Abstract
The current paper is aimed at understanding the environmental fate of linear low density polyethylenes (LLDPE) films designed for mulching purposes and loaded with different pro-degradant additives. These were analyzed, upon exposure to natural sunlight for a period intended to mimick a general crop season in the mediterranean region. The selected samples underwent a relatively low extent of degradation as monitored by carbonyl index, molecular weight variation, extractability by solvent, changes in the onset of the decomposition temperature and crystallinity. The tendency to biodegradation of outdoor exposed LLDPE was then assessed under different environmental compartments including soil medium, aqueous medium as well as in axenic culture of white-rot fungus Phanerochaete chrysosporium. That fungus is known to be effective in the degradation of recalcitrant organic materials and plastic items. During the soil burial biodegradation test, lasted for 27 months, samples specimen were withdrawn at time intervals and characterized by means of structural and thermal analysis. These analytical assessments allowed to monitor any progress of oxidative degradation as a direct effect of the incubation in an active microbial environment. Analogous characterizations were carried out at the end of the biodegradation tests in aqueous medium and in P. chrysosporium axenic cultures. Data presented here are in keeping with the initial abiotic oxidation via a free radical chain reaction promoted by a pro-degradant additive acting on hydroperoxides and peroxide moieties present initially in the polymer bulk. This step was followed by a free radical cascade reactions leading to degradation once the oxidation started under relatively mild conditions (sunlight exposure). During the incubation step in soil, the abiotically degraded samples underwent significant variation in the level of oxidation and degradation with respect to the detected starting values. Indications were gained on the synergistic effect of a random fashion microbial metabolization coupled to biotically mediated oxidation of the original abiotically fragmented samples. Similar results were obtained in the biodegradation tests carried out in the aqueous media and in presence of P. chrysosporium axenic cultures. These evidences are suggesting the role of natural occurring microorganisms in promoting both partial oxiditation and degradation of LLDPE samples in combination with contextual mineralization process of the oxidized fragments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Among the numerous applications of thermoplastics commodities, polyethylene (PE) occupies a major role in the production of films for food and non-food packaging, carrier bags, wrapping and agricultural films. The revolution in the production of PE based plastic items is connected to its low cost, easy processability and good mechanical properties. Mainly, the use of plastics in agriculture have increased 2–3 folds, as they have become increasingly useful in practices implying early harvest, higher yields, less reliance on herbicides and pesticides, better protection of crop practice (fruit and vegetables) and more efficient water conservation [1]. In accordance almost half of the use of plastics are employed in protecting cultivation practices (greenhouses, mulching, small tunnels, temporary coverings of structures for fruit trees, etc.) [2]. In particular, linear low-density polyethylene (LLDPE) is the most widely used polyethylene grade, due to its relatively good mechanical and optical properties, combined with a competitive market price [3, 4]. A major concern bound to the plastic items used in packaging and agriculture segments is represented by the issue bound to their disposal at the end of their service life. Therefore, the introduction on the market of PE characterized by a controlled lifetime would be a very useful input for the mitigation of plastic waste disposal burden [5–10]. Unfortunately, commercially available polyolefins and most other man-made polymers are recalcitrant to degradation into functional lower molar mass fragments eventually vulnerable to microorganisms ubiquitous in various environmental compartments.
In this regard, the first attempt aimed at mitigating the plastic waste burden was done by implementing, whereby traditional recycling procedures are not viable, research activities intended to reengineer man-made full carbon backbone polymers. As a part of the continuing activities ongoing at our BioLab centre, attention was focused on the physical (e.g., thermal and photolytic degradation) of polyolefins with particular reference to PE. The mitigation and the final overcome of the intrinsic recalcitrance of PE to biological attacks were the ultimate goal of the undertaken activities. The introduction of pro-degradant/pro-oxidant additives capable of promoting the initiation of free-radical degradation of the macromolecules, leading to oxidized low-molar mass fragments, should be the key to break down the recalcitrance of PE to ultimate biodegradation [7, 9]. The use of degradation promoters, was early approached with the introduction in the late seventies, of phenolic agents (Scott and Gilead) [11, 12]. This approach was later revisited in the nineties by Fabbri [13]. The last approach is also reminiscent of the early studies aimed at the introduction either into the main chain or directly grafted to it of carbonyl groups such as in copolymers of ethylene with carbon monoxide produced by Shell company. Copolymers of propylene with methyl vinyl ketone were introduced as “ecolytes” by Guillet [14, 15].
