.com/desktopdefault.aspx/tabid-597/343_read-6203. Accessed 1 July 2013
Poole AJ, Church JS, Huson MG (2009) Biomacromolecules 10:1–8
Rampon V, Robert P, Nicolas N, Dufour E (1999) J Food Sci 2:313–316
Jong L (2005) J Polym Environ 4:329–338
Wang Y, Sun XS, Wang D (2006) T Asabe 3:713–719
Lodha P, Netravali AN (2005) Compos Sci Technol 65:1211–1225
Lodha P, Netravali AN (2002) J Mater Sci 37:3657–3665
Paetau I, Chen CZ, Jane J (1994) Ind Eng Chem Res 33:1821–1827
Yu L, Yan D, Sun G, Gu L (2008) J Appl Polym Sci 108:1100–1108
Huang HC, Hammond EG, Reimeier CA, Myers DJ (1995) Jaocs 12:1453–1460
Yu L, Gu L (2009) Polym Int 58:66–73
Katsuta K, Hayakawa I (1984) Agric Biol Chem 48:727–731
Zhang X, Min BG, Kumar S (2003) J Appl Polym Sci 90:716–721
Xiao R, Yin D, Gu L (2008) J Appl Polym Sci 110:1961–1966
Zhang Y, Ghasemzadeh S, Kotliar AM, Kumar S, Presnell S (1999) J Appl Polym Sci 71:11–19
Cho D, Nnadi O, Netravali A, Joo YL (2010) Mater Eng 295:763–773
Vega-Lugo AC, Lim LTJ (2008) Biobased Mater 2:223–230
Phiriyawirut M, Rodchanacheewa N, Nensiri N, Supaphol P (2008) Adv Mat Res 55–57:733–736
Winterton N (2006) J Mater Chem 16:4281–4293
Kilpelaineni I, Xie H, King A (2007) J Agric Food Chem 55:9142–9148
Swatloski RP, Spear SK, Holbrey JD, Rogers RD (2002) J Am Chem Soc 124:4974–4975
Zhu S, Wu Y, Chen Q (2006) Green Chem 8:325–327
Zhang H, Wu J, Zhang J, He J (2005) Macromolecules 38:8272–8277
Haibo X, Shenghai L, Suobo Z (2005) Green Chem 7:606
Fujita K, Macfarlane DR, Forsyth M (2005) Chem Commun 4804–4806
Mantza RA, Foxb DM, Green JM III, Fylstrac PA, Long HCD, Trulove PC (2007) J Phys Sci 62(5–6):275
Kubisa P (2004) Prog Polym Sci 29:3–12
Phillips DM, Drummy LF, Naik RR, Long HCD, Fox DM, Trulove PC, Mantz RA (2005) J Mater Chem 15:4206–4208
Soy protein isolate material safety data sheet, http://www.adm.com/en-US/worldwide/australia/Documents/Soy%20Protein%20Isolate%20AU.pdf. Accessed 1 July 2013
Wang XZ, Jie L, Gang W (2003) Carbon 41:2805–2812
Sutasinpromprae J, Jitjaicham S, Nithitanakul M, Meechaisue C, Supaphol P (2006) Polym Int 55:825–833
Acknowledgments
The authors are grateful for the financial support from the United Soybean Board, Project # 9456.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Deng, S., Cheng, J., Guo, X. et al. Fiber Spinning of Polyacrylonitrile Grafted Soy Protein in an Ionic Liquid/DMSO Mixture Solvent. J Polym Environ 22, 17–26 (2014). https://doi.org/10.1007/s10924-013-0617-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-013-0617-8