Abstract
In this study we investigated the role of the water content of extrudates had in foaming capacity and searched for the water content giving the greatest expansion of starch extrudates. Porous structures based on potato amylopectin starch were prepared by extrusion followed by a microwave foaming process. Starch was first extruded with water, in order to incorporate water in the granular structure and achieve gelatinization. Extrudates were conditioned at humidities ranging from 11% to 97%. The water content in the starch extrudates was studied by a water vapor sorption isotherm study. Extrudates were analyzed with light microscopy and wide angle X-ray scattering studies to determine degree of crystallinity. In the second step, extrudates were foamed in a microwave oven. As the water started to boil, it acted as a blowing agent, leaving a porous closed-cell starch structure. The densities and the expansion ratios of the foamed samples are determined. Porosity was studied with environmental scanning electron microscopy. Mechanical properties as a function of the surrounding humidity were analyzed with dynamic mechanical analysis. We found that the maximal degree of expansion was in extrudates conditioned at 33% and 54% RH and having water content of 11.2% and 13.4%, respectively. This level of water is sufficient to expand the extrudate to a maximum level but not high enough to plasticize the starch and cause cell collapse after treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Starch is abundant all over the world as it is produced by most higher plants as energy storage. Starch is an important source of nutrition for man, but it is also used for industrial applications. Starch is composed mainly of the two polysaccharides amylose and amylopectin. Both amylopectin and amylose have a α(1→4) linked d-glucan backbone. Amylose is essentially linear and amylopectin is extensively (1→6) branched. Amylose has a molecular weight of 105–106 and amylopectin 2 × 107–5 × 108 [1–3].
Because of the growing greenhouse effect due to the use of non-renewable resources such as crude oil and the incineration of fossil fuels, starch has gained interest as a raw material for disposable products such as films and foams [4, 5]. In the past two decades starch has been used in many different applications in attempts to replace synthetic polymers [5]. Starch is abundant and a relatively cheap raw material. In addition to being biodegradable, starch has the advantage of being carbon dioxide neutral, which means that the same amount of carbon dioxide that is produced when the plant, or products thereof such as starch plastics, is degraded it is consumed as the plant grows. Starch has been used as a filler in synthetic polymers [6] and as a raw material for products such as bags, packaging chips and other disposable products [4, 5]. To create a thermoplastic material of native starch, it is necessary to disrupt and melt the semi crystalline granular structure of starch [7, 8]. Several methods have been used in preparing starch-based thermoplastic materials, such as baking in a hot mould [9] to create shaped articles, freeze drying [10] and extrusion all of which are commonly used in the food industry. Foams have also been prepared by a microwave technique [11].
Starch granules are very sensitive to humidity and quickly adapt their moisture content to the surrounding humidity. Physical properties and the material properties of starch vary significantly with its moisture content [12]. Water molecules break the hydrogen bonds between chains in the starch granules, the mobility of the chains increases and the glass transition temperature, T g, is thus reduced. T g depends on the mobility of the polymer. Water molecules decrease the interaction energy between starch chains and increase the distance between them, resulting in a decreased T g [13]. It has been shown that there is a relationship between T g and the foaming capacity. Kokini and co-workers [14] state that the largest expansion in cereal starches is achieved in a temperature region, ranging from T g to 100 degrees above T g, where after thermal degradation of the sample starts. Water is a strong plasticizer for many biopolymers [15]. In addition to having a plasticizing effect on starch, water is also the blowing agent in the preparation of foams in a microwave oven. During heating in a microwave oven starch extrudates will expand at the same time as their water contents are reduced [16]. Boiling water introduces steam bubbles in the structure the water eventually evaporates and leaves a porous closed-cell structure.
Studies by Shogren and co-workers [9] showed that foams prepared from amylopectin rich starch have lower densities than foams rich in amylose. This is valid for foams prepared by both extrusion [9] and microwave treatment [11]. In literature contradictory results have been found, were amylose rich starches have a larger foaming capacity [17, 18]. Based on our previous results [11] we choose to focus this study on potato amylopectin starch.
Microwave treatment is a fast way of adding energy and is used in many industrial applications. Buffler [19] described the way in which microwave energy is converted to heat. Microwave energy can be used for cooking and heating as well as for baking and expansion of foods [20]. A study of popping popcorn shows that the best expansion was achieved when the corn kernels had a moisture content of 10–15% [21]. When starch extrudates are heated by microwaves the moisture will generate superheated steam, which creates high internal pressure [22]. During heating, the starch in presence of water undergoes a phase transition from a glassy to a rubbery state. The walls inside the starch extrudates will start to yield to the pressure created by the superheated steam. As a result of moisture loss during microwave treatment and cooling after treatment, the structure will return to the glassy state and the porous structure will become permanent [23].
The purpose of this study was to identify the effect of moisture content on the foaming ability of high amylopectin starch during microwave processing and to identify the optimum degree of moisture.
Materials and Methods
Materials
Potato amylopectin starch in granular form was kindly supplied by Lyckeby Stärkelsen AB (Kristianstad, Sweden). This type of potato starch was genetically engineered by Lyckeby Stärkelsen AB and Svalöf Weibull AB (Svalöv, Sweden) in order to suppress the synthesis of amylose [24]. Deionized ultra filtered water, millipore®, was used.
Extrusion
Starch was gelatinized and blended with water by an extrusion process. Starch and water were extruded in a Haake Minilab, Germany, twin screw co-rotating extruder. The screws had a length of 110 mm and a diameter of 14 mm. The die of the extruder had a diameter of 2.5 mm. Starch and water were mixed prior to extrusion, total water content in starch was 20 wt%. The extrusion temperature was 95 °C. The extruder had a screw speed of 100 revolutions per minute (rpm). The starch/water mixture was circulated in the extruder for 2 min.
Conditioning
Samples of extruded amylopectin starch were conditioned in five different levels of ambient humidity. Pieces of extrudates were placed in closed vessels containing saturated salt solutions. The salts used were lithium chloride (Sigma-Aldrich R31413), magnesium nitrate hexahydrate (Sigma-Aldrich F63079), sodium chloride (Sigma-AldrichF71381) and potassium sulphate (Sigma-Aldrich R31270). According to ASTM standard E104 [25] saturated solutions of these salts result in relative humidities of, 11%, 33%, 54%, 75% and 97%, respectively, at room temperature, 23 °C. Extrudates were first placed in the vessel with the lowest relative humidity, 11%, when equilibrium water content was reached, they were successively moved to a higher degree of relative humidity. Samples were weighed on a Mettler AE260-DR balance to determine the mass after water absorption. Samples were weighed every day. When no weight increase could be noted saturation was considered to have been reached and the samples were moved to a higher humidity. When the highest relative humidity was reached, extrudates were successively moved to the next lower humidity. When the lowest level was reached, the samples were dried in an oven at 120 °C for 24 h in order to achieve complete dryness. The true water percentage at each level of relative humidity was calculated. All measurements were made in triplicate. The deviations were small.
Light Microscopy
Extruded amylopectin starch samples were optically studied with light microscopy to investigate the efficiency of the gelatinization process. Extrudates were pressed to 1 mm thick films prior to measurements. Films were pressed in a Fontyne table press, TP 200, at 95 °C with an initial pressure of 5 kN for 5 min and at the higher pressure of 100 kN for 5 min. Photos were taken in a Nikon FXA light microscope with 10 times enlargement.
Foaming
Starch extrudates were foamed by microwave treatment. Extruded and subsequently conditioned samples of amylopectin starch were placed in a microwave oven. The microwave oven was a Sharp (Hamburg, Germany) R-202N that emits microwaves with a frequency of 2450 MHz. Samples were exposed to microwaves for 2 min and then left to dry in 33% RH.
Wide Angle X-ray Diffraction
After extrudates were expanded into foams by microwave treatment, the foams were ground in a mortar in liquid nitrogen and reconditioned for 7 days in closed vessels in 54% relative humidity using a saturated aqueous salt solution of magnesium nitrate. Analyses were made in triplicate, and diffractograms of the powdered samples were recorded in the reflection geometry on a Siemens D5000 Diffractometer using nickel filtered CuKa (l = 0.154 nm) radiation. Diffractograms were taken between 5 and 30°(2θ) at a rate of 1°(2θ) per minute and a step size of 0.1°(2θ).
Density
Foam density was measured by a sand volumetric displacement method. A similar method has been used by Segnini and co-workers [26]. Prior to measurement, samples were conditioned in 33% RH. Three different foams were analyzed for each sample. The measurements were made in triplicate, and deviations were small. Pieces of the foams were weighed on a Mettler AE260 analytic balance. The pieces were then placed in a 25-mL graduated cylinder, and a known volume of sand was added to the cylinder. The total volumes of foams and sand were recorded after tapping the graduated cylinder for 1 min. Foam density was calculated from the mass divided by the displaced volume.
Expansion Ratio
The volume of conditioned amylopectin extrudates was measured by a sand volumetric replacement method. The extrudates were foamed in a microwave oven, and the volume was measured. The expansion ratio was calculated according to Eq. 1. The values given are an average of measurements made in triplicate.
Environmental Scanning Electron Microscopy
The porous structures of foams prepared by microwave treatment of extruded amylopectin starch were studied by taking images with an environmental scanning electron microscopy (ESEM). Pieces of foams were immersed in liquid nitrogen prior to being cut with a scalpel to achieve sharp incisions and to avoid crushing the samples. ESEM images were received in a Philips XL 30 operated at 12 kV. Images were taken at 50 times enlargement at 0.9 torr.
Dynamic Mechanical Analysis
The mechanical behavior of the cell walls in the foams was studied with dynamic mechanical analysis, DMA. Extruded amylopectin was pressed to a thin film to mimic the cell wall. A Perkin Elmer DMA7 was used with an extension assembly connected to a humidity controller. Films were cut into 5 mm × 15 mm strips with a thickness of 0.06 mm. The strips were mounted between two holders. Samples were tested in tensile mode with a frequency of 1 Hz and an amplitude of 4 μm. The relative humidity in the sample chamber followed a program that held RH at 10% for 30 min and then increasing it by 1% per minute until 95% RH was reached; after which 10% RH was held for 1 min. Three different testing temperatures were used, 40, 60 and 80 °C, and all measurements were made in duplicate. It was of interest to determine at which humidity the softening occurred at each testing temperature.
Results and Discussion
Extrusion
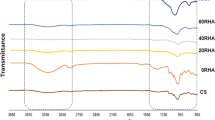
After extrusion, amylopectin starch was collected as a clear, transparent homogeneous material with thermoplastic properties. The X-ray diffractogram, Fig. 1, shows a lack of crystallinity. There are no visible peaks indicating residual crystallinity or peaks as signs of recrystallization. An amorphous material has been created. The starch granules were destructurized and gelatinization took place in the extruder. The diffractograms show that the starch extrudates did not recrystallize during conditioning for 5 days, even at the highest surrounding humidity 97%, yielding a starch extrudate with moisture content 25.3%.
Light microscopy images were taken in to verify the results of WAXS diffractograms. Images, Fig. 2a and b, show only a few individual undisrupted granules as signs of residual crystallinity. Undisrupted granules are identified by the significant Maltese cross. With so few granules present, the material can still be considered amorphous.
Water Vapor Sorption Isotherms
After conditioning extrudates at five different levels of room humidity the moisture contents at each level were calculated and the values are compiled in a water vapor sorption isotherm graph, Fig. 3. The graph shows hysteresis; the water content is dependant on whether the sample comes from a lower or a higher relative humidity. Samples from a lower relative humidity have low water content than samples from a higher relative humidity. Hysteresis of native starch is well known and has been described by Sala and Tomka [27] and Slade and Levine [28]. This is also the case for the potato amylopectin used in this study. The graph in Fig. 3 can be divided into three regions. In the first region, from 0% to 11.4%, there is a steep increase in water content. The dry starch has polymer–polymer hydrogen bonds as well as free hydroxyl groups that can absorb water, and the water content thus increases rapidly. The second region has a more modest increase in water content. There is an equilibrium of polymer–polymer hydrogen bonds and polymer–water hydrogen bonds. The highest moisture content was 25.3% when conditioned at 97% RH. There is another significant increase in water uptake at approximately 75% RH. There is a majority of polymer–water hydrogen bonds [23].
Density and Expansion
The density of foams prepared by microwave treatment as a function of conditioning level is listed in Table 1. The lowest foam density was found for extrudates containing 13.4% water and that had been conditioned in 54% RH prior to foaming in the microwave oven. Extrudates conditioned at 33% RH, and that had a water content of 11.2% also yielded foams with a low density. Extrudates conditioned at 11% RH had a higher density; we believe that the relatively low water content, 8.3%, is not sufficient to expand the extrudate. Even though the difference in moisture content is low, 2.9%, this seems to be important for the foaming effect on the sample. At the higher degrees of moisture, conditioning at 75% and 97% RH, the foam densities are higher than at 54%. We think that a more compact material was created due to collapse of the expanding cells. Water in the extrudates causes expansion and the creation of a porous structure, but water also plasticizes starch and gives the cell walls increased elasticity. The elasticity in the cell wall prevents rupture of the cell during foaming. However, cell wall contains water after foaming, it retains its melt tenacity and can cause the cell to collapse after expansion. The expansion ratios correlate well with the results of the density measurements, Fig. 4. The highest expansion ratio is observed for foams for which the extrudates with water contents of 11.4% and 13.4%, conditioned at 33% and 54% RH, respectively.
ESEM
Images taken with ESEM show that porous structures are created from extrudates conditioned at all five levels of ambient humidity. The degree of porosity varies, however, as do the size and shape of pores. Foam conditioned at the lowest level of humidity, 11%; Fig. 5a, are porous although not to the same degree as foams in Fig. 5b and c, conditioned at 33% and 54%, respectively. Density measurements and expansion ratio calculations show that the highest porosity is achieved for foams conditioned in 33% and 54% RH. These results are in agreement with results observed in ESEM images. Foams conditioned at 75% and 97%, Fig. 5d and e, are not as porous as those shown in the previous figures. A greater number of collapsed cells can be seen at the highest relative humidity, Fig. 5e. Figure 6 shows the edge of a foam piece. The foam has a rather thick skin with low porosity. Cells at the edges of the foam are compressed, and this is the case regardless of the humidity at which the sample was conditioned. This may be because the edges of the sample dry out first. Directly inside the low porosity skin pores are evenly distributed and are even in size and shape.
(a) ESEM image of foam conditioned at 11% RH, 50 times enlargement, (b) ESEM image of foam conditioned at 33% RH, 50 times enlargement, (c) ESEM image of foam conditioned at 54% RH, 50 times enlargement, (d) ESEM image of foam conditioned at 75% RH, 50 times enlargement, (e) ESEM image of foam conditioned at 97% RH, 50 times enlargement
Theoretical Prediction of the Glass Transition Temperature
The glass transition temperature, T g, of a starch extrudate is dependent on its moisture content. T g can be predicted with the Couchmann-Karasz Eq. 2 [29]. The equation was originally developed only for compatible, homogeneous mixed systems, but Brinke et al. [30] expanded its use by showing it to be valid for polymer networks/dilutent systems as well.
W 1 is the weight fraction of the dilutent (here water), W 2 is the weight fraction of the polymer, T g1 is the glass transition temperature of the dilutent, T g2 is the glass transition temperature of the polymer, ΔC p1 is the change heat capacity at T g1 and ΔC p2 is the change in heat capacity at T g2. We calculated T g at the different relative humidities using the water content obtained from the sorption isotherm graph measurements, Fig. 7. The values of T g1, T g2, ΔC p1 and ΔC p2 are taken from Bizot et al. [27], where T g, amylopectin = 558 K, T g, water = 134 K, ΔC p, amylopectin = 0.295 J/gK and ΔC p, water = 1.83 J/gK. Figure 7 shows the predicted T g values as a function of the ambient relative humidity. According to Kokini et al., the greater expansion is achieved in a temperature region of T g and 100 °C and above. The water content in the sample during foaming steadily decreases due to evaporation. The samples conditioned at 33% and 54% RH start with water contents of 11.2% and 13.4% and T g values of 132.5 and 98.9 °C, respectively. They will immediately be within their optimum expansion temperature region and thus have better conditions for expansion.
Dynamic Mechanical Properties
It is of great interest to measure the strength of the walls of cells that are created during foaming, since this will determine whether the cell will collapse. If water is still present after evaporation, the cell wall will have high melt tenacity owing to a low T g, and the cell will be able to flow and collapse. Samples of amylopectin starch were investigated at three different temperatures, 40, 60 and 80 °C. The humidity at which softening of the material occurred was recorded for each temperature. Foaming started at 100 °C, when water was brought to boiling. The results were extrapolated to 100 °C, Fig. 8. Softening occurs at approximately 23% ambient humidity at 100 °C. The corresponding moisture content in the amylopectin extrudate was 10.5–12%, Fig. 1. The best expansion was achieved for extrudates with slightly higher moisture contents, 11.2% and 13.4%.
Conclusions
We have shown that the water content of amylopectin starch extrudates has a significant effect on their expansion capacity during microwave foaming and stability towards shrinkage after treatment. The largest expansion is achieved when extrudates are conditioned at 33% and 54% RH, which corresponds to moisture contents of 11.2% and 13.4%, respectively. This is calculated from measurements of density and is confirmed by observations in ESEM images. Lower water content did not expand the extrudate to the same degree, and thus the foams had higher densities. One reason for this may be the evaporation of all water before maximum expansion can take place. Extrudates with water content, 16.9–25.8%, yield foams with comparatively high densities. We believe these extrudates have been expanded to the same degree as extrudates with optimum water content, 11–13.5%. At high levels of water content, the evaporating water will expand the samples. However after treatment, water is still present in the structure, giving the cell wall a melt tenacity that allows it to shrink, which results in cell collapse. Water has two major functions in creating starch foam; first in forming cells and second in plasticizing the cell wall. Water that forms bubbles creates cells as the water evaporates. As a plasticizer, water lowers T g and increases the melt tenacity. The plasticizing effect will not be great enough at low water content and cells will be disrupted. We have found that the optimum water content in an amylopectin starch extrudate is 11–13.5%. At this level, the extrudate has enough water to prevent breakage of cells during foaming but a low enough water content after treatment to give a fixed structure.
References
Swinkels JJM (1985) Starch/Stärke 37:1
Zobel HF (1988) Starch-Stärke 40:44
Buleon A, Colonna P (1998) Int J Biol Macromol 23:85
Lourdin D, Della Valle G, Colonna P (1995) Carbohydr Polym 27:261
Shogren RL, Fanta GF, Doane WM (1993) Starch-Stärke 45:276
Griffin GJL (1977) U.S. patent No. 4.021.388
French D (1984) Organization of starch granules. In: Whistler RL, Bemiller JN, Paschall EF (eds) Starch chemistry amd technology 2nd edition. Academic Press, New York, USA, 184–247
Blanshard JMV(1987) Starch granule structure and function: a physiochemical approach. In: Galliard T (ed) Starch: properties and potential.8 edn. New York, Wiley, 16–54
Shogren RL et al (1998) Polymer 39(25):6649
Glenn GM, Irving DW (1995) Cereal Chem 72:155
Sjöqvist M, Gatenholm P (2005) J Polym Environ 13:29
Stading M, Rindlav-Westling Å, Gatenholm P (2001) Carbohydr Polym 45:209
Trommsdorff U, Tomka I (1995) Macromolecules 28:6128
Kokini JL, Cocero AM, Madeka H, Degraaf E (1994) Trends Food Sci Technol 5:281
Trommsdorff U, Tomka I (1995) Macromolecules 28:6128
Ernoult V, Moraru CI, Kokini JL (2002) Cereal Chem 79:265
Chinnaswammy R, Hanna MA (1988) Cereal Chem 65:138
Willett JL, Shogren RL (2002) Polymer 43:5935
Buffler CR (1993) Microwave cooking and processing: engineering fundamentals for the food scientist. New York, Van Nostrand Reinhold
Moraru CI, Kokini JL (2003) Compr Rev Food Sci Safety 2:120
Singh J, Singh N (1999) J Food Eng 42:161
Boischot C, Moraru CI, Kokini JL (2003) Cereal Chem 80:56
Kokini JL, Lai LS, Chedid LL (2002) Food Technol 46:124
Hofvander P, Persson PT, Tallberg A, Wikström O (1992) Swedish patent SE 9004096 5
ASTM (2001) E104-85 Standard practice for maintaining constant relative humidity by means of aqueous solutions. Annual Book of ASTM Standards
Segnini S, Pedreschi F, Dejmek P (2004) Int J Food Properties 7:37
Sala RM, Tomka I (1992) Sorption Angew Makromol Chem 199:45
Slade L, Levine H (1991) Crit Rev Food Sci Nutr 30:115
Couchmann PR, Karasz FE (1978) Macromolecules 11:117
Brinke G, Karasz FE, Elllis TS (1983) Macromolecules 16:244
Acknowledgments
This work was funded by SCA Hygiene Products and KK foundation, through the Marchal program. Lyckeby stärkelsen is gratefully acknowledged for material supply. We appreciate our discussions with Dr Shabira Abbas at SCA and Dr Karin Svegmark. Thanks to Camilla Bemm at SCA for ESEM images and to Anne-Mari Olsson and Dr Lennart Salmén at STFI for help with DMA measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sjöqvist, M., Gatenholm, P. Effect of Water Content in Potato Amylopectin Starch on Microwave Foaming Process. J Polym Environ 15, 43–50 (2007). https://doi.org/10.1007/s10924-006-0039-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-006-0039-y