More recent approach is represented by the generation of free radicals by action, in combination with pro-degradants, of heat, light, mechanical stress, susceptible to react with molecular oxygen to produce mainly hydroperoxides [5, 9, 16, 17]. The hydroperoxides decomposition under the effect of heat, light and metal ions, leads to the formation of macroalkoxy radicals through the classical free radical chain cascade reactions [10]. As a result, main chain scission and eventually grafting, turned out to be the major consequences of physical–chemical, promoted oxidation of polyolefins. Polyolefins, during the processing at temperature around 200 °C, tend to give rise under shear to free radicals, that in presence of oxygen, can produce hydroperoxides, eventually remaining dormant in the polymer bulk unless pro-degradant additives are present. These latter may catalyze the hydroperoxides decomposition thus initiating a free radical chain reactions leading to oxidation of carbon chain backbone followed by degradation with formation of ultimate functional fragments (Fig. 1). Several studies have reported significant reduction of both Mn and Mw of poly(ethylene) samples containing pro-degradant additives after thermal degradation [17]. Further studies reported the identification of oxidation products as carboxylic acids, ketones, lactones and low molar mass hydrocarbons [17–23]. Rate and extent of free-radical oxidation of polyolefins are also affected by structural parameters such as chain kinks and branching, identified as weak points liable to free radical productions [19–23]. However, relatively scarce information on the influence of physical parameters such as natural sunlight exposure as well as the whole biological environmental conditions on the propensity to degradation of PE films, are available [24–26].
The purpose of the present study is to investigate the effects of outdoor exposure to natural sunlight for a period intended to mimick a summer crop season on the oxo-biodegradation behavior of LLDPE samples containing proprietary pro-degradant additives. Standardized respirometric tests [27] simulating the soil burial biodegradation conditions, were utilized to assess the biodegradation extent of the LLDPE samples aged outdoor. In addition, the tendency to biodegradation was assessed in aqueous medium as well as in axenic culture of white-rot fungus Phanerochaete chrysosporium in order to investigate the role of natural occurring microorganisms in promoting the ultimate environmental degradation of the tested materials.
Experimental
Test Materials
Test samples of linear low density polyethylene (LLDPE) were two commercially produced for agricultural clear mulch film (12 micron thickness) supplied by CIBA SpA, Italy. Each of the received film samples contained different proprietary pro-degradant additives and were indicated as LLDPE-TD1 and LLDPE-TD2. A control sample with the same formulation and thickness, but pro-degradant additive-free (LLDPE-TD0) was also supplied and tested as received.
Abiotic Degradation
Outdoor Exposure Degradation Tests
The tests were carried out in accordance with ASTM D5272-08 “Standard practice for outdoor exposure testing of photodegradable plastics” [28]. Approximately, 1 square meter of each film sample was allocated in a nylon net (4 mm mesh) and directly exposed to the south-west direction in outdoor conditions at 30° inclination for 3 months during summer (June 4 to September 4, 2007) in north-central Italy (43°40′48″N, 10°20′55″E). During the exposure time, the minimum and maximum temperatures were recorded on a daily basis and were between the range of 20 and 35 °C. At time intervals, the samples were analysed by FT-IR spectroscopy by taking relevant spectra from 5 different positions of each test specimen.
Biodegradation
The following biodegradation experiments were carried out under both aqueous and solid media conditions.
Respirometric Biodegradation Tests in Aqueous Medium
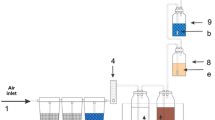
Aerobic biodegradation tests were carried out in aqueous medium by using as respirometric apparatus 300 ml Erlenmeyer flasks, containing 100 ml of mineral salt medium having the following composition per liter of distilled water: KH2PO4 85.0 mg, K2HPO4 218.0 mg, Na2HPO4 334.0 mg, (NH4)2SO4 10.0 mg, NH4NO3 10.0 mg, CaCl2 36.0 mg, MgSO4·7H2O 23.0 mg, and FeCl·6H2O 0.3 mg, pH 7.4 ± 0.2. Each flask was equipped with silicone rubber stoppers hanging 40 ml capacity plastic vials filled with CO2 absorbing 0.05 N KOH solutions (Fig. 2). The microbial inoculum was prepared by cultivating 1 ml of a forest soil suspension (1 g/10 ml 0.9 % NaCl solution) in 300 ml mineral salt medium containing 100 mg of the sample fraction of thermally oxidized LDPE-TDPA sample as sole carbon and energy source. This soil culture was used as microbial source in 10 % by volume ratio to inoculate each test flask once the stationary phase, as determined by the optical density at 660 nm in a UV/Vis410 Jasco spectrophotometer, was reached. All the test samples were supplied to the microbial cultures as sole carbon sources at approximately 0.1 % by weight concentration level.
Test flasks were incubated at room temperature (25 °C) in the dark on a rotatory shaker (120 rpm). All the experiments were carried out in triplicate. Every 5–7 days CO2 absorbers, containing 20 ml of 0.05 N KOH solution were retrieved and replaced with fresh KOH solution in known aliquots. The retrieved KOH solutions were back-titrated with 0.05 N HCl and the amount of adsorbed CO2 was computed.
The head space of flasks was sufficiently large to provide the cultures with oxygen; thus avoiding anoxic conditions. Moreover the flasks and vials were opened weekly so that the head-space air could be replenished. Gas-tight sealing of the vessels was necessary to prevent water evaporation during the long incubation time and any minimal loss of CO2 deriving from the microbial bioactivity. At the end of the test all the test samples were withdrawn from aqueous cultures, carefully washed and characterized by gravimetric analysis (e.g., weight variation), FT-IR spectroscopy and thermal analysis (TGA and DSC).
Biodegradation Tests in Soil Medium
Soil burial biodegradation tests were carried out in cylindrical glass vessels (Biometer Flask 500 ml capacity) containing a multilayer substrate in which defined amounts of forest sandy soil (20 g) were placed (Fig. 2). Soil samples sieved at 0.6 mm, mixed with 10 g perlite and supplemented with 10 ml of 0.1 % (NH4)2HPO4 solution, were sandwiched between two layers of 15 g perlite wetted with 15 ml of distilled water. Accordingly perlite was used to ensure satisfactory incubation conditions, whereas soil samples were used mainly as microbial inoculum. This arrangement guarantees more favorable and reliable signal-to-noise ratio resulting in improved test accuracy, particularly when limited carbon dioxide emissions are expected from the test samples [27].
Every 3 months of incubation each culture was carefully aerated by turning up the middle soil layer, re-wetting and adding other 5 g of fresh soil/perlite mixture in order to mimick the situation of a real agricultural soil. At the same time small fragments of the analyzed test samples were withdrawn from the relevant culture, carefully washed with distilled water and dried under vacuum. Before any analytical determination samples were pre-conditioned at room temperature in a desiccator and were submitted to spectroscopic and thermal characterization in order to evaluate the progress of chemical degradation of the test samples as a consequence of the incubation in a microbiologically active soil medium.
Biodegradation Tests with Single Microbial Species in Agar Plates
The lignin degrading Phanerochaete chrysosporium ATCC 34541 strain purchased from Deutsche Sommlung von Mikroorganismen und Zellkulturen (DSMZ), was utilized in pure culture.
Tests were carried out in 9.0 cm diameter Petri dishes containing approximately 20 g of sterilized agar medium having the following concentration of each ingredient per liter of distilled water: K2HPO4 2.2 g, KH2PO4 0.8 g, MgSO4 7H2O 0.2 g, NH4NO3 0.25 g, MnSO4 H2O 0.021 g, CuSO4 5H2O 0.006 g, CoCl2 6H2O 0.006 g, Glucose 1.0 g, Bacto Agar 20.0 g.
The test specimens relevant to outdoor exposed LLDPE films, were sterilized under UV lamp for 12 h before to be aseptically transferred onto the agar surface, after that each plate was inoculated with the white rot fungus. Tests were carried out in triplicate.
The effect of the microbial activity was monitored after 20, 45, 65 and 180 days of incubation, film specimens were withdrawn from the agar plates and characterized by FT-IR spectroscopy and relevant determination of the level of oxidation by evaluation of carbonyl index (COi). Un-inoculated control samples of outdoor exposed specimens were incubated aseptically under the same conditions.
At the end of the biodegradation tests and prior to the analytical characterization all the sample specimens were submitted to a cleaning procedure carried out in order to remove any microbial components from the film sample surfaces. In particular, the specimen were carefully washed with distilled water in an unltrasound bath. After this treatment, the specimens were suspend in distilled water (40 ml) containing 25 mg of sodium dodecyl sulphate surfactant (SDS) and stirred at 120 rpm for 2 h, in order to remove any traces of biofilm. The specimens were therefore washed twice with distilled water and recovered on nylon membrane having 0.45 μm porosity by vacuum filtration. Finally all the specimen were dried over night under vacuum in a freeze dryer apparatus.
Analytical Characterization
The following analytical characterizations have been undertaken as aimed at monitoring the oxidative degradation and biodegradation of the investigated polymeric materials and hence at understanding their ultimate environmental fate.
FT-IR Analysis and Carbonyl Index Determination
Fourier transformed infrared (FT-IR) spectroscopic characterization of the analyzed specimens was carried out with a Jasco model 410 spectrophotometer. The spectra were taken as an average of 16 scans with 2 cm−1 resolution. The carbonyl index (COi) was calculated as the ratio of the optical density of the absorption band comprised between 1,650 and 1,830 cm−1 (carbonyl peak), and the optical density of the absorption band at 1,463 cm−1 (CH2 scissoring peak) [26, 29].
Differential Scanning Calorimetry (DSC)
The thermal analysis of polymeric materials was carried out in a DSC 822 (700 °C) module equipped with FRS5 sensor and operated by means of STARe software. Measurements were performed under nitrogen flow rate of 80 ml/min according to the following protocol:
-
1.
First heating scan from−20 to 140 °C at 10 °C/min and 2 min of isotherm at the end;
-
2.
First cooling scan from 140 to −20 °C at −10 °C/min and 4 min of isotherm at the end;
-
3.
Second heating scan from −20 to 140 °C at 10 °C/min.
All the thermal parameters [Melting temperature, (Tm) and Crystallinity (%)] were taken from DSC traces recorded during second heating scan. In order to assess the degree of crystallinity of samples, the enthalpy of fusion of 100 % crystalline PE was taken as 293 J/g [30].
Thermal Gravimetric Analysis (TGA)
TGA experiments were performed in the thermogravimetric analyzer series Q500 of the TA Instruments. Generally, sample amount was between 10 and 15 mg. TGA experiments were performed in the thermogravimetric analyzer under nitrogen atmosphere at 60 ml/min flow rate according to following protocol:
-
1.
Heating from 25 °C up to 610 °C at heating 10 °C/min rate.
Degradation temperature onsets at 2 % weight loss (TON) and residue weight at 600 °C were recorded during the TGA courses.
Gravimetric Analysis
Weight analyses of samples from degradation studies were carried out by recording the samples weight in an analytical laboratory balance with ±0.1 mg precision. Before any determinations the samples were pre-conditioned at room temperature in a desiccator.
Solvent Fractionation of Test Samples
To analyze the low molecular weight fractions produced during the abiotic degradation tests, exposed samples were submitted to an extraction procedure by using in sequence boiling distilled water, acetone and dichloromethane under reflux conditions for 2 h in a Kumagawa apparatus. The obtained extracts were dried to constant weight under vacuum and the relevant molecular weight (Mw) and molecular weight distribution (ID) were evaluated by means of GPC analysis.
Gel Permeation Chromatography (GPC)
Molecular weight (Mw) and molecular weight distribution (ID) of the solvent extracted fractions from LLDPE film samples were analyzed by gel permeation chromatography (GPC) carried out with a Jasco PU-1580 liquid chromatograph equipped with a Jasco 830RI refractive index detector and Perkin Elmer LC-75 UV Vis detector. A PLgel guard column and two PLgel Mesopore (30 cm, 10 mm) columns were used. Chloroform was used as mobile phase at 1 ml/min flow rate. Relative calibration was obtained by analyzing monodisperse polystyrene standards.
Results and Discussion
Outdoor Exposure Test of LLDPE Films Containing Pro-Degradant and Pro-Degradant Free Control Film
The test lasted for 93 days from June 4 up to September 4, 2007 During this period the tendency to oxidation and degree of oxidation of LLDPE films [LLDPE-TD1, LLDPE-TD2 (pro-degradant loaded) and LLDPE-TD0 (pro-degradant free)] were monitored by means of FT-IR spectroscopy and relevant evaluation of COi. The average COi value relevant to measurements at various spots of the specimen, as reported elsewhere [26], showed remarkably different oxidation behaviours in the two different film samples containing pro-degradant additive. The COi profile relevant to LLDPE-TD1 sample approached a plateau phase after 3 weeks of exposure, followed later by a new exponential phase. The COi profile of LLDPE-TD2 film was maintaining a positive trend during the aging time and especially in the last 10 days of outdoor exposure. After 93 days outdoor exposure, an appreciable intensity of carbonyl absorption band due to different carbonyl functional groups was observed in the test samples containing the pro-degradant additives. The shape of carbonyl band was almost identical as formerly detected [26]. A lower intensity of carbonyl absorption in the control LLDPE-TD0 film sample was observed in the same timeframe with a trend comparable to that recorded for the samples LLDPE-TD2 up to 75 day exposure. Moreover, the LLDPE samples containing pro-degradant additives started to undergo fragmentation at the end of 93 day outdoor exposure.
In order to evaluate the tendency of the outdoor exposed LLDPE films to be fragmented into low molecular weight oxidized fractions, a solvent extraction procedure was carried out by using the following series of solvents: distilled water, acetone and dichloromethane (DCM), in that order. The COi of all the solvent extracted fractions and relevant residues to extraction were evaluated by FT-IR, thus recording higher values with respect to the corresponding COi of film specimens in the case of the extractable fractions, and lower values for the relevant residues to extraction (Table 1). The formation of heavily oxidized low molecular weight fractions, particularly in the case of LLDPE-TD1 sample, was detected. No significant extractable fractions were obtained in the control film samples (LLDPE-TD0) submitted to the same extraction procedure.
All the solvent extracted fractions were submitted to structural characterization with particular attention to the determination of molecular weight (Mw) and molecular weight distribution (ID) by GPC analysis. Data collected on the analyzed samples are reported in Table 2.
The reported data were showing that all the types of extract contained low molecular weight oxidized fractions. Nevertheless, slight but significant differences were observed between the extracts obtained from the two LLDPE samples submitted to outdoor exposure. In particular, it was evidenced that the lowest molecular weight compounds were extracted from outdoor exposed LLDPE-TD2 sample, whereas fractions with similar molar mass were detected in the acetone and DCM extracts (Table 2).
The FT-IR analysis showed that the solvent extracts, attainable from abiotically aged LLDPE samples, are characterized by the presence of oxidized carbon moieties, whose type and relevant amounts were depending upon the solvent used, and in a little extent, upon the kind of aged LLDPE sample. In the first case, it was evident that both acetone and DCM extracts are characterized mostly by the presence of aliphatic keto groups (Figs. 3, 4, 5). The corresponding water extracts (Fig. 3) were indeed characterized by the presence of much more polar compounds, as evidenced by the appearance of strong absorption band in the hydroxyl regions (3,300–3,500 cm−1). Similar profiles have been reported by Karlsson and Erlandsson [19, 31] for the degradation products obtained from polyethylene samples not containing pro-degradant additives exposed to thermal and photolytic treatments.
Outdoor exposed LLDPE-TD1 and LLDPE-TD2 aged samples were previously characterized by both TGA and DSC analysis [26]. The thermal stability (TGA in nitrogen atmosphere) of the analyzed samples was shown to be reduced as consequence of the outdoor exposure, thus recording in both cases a significant drop of the onset temperatures (TON) with respect to the corresponding temperature values observed in the case of pristine specimens. On the other hand, only minor changes were recorded in the case of the melting temperature and crystallinity degree as determined by DSC analysis in comparison with the pristine specimen [26].
Biodegradation Test in Aqueous Medium
A preliminary investigation, aimed at evaluating the biodegradation tendency of the analyzed LLDPE-TD1 and LLDPE-TD2 film samples, was carried out in a respirometric test in aqueous medium by using original and outdoor exposed samples as sole carbon source. The cumulative CO2 emissions, expressed as average value of three replicates, recorded after 90 days of incubation from the aqueous cultures supplemented with test materials and blanks, are reported in Fig. 6.
Only slight differences were recorded in the CO2 production from liquid culture supplemented with original film, outdoor exposed specimens and blank. Nevertheless it can be evidenced a fairly good correlation between the amount of low molar mass compounds as detected after the solvent fractionation and the CO2 emission. Consequently, higher CO2 productions were recorded in the aqueous cultures supplemented with outdoor exposed LLDPE-TD1 specimen that was characterized by an overall higher degree of oxidation (i.e., higher COi and solvent extractable fraction) with respect to LLDPE-TD2 sample.
At the end of the test all the analysed materials were withdrawn from the aqueous cultures, carefully washed and characterized by gravimetric analysis, FT-IR spectroscopy and thermal analysis.
A significant increase of the COi values was detected in the case of outdoor exposed specimens submitted to biodegradation (Table 3). In this connection also an increase of the specimens weights was recorded, it can be attributed either to the oxygen uptake and/or to the formation of tightly bound microbial biofilms onto the specimens surface.
By contrast, a different behavior was observed in the case of pristine samples submitted to biodegradation test in aqueous medium under the same experimental conditions (Table 4). In this latter case, no significant increase of the level of oxidation was detected. A marked weight increase was indeed detected, it could be attributed, however, almost exclusively to lightly bound biofilm formation onto the specimen surfaces.
The recorded observations were confirmed by TGA analysis carried out on the test samples before and after the biodegradation process in the aqueous medium. In accordance, the thermal stability of outdoor exposed samples was shown to decrease after the incubation in the aqueous microbial cultures. In keeping with that, the temperatures, corresponding to 2 % weight loss (TON), of these samples decreased from 353.5 and 304.2 °C to 218.0 and 226.0 °C before and after biodegradation, in the case of LLDPE-TD1 and LLDPE-TD2 samples, respectively.
Different results were indeed recorded in the case of pristine samples submitted to the same aqueous biodegradation test. In this case, in fact, the thermal stability did not change significantly for LLDPE-TD1 specimens, whereas a decreased thermal stability was detected for LLDPE-TD2 sample specimen (Table 5).
All the samples submitted to the aqueous biodegradation tests were also analyzed by DSC and the data relevant to the melting temperatures and crystallinity are reported in Table 6. DSC traces, relevant to the second heating, did not reveal, however, substantial variation in the thermal parameters before and after the incubation in aqueous microbial cultures. Nevertheless, a slight decrease of the crystallinity was observed in the case of pristine samples submitted to the biodegradation test, whereas, this parameter was found to slight increase after the incubation in the aqueous medium within the same incubation time in the case of outdoor exposed specimens (Table 6).
It has to be remarked that in the case of LLDPE sample, characterized by an initial low COi value, an increase of the oxygen content (COi) and a corresponding lower thermal stability were observed. These data, along with the slight differences recorded in the crystallinity degree of biodegraded samples in aqueous medium, are also suggesting that the microbial population present in the aqueous medium may stimulate directly a certain level of oxidation of the polymer matrix.
Soil Burial Biodegradation Tests of Outdoor Exposed LLDPE Film Samples
The second stage in the assessment of the environmental fate of degradable PE for mulching application is represented by the evaluation of the ultimate biodegradation in littering or disposal environments. In fact, soil environment can be considered as one of the most probable throwing away habitats where plastic items, such as mulching films, carrier bags and commodities in general, even after composting treatment, may ultimate their degradation and biodegradation processes
Outdoor exposed specimens of both LLDPE samples, which were characterized by a fairly low initial level of oxidation, interestingly showed, even at fairly low mineralization level (Fig. 7), a substantial increase in the COi as high as two–three fold after one year incubation in soil with respect to that recorded at the beginning (Fig. 8). The overall increase of the level of oxidation of the buried samples is confirmed by appearance in the FT-IR spectra of oxidized functional groups such as hydroxyl and double bond with respect to the starting materials (Fig. 9). It can be therefore suggested that the oxidative degradation of these materials, still proceeds, once they are confined in an active microbiological environment, even at room temperature and in the absence of light irradiation.
In accordance with these data, also the thermal stability, as determined by TGA, of the LLDPE-TD1 and LLDPE-TD2 outdoor exposed specimen, was found to change upon the incubation in soil cultures. In particular, after 3 months incubation, a significant drop in the thermal stability was recorded with respect to the corresponding values attained before the biodegradation test (Table 7). Nevertheless, at longer incubation times (12 and 27 months) the onset temperatures (TON) were found to further increase with the exception of LLDPE-TD1 specimen at month 27, most likely because of the assimilation of degraded LLDPE fractions by soil microorganisms.
