Abstract
The aim of this study was to investigate the alterations in regional homogeneity assessed by fMRI in patients with migraine without aura (MWoA). Fifty-six eligible MWoA patients and 32 matched healthy volunteers were enrolled in this study. MWoA patients were divided into three groups according to the headache days per month within 3 months: infrequent episodic migraine (IEM) group, frequent episodic migraine (FEM) group, and chronic migraine (CM) group. Data collection and rest-state fMRI examination were performed in all cases. The ReHo method was used to analyze the blood oxygen level dependent (BLOD) signals of the adjacent voxels in the brain regions of each patient, and the consistency of their fluctuations in the sequences of same time. Compared with normal controls, ReHo values of bilateral thalami, right insula and right middle temporal gyrus increased and both precentral gyri decreased in the IEM group; ReHo values of bilateral thalami and the right middle temporal gyrus increased; ReHo values of both anterior cingulate cortex, precentral gyri and putamen decreased in the FEM group. Compared with control group, ReHo values of left olfactory cortex, right hippocampus, parahippocampal gyrus, suboccipital gyrus and precuneus increased, both precentral gyri, precuneus, putamen and anterior cingulate cortex decreased in the CM group. Compared with IEM group, ReHo values of both putamen, left middle frontal gyrus, right superior frontal gyrus increased, and the left precuneus decreased in the FEM group. Compared with FEM group, ReHo values of left olfactory and left precuneus increased, and the right superior frontal gyrus, insula, middle temporal gyrus, thalami, both superior temporal gyri decreased in the CM group. In the IEM group, the changes of function focus on the regions associated with coding, conduction and regulation of pain signals. In the FEM group, functional alterations mainly concentrated on the regions associated with pain regulation and emotion cognition. In the CM group, the changes focus on the regions related to spatial attention and cognition, affective disorders and pain feedback, which may be associated with migraine production, development and chronification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Migraine is a common disabling disease, usually accompanied by nausea, vomiting and heightened sensitivity to light and sound, which seriously affects people’s health and quality of life. Migraine has been listed as one of the four diseases affecting human health by the World Health Organization [1, 2]. According to the headache days per month, it can be divided into episodic migraine (EM) and chronic migraine (CM). It has been reported that about 2% to 2.5% of EM undergoes chronic transformation each year [3]. Chronic transformation of migraine (Chronification) causes changes not only in the frequency of headache but also in the nature of headache. The mechanism of chronic transformation of migraine has not yet been fully clarified. Resting state BLOD-fMRI is widely used in the functional study of neurological diseases, but there isn’t relevant functional imaging study for the migraine patients with different frequency of attack.
Functional MRI (fMRI) has been used to investigate the mechanisms leading to sensory hypersensitivity by measuring brain responses to visual, olfactory, and painful cutaneous stimulation in migraine cases, and functional connectivity analyses have shown the reconstruction of specific brain regions and networks responsible for sensory processing. These studies have consistently shown atypical brain responses to sensory stimuli, absence of the normal habituating response between attacks, and abnormal functional connectivity of sensory processing regions. Identification of the mechanisms related to sensory hypersensitivity might help to better understand neurological dysfunction in migraine and provide new targets for prevention therapy, and also provide fMRI image markers indicating early responses to preventive therapy. In this study, the Regional Homology Analysis Method (ReHo) was used to analyze the blood oxygen level dependent (BLOD) signals of the adjacent voxels in the brain regions of migraine cases without aura, who were grouped according to the headache days per month within 3 months. We used the healthy subjects as the control group to compare the difference of brain activity in the resting state with migraine patients, which would provide the basis for further investigation of the mechanisms of migraine occurrence and development.
Objects and methods
General information
Case group
A total of 65 migraine patients (age range, 14–76 years) without aura presented in the Department of Neurology in the First Affiliated Hospital of Soochow University from December 2016 to September 2017. All the subjects met the following criteria: (1) Conform to the diagnostic criteria for migraine without aura in the International Classification of Headache Disorders, 3rd edition; (2) the patients without headache attacks within 3 days before scanning; (3) No history of overuse of analgesics; (4) No abnormal signal in the brain in conventional MRI scanning; (5) Female subjects were not pregnant or during menstrual periods. Exclusion criteria were as follows: (1) Patients with other types of headache; (2) Patients accompanied with cardiovascular and cerebrovascular diseases and other medical diseases; (3) Patients with a history of drug or alcohol addiction or abuse; (4) Patients with contraindