Abstract
The production conditions of 3-hydroxybutyrate (3-HB) monomers in submerged culture using Pseudomonas mendocina DS04-T as a degrading strain were optimized. The optimal culture medium constituents (w/v) were determined as follows: 0.5 % PHB, 0.15 % NH4Cl, 1.2 % Na2HPO4·12H2O, 0.3 % KH2PO4, 0.05 % MgSO4·7H2O, and 0.0005 % CaCl2·2H2O. The optimum parameters for liquid fermentation were as follows: temperature, 30 °C; inoculum content, 1.0 %; cultivation time, 18 h; initial pH, 7.0; volume of medium, 100 mL; and rotary speed, 180 rpm. Yield of 3-HB monomer and PHB depolymerase activity at optimized conditions were (56.4 ± 0.8) % and (57.4 ± 2.6) U/mL, respectively. The 3-HB monomer concentration obtained under optimized conditions was 1.5 times that obtained under the basic culture medium and initial conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyhydroxyalkanoates (PHAs) are natural polyesters that are intracellularly synthesized and accumulated during the unbalanced growth of a wide variety of microorganisms [1]. Over the last few decades, PHAs have attracted commercial attention for the production of green plastics because of their biodegradability, biocompatibility, and mechanical properties, which are similar to those of petrochemical-derived plastics. Most current applications of PHAs are based on these features and used in the environmental and medical fields [2, 3]. PHAs are ecological plastics used in packaging and coating as substitutes for non-biodegradable petrochemical polymers. Their main medical applications include fabrication of biodegradable body implants and control of drug delivery; they are also utilized in pharmacy, agriculture, and veterinary medicine [4–6]. PHAs have over 150 different types of monomer compositions that provide different properties and functionalities [1, 2]. The most common PHAs is poly (3-hydroxybutyrate) (PHB), a homopolymer of 3-hydroxybutyric acid (3-HB).
The increased utilization of PHAs may cause ecological problems because of their incomplete degradation over a desired period of time or formation of intermediates during degradation in the environment. Slow rates for complete degradation have been observed in a field test of biodegradable plastics in the soil. Accumulation of PHAs in soil may negatively affect the ecological system [7]. Therefore, the study of PHAs degradation is necessary to formulate guidelines regarding their appropriate use. In nature, PHAs can be degraded by many microorganisms. The ability to degrade extracellular PHA in the environment and use its degradation products as a source of carbon and energy depend on the specific extracellular PHAs depolymerase released by microorganisms [8, 9]. Generally, the end products of PHAs degradation are monomers (3-hydroxycarboxylic acids, 3-HAs), dimers, or a mixture of oligomers [10]. PHB is a source of optically active 3-hydroxyalkanoic acid (3-HB) monomers [11], which can be used to synthesize chiral compounds such as amino acids, vitamins, antibiotics, pheromones, and perfumes or biodegradable solvents. 3-HB monomers are applied not only in the biomedical and pharmaceutical fields [11, 12] and also as starting materials to obtain other new polyesters [4]. Thus, the development of a cost-effective industrial process for the production of 3-HB monomers is of considerable interest.
Some 3-HAs production methods have already been reported. 3-HAs can be chemically synthesized by enantioselective reduction of the corresponding 3-keto acids [13]. For certain products, this process requires the synthesis of precursor molecules, which complicates the synthetic procedure and reduces the product yield [14, 15]. Other synthetic approaches include stereoselective functionalization through Sharpless’ asymmetric epoxidation and hydroxylation or through Brown’s asymmetric allyboration [16]. These approaches require expensive metal-complex catalysts, which might contaminate the final products. For some conversions vigorous reaction conditions, have to be applied such as high pressure, flammable reaction media, or cryogenic conditions [17]. Alternative to chemical synthesis, bacterial PHAs could be used as an important source of 3-HAs. To obtain 3-HAs from PHAs, in vivo depolymerization has been investigated and efficiently accomplished for PHB [18].
Pseudomonas mendocina DS04-T was isolated by the authors, and it showed special degrading activity of PHB, poly (hydroxybutyrate-co-hydroxyvalerate) (PHBV), poly(3-hydroxybutyrate-co-4-hydroxybutyrate) [P(3HB-co-4HB)], poly(ε-caprolactone) (PCL) and poly lactic acid (PLA) [19]. As a multi-substrate degrading microbe, the study of degradation behavior of different substrate is necessary. The present work attempts to establish suitable culture conditions for 3-HB production by strain-degraded PHB in a submerged culture. This work assists in the analysis of the strain-induced PHB-degradation process and presents a reference for the use of strains in future 3-HB mass production.
Materials and Methods
Materials
PHB powder with a molecular weight of 7.31 × 105 was obtained from the Institute of Microbiology, Chinese Academy of Sciences, PR China. 3-HB monomer was obtained from Sigma. Other biochemical reagents, solvents, and chemicals were obtained from Beijing Chemical Co. (PR China) and used without further purification.
Strain and Medium
Pseudomonas mendocina DS04-T was obtained from the microbiology laboratory of the Northeast Normal University (Changchun, China). The basal medium contained (in w/v) PHB powder 0.15 %, Na2HPO4·12H2O 1.2 %, KH2PO4 0.45 %, NH4Cl 0.1 %, MgSO4·7H2O 0.05 %, and CaCl2·2H2O 0.0005 %, and its pH was adjusted to 6.5. The solid medium contained 2.0 % (w/v) agar [20].
Inoculum Preparation
After incubation in the basal medium slant at 30 °C for 24 h, the strain was collected with sterile water, and the cell concentration was adjusted from 1.0 × 108 to 1.5 × 108 cell/mL. This bacterial suspension was used as the seed for the next liquid culture.
Optimization of Culture Medium for 3-HB Production
The carbon source (PHB) concentration and nitrogen source type and concentration were selected and screened. Carbon sources of different concentrations and nitrogen sources of different types and concentrations were used to replace which in the basic medium, respectively. A four-factor, three-level orthogonal test was subsequently applied to optimize the medium composition for 3-HB production after the determination of optimal carbon and nitrogen sources. The orthogonal design of the culture medium with variable sources and levels is shown in Table 1. Approximately 1 % (v/v) of the bacterial suspension was inoculated and cultured in a 250 mL flask containing 100 mL of culture medium in a rotary shaker (200 rpm) at 30 °C for 24 h in all experiments.
Cultivation Condition Optimization for 3-HB Production
The cultivation temperature (25, 28, 30, and 35 °C), inoculum content (0.5, 1, 1.5 2, and 2.5 %, v/v), cultivation time (6, 12, 18, 24, 30, and 36 h), culture medium initial pH (5.5, 6.0, 6.5, 6.8, 7.0, 7.5, and 8.0), culture medium loading volume (50, 75, 100, 125, and 150/250 mL), and rotary speed (140, 160, 180, and 200 rpm) were investigated for 3-HB production. All trials were conducted in the optimum liquid medium obtained according to above section.
3-HB Monomer Assay and PHB Depolymerase Activity Assay
The fermented culture medium was centrifuged at 18,000×g for 20 min. The supernatant was analyzed via ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS, Waters, USA). The instrument parameters were as follows: mass range, m/z 0–800; exclusion voltage, 30 kV; attraction voltage, 9.3 kV; detection voltage, −4.75 kV; and vacuum degree, 1.9 × 10−4 Pa.
The supernatant was concentrated using a centrifugal ultrafiltration pipe (Pall Filtration Co., USA) with a molecular weight cut off at 3 kDa. The liquid under the membrane was analyzed by high performance liquid chromatography (HPLC, HITACHI L2000, Japan) according to Gao et al. [21] with several modifications. The liquid under the membrane was acidified to pH 2 with 1 M H2SO4 to facilitate HPLC analysis using an Inertsil C18 column (4.6 × 250 mm) (SHIMADZU Inc., Japan). The HPLC parameters were as follows: mobile phase, H2O (adjusted to pH 2.8 with HCl)-acetonitrile (85:15, v/v); flow rate, 1 mL/min; detection wavelength, 210 nm; and column temperature, 10 °C. The yield (%) of 3-HB was calculated as the content of 3-HB divided by PHB added in the medium.
A PHB emulsion (2 mg/mL) in 20 mM phosphate buffer (pH 6.8) was used as the substrate for PHB depolymerase activity assay. The reaction system contained 3 mL of the substrate and 1 mL of the supernatant incubated at 50 °C for 20 min. The decrease in turbidity of the PHB emulsions was measured at 650 nm using a UV–VIS spectrophotometer (Unico UV2600, USA). One unit of the PHB depolymerase activity was defined as a 0.001 OD decrease in the absorbance per minute at 650 nm under the previously described assay conditions [22].
Results
MS Analysis of PHB Degraded Product by P. mendocina DS04-T
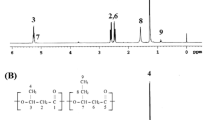
Figure 1 shows the MS results of the ferment supernatant with different degradation time. When the degradation time is less than 12 h, degradation products exist some oligomer besides 3-HB monomer. While when degradation time is higher than 18 h, the products obtained after strain degradation of PHB were only identified as 3-HB monomers without other oligomers. Figure 2a shows the HPLC retention times of 3-HB standard monomer is 2.52 min, and Fig. 2b shows the HPLC result of PHB hydrolysis product of P. mendocina DS04-T degraded for 18 h. There only has one peak with the same retention times with 3-HB standard monomer. From Figs. 1 and 2, it can be found 3-HB monomer is the only product when degradation time is greater than 18 h. Therefore, the use of P. mendocina DS04-T to obtain 3-HB monomers after PHB degradation is appropriate because the strain does not produce oligomers with similar properties during the 3-HB monomer purification process.
Medium Optimization for 3-HB Monomer Production
Figure 3 shows the change in 3-HB monomer production with increasing PHB content. When the added amount of PHB reached 0.45 % (w/v), the highest yield of 3-HB monomer was 34.8 ± 1.7 %. PHB depolymerase activity showed no significant increase when the added amount of PHB continued to increase beyond 0.15 %, which is the PHB content in the basic culture medium. In other words, the basic medium is applicable to the production of PHB depolymerase but not completely suitable for the production of 3-HB monomers. Thus, the basic medium requires further optimization.
Figure 4a shows the changes in the monomer production when different nitrogen sources were used in the medium. When NH4Cl was used as nitrogen source, yield of 3-HB monomer and PHB depolymerase activity are significantly higher than those obtained using other nitrogen sources. It indicates that ammonium salt is better than nitrate for promoting monomer production. Nitrate affects the growth rate of bacteria and influences the generation of monomers and depolymerase activity. (NH4)2SO4, a type of ammonium salt, stimulates microorganisms with excess SO4 2− and reduces the digestive and absorptive activity of the microbes, thereby affecting 3-HB monomer production and PHB depolymerase activity. In particular, when peptone is specially designed as a nitrogen source in the culture medium, the production of 3-HB monomers and PHB depolymerase activity are significantly lower than those in culture media with inorganic nitrogen sources. Organic nitrogen sources are generally more advantageous to the growth of microorganism but they did not appear to promote the generation of monomers in this study. Figure 4b shows that the production of 3-HB monomers and PHB depolymerase activity are higher when the added amount of NH4Cl in the medium is between 0.15 and 0.20 %.
After the carbon source amount and nitrogen source type and amount were determined, an orthogonal test design was used for further study (Table 1). The results and analysis are shown in Table 2. The R-value in Table 2 shows that the effect of these variables decreased in the order of D > A > B > C. KH2PO4 is a significant factor and should be controlled at high levels. The R-value of KH2PO4 indicates that it is also a significant factor for PHB depolymerase activity. The optimized culture medium was the combination A3B2C2D1, which is composed of (in w/v): 0.5 % PHB, 0.15 % NH4Cl, 1.2 % Na2HPO4·12H2O, 0.3 % KH2PO4, 0.05 % MgSO4·7H2O, and 0.0005 % CaCl2·2H2O.
Cultivation Condition Optimization for 3-HB Monomer Production
Table 3 shows the effects of cultivation conditions on 3-HB monomer production and PHB depolymerase activity. Both 3-HB monomer production and PHB depolymerase activity were apparently higher when the culture temperature was 30 °C. When the inoculum content was lowered to 1 %, both 3-HB monomer production and PHB depolymerase activity were significantly lower. When the inoculum content was higher than 1 %, no significant effect on the 3-HB monomer production was observed. PHB depolymerase activity had great influence when the inoculation amount was over 1.5 %. 3-HB monomer production showed two conspicuous steps with increasing incubation time. 3-HB monomer production underwent a growth process in the first stage (0–18 h) and then showed no obvious changes in the second stage (18–30 h). PHB depolymerase activity presented similar changes with increasing incubation time, becoming stable after 12 h of cultivation. When the initial pH of the culture medium ranged from 5.5 to 8.0, 3-HB monomer production and PHB depolymerase activity presented a downward trend after an initial increase. When the initial pH was 7.0, 3-HB monomer production and PHB depolymerase activity reached peak values. The liquid medium volume of the culture medium in the flask and shaking speed can influence the amount of dissolved oxygen in the medium. 3-HB monomer production and PHB depolymerase activity were low in large liquid medium volumes (over100 mL) because low dissolved oxygen levels affect the growth of bacteria. 3-HB monomer production and PHB depolymerase activity increased gradually with rotary speeds ranging from 140 to 180 rpm. The optimal cultivation conditions were thus determined as follows: temperature, 30 °C; inoculum content, 1.0 %; cultivation time, 18 h; initial pH, 7.0; volume of medium, 100 mL; and rotary speed, 180 rpm.
Comparison of Culture Medium and Conditions
Pseudomonas mendocina DS04-T was cultured under initial and optimal conditions. 3-HB monomer production and PHB depolymerase activity in initial conditions were (36.2 ± 1.7) % and (37.4 ± 2.5) U/mL, respectively, compared with (56.4 ± 0.8) % and (57.4 ± 2.6) U/mL, respectively, under optimized conditions. The yield of 3-HB obtained under optimized conditions was 1.5 times than that obtained under the basic culture medium and initial conditions.
Conclusion
The current research on PHB degradation mainly focuses on the purification of PHB-degrading enzymes and elucidation of the PHB degradation mechanism. No reports are currently available in the literature regarding the optimization of 3-HB monomer production. In the present study, conditions for 3-HB monomer production by P. mendocina DS04-T were optimized. The results provide a reference for the large-scale fermentation production of 3-HB monomers in future studies. The harvested 3-HB monomer can be used for the re-synthesis of PHB, realizing the recycling of PHB and sustainable development. 3-HB monomer purification is currently under study in our laboratory.
References
Lenz RW, Marchessault RH (2005) Biomacromolecules 6:1–8
Hazer B, Steinbüchel A (2007) Appl Microbiol Biotechnol 74:1–12
Ueda H, Tabata Y (2003) Adv Drug Deliv Rev 55:501–518
Chen GQ (2009) Chem Soc Rev 38:2434–2446
Cousley RR (2009) J Clin Orthod 43:403–407
Xu XY, Li XT, Peng SW, Xiao JF, Liu C, Fang G, Chen KC, Chen GQ (2010) Biomaterials 31:3967–3975
Tokiwa Y, Calabia BP (2004) Biotechnol Lett 26:1181–1189
Kim DY, Kim HW, Chung MG, Rhee YH (2007) J Microbiol 45:87–97
Luckachan GE, Pillai CKS (2011) J Polym Environ 19:637–676
Jendrossek D, Handrick R (2002) Annu Rev Microbiol 56:403–432
Chen GQ, Wu Q (2005) Appl Microbiol Biotechnol 67:592–599
de Roo G, Kellerhals MB, Ren Q, Witholt B, Kessler B (2002) Biotechnol Bioeng 77:717–722
Noyori R, Kitamura M, Ohkuma T (2004) Proc Natl Acad Sci USA 101:5356–5362
Wang Z, Zhao C, Pierce ME, Fortunak JM (1999) Tetrahedron Asymmetry 10:225–228
Nakahata M, Imaida M, Ozaki H, Harada T, Tai A (1982) Bull Chem Soc Jpn 55:2186–2189
Brown HC, Ramachandran PV (1991) Pure Appl Chem 63:307–316
Ikunaka M (2003) Chem Eur J 9:379–388
Lee SY, Lee Y, Wang FL (1999) Biotechnol Bioeng 65:363–368
Wang ZY, Wang Y, Guo ZQ, Li F, Chen S (2011) Polym Eng Sci 51:454–459
Zhou HL, Wang ZY, Chen S, Liu DB, Xia HM (2009) Polym-Plast Technol 48:58–63
Gao D, Maehara A, Yamane T, Ueda S (2001) FEMS Microbiol Lett 196:159–164
Shirakura Y, Fukui T, Saito T, Okamoto Y, Narikawa T, Koide K, Tomita K, Takemasa T, Masamune S (1986) Biochem Biophys Acta 880:46–53
Acknowledgments
This work was supported by National Natural Science Foundation of China (Grant No. 31100099), Science Project of Liaoning Province Education Office (L2011060) and Science Foundation of Liaoning Shihua University (No. 2011XJJ-025).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Ll., Gao, J., Jiang, Hs. et al. Production of 3-Hydroxybutyrate Monomers by Pseudomonas mendocina DS04-T Biodegraded Polyhydroxybutyrate. J Polym Environ 21, 826–832 (2013). https://doi.org/10.1007/s10924-012-0553-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-012-0553-z