LLDPE samples submitted to biodegradation in soil were also characterized by DSC analysis, thus evidencing in all the analyzed specimens the overall increase of the degree of crystallinity from 46.3 to 53.8 % in the case of LLDPE-TD1 specimens, and from 47.5 to 50.2 % in the case of LLDPE-TD2 specimens as a consequence of soil incubation. On the contrary, the melting temperature (Tm) was not significantly affected by the incubation in the soil medium. DSC characterizations also suggested that the noticeable changes of crystallinity can be considered as a result of oxidation and assimilation of lower molecular fractions under biotic conditions.
Biodegradation of LLDPE Films in Solid Medium in the Presence of Phanerocheate chrysosporium Fungal Strain
In order to confirm the ability of the soil-borne microorganisms to promote the oxidation of LLDPE specimens, analogous tests were carried out by using the lignin-degrading fungus Phanerocheate chrysosporium. This strain has been utilized to study the degradation of a wide variety of recalcitrant organic pollutants because of the powerful oxidizing enzymatic tool box associated to lignin-degrading or wood-rotting activities [32].
The biodegradation test was carried out onto solid media under co-metabolic conditions (e.g., in the presence of an easily assimilable carbon source such as glucose) by using outdoor exposed LLDPE-TD1, LLDPE TD2 and relevant pristine specimens. After 6 months of incubation on solid cultures inoculated with P. chrysosporium, LLDPE-TD1 and LLDPE-TD2 test specimens were carefully cleaned up and characterized by means of TGA, DSC and determination of COi by FT-IR spectroscopy. Relevant data are collected in Table 8.
Analogously to that previously recorded in aqueous and soil burial biodegradation tests, significant increase of COi values along with a marked decrease of the decomposition temperatures (TON) were observed in the test samples submitted to incubation in P. chrysosporium cultures. A slight increase in the degree of cristallinity was also recorded in the case of outdoor exposed sample (data not shown).
The collected results clearly indicated the progress of oxidation of the samples submitted to outdoor exposure as a consequence of the metabolic activity of the P. chrysosporium, thus demonstrating that a moderate level of oxidation in the LLDPE matrix is sufficient to induce further microbial attack as previously recorded in the tests carried out in the presence of the selected fungal strains [26]. Nevertheless, it was also interestingly observed that the selected fungal strain was capable to induce a significant oxidation of the polymer matrix in the pristine specimen of LLDPE-TD1. In this latter case, either a decrease in the overall thermal stability (Table 8) as well as the formation of carbonyl groups (Fig. 10) were recorded after 180 days of incubation.
Conclusion
From the undertaken investigation on the behaviour of LLDPE film samples loaded with pro-degradant additives, some interesting conclusive remarks can be drawn from the relevant degradation tests carried out under abiotic and biotic conditions on the samples not exposed and exposed outdoor for 3 months. The tendency to oxidation of LLDPE matrix is depending upon the pro-degradant additive used in the formulation of the film samples. Most importantly, the LLDPE containing pro-degradant can accelerate oxidative degradation when film samples are submitted to outdoor exposure. The rate and degree of oxidation detected by FT-IR spectroscopy, and recorded as COi index of the tested samples appear to be a simple straightforward parameter for the assessment of the relative extent of degradation and for predicting the potential assimilation of the oxidized fragments by microorganisms.
A second point to be remarked is that the LLDPE samples characterized by an initial low COi value, experience an increase of the oxygen content (COi) and a corresponding lower thermal stability once they are submitted to biodegradation under different environmental conditions, as well as to the metabolic activity of a specific microorganism strain such as P. chrysosporium. In this respect, it is worth noting that usually a decrease of the oxidized fraction is found when abiotically degraded PE is submitted to biodegradation experiments due to their preferential assimilation. On the contrary, the present investigation is suggesting for the first time that a fairly low level of oxidation is stimulating the degrading activity of microorganisms, thus promoting further oxidation and degradation of the full carbon backbone chains in a continuous process during which assimilation and oxidation seems to proceed simultaneously even tough at fairly low rate and relevant extent.
In accordance it looks like that the combination of different environmental factors such as oxygen, temperature, sunlight and action of living microorganisms appear to be responsible for the degradability/biodegradability of LLDPE containing pro-degradant additives. The testing procedure applied to samples, previously submitted to a preliminary degradation step aimed at closely mimicking the field scale conditions, such as those related to outdoor exposure, appears to be convenient for the assessment of their tendency to biodegradation. The obtained results have significance in achieving effective and sustained biodegradation of LLDPE and are critical for the design of LLDPE based products and somehow able to predict their ultimate fate once disposed into various environmental compartments under controlled or uncontrolled conditions.
References
Kyrikou I, Demetres B (2007) J Polym Environ 15:125
Briassoulis D (2005) Polym Degrad Stab 88:489
von Elsner B, Briassoulis D, Waaijenberg D et al (2000) J Agr Eng Res 75:1
Dilara PA, Briassoulis D (1998) Polym Test J 17:549
G. Scott (1995) In: Scott G, Gilead D (eds) Introduction to the abiotic degradation of carbon chain polymers. In: Degradable polymers: principles and applications. Chapman & Hall, London
Al-Malaika S, Scott G (1983) In: Allen NS (ed) Degradation and stabilisation of polyolefins. Applied Science Publishers, London
Wiles DM, Scott G (2006) Polym Degrad Stab 91:1581
Billingham NC, Calvert PD, Allen NS (1983) In: Allen NS (ed) Degradation and stabilization of polyolefins. Applied Science Publishers, London
Scott G, Wiles DM (2001) Biomacromolecules 2:615
Scott G (1994) In: Doi Y, Fukuda K (eds) Biodegradable plastics and polymers. Amsterdam, Elsevier, pp 79–91
Scott G, Gilead D (1978) British Patent 1, 588:344
Scott D, Gilead D (1982) In: Scott G (ed) Developments in polymer stabilisation. Applied Science Publishers, London, pp 71–106
Fabbri A (1995) In: Scott G, Gilead D (eds) Degradable polymers: principles and applications, 1st edn. Chapman & Hall, Chapter 10
Guillet JE (1973) US patent 3, pp 753–952
Guillet JE (1973) in: J.E. Guillet ed. Polymers and ecological problems. Polymers with controlled life times. Plenum, New York
Roy PK, Sureka P, Rajagopal C, Chatterejee SN, Choudhary V (2006) Polym Degrad Stab 91:1791
Osawa P, Kurisu N, Nagashima K, Nankano K (1979) J Appl Polym Sci 23:3583
Khabbaz F, Albertsson A-C, Karlsson S (1999) Polym Degrad Stab 63:127
Karlsson S, Hakkarainen M, Albertsson A-C (1997) Macromolecules 30:7721
Roy PK, Surekha P, Rajagopal C, Chatterjee SN, Choudhary V (2005) Polym Degrad Stab 90:577
Setnescu R, Silviu J, Osawa Z (1998) Polym Degrad Stab 60:377
Weiland M, Daro A, David C (1995) Polym Degrad Stab 48:275
Jakubowicz I, Yarahmadi N, Arthurson V (2011) Polym Degrad Stab 96:919
Chiellini E, Corti A, D’Antone S (2007) Polym Degrad Stab 92:1378
Koutny M, Sancelme M, Dabin C, Pichon N, Delort A-M, Lemaire J (2006) Polym Degrad Stab 91:1495
Corti A, Sudhakar M, Vitali M, Imam SH, Chiellini E (2010) Polym Degrad Stab 95:1106
Chiellini E, Corti A, Swift G (2003) Polym Degrad Stab 81:341
ASTM D5272-08 standard practice for outdoor exposure testing of photodegradable plastics, Book of Standards vol. 08.03
Sudhakar M, Doble M, Murthy PS, Venkatesan R (2008) Int Biodeterior Biodegrad 61:203
Leskovics K, Kollár M, Bárczy P (2006) Mater Sci Eng A 419:138
Erlandsson B, Karlsson S, Albertsson A-C (1997) Polym Degrad Stab 55:237
Iiyoshi Y, Tsutsumi Y, Nishida T (1998) J Wood Sci 44:222
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Corti, A., Sudhakar, M. & Chiellini, E. Assessment of the Whole Environmental Degradation of Oxo-Biodegradable Linear Low Density Polyethylene (LLDPE) Films Designed for Mulching Applications. J Polym Environ 20, 1007–1018 (2012). https://doi.org/10.1007/s10924-012-0493-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-012-0493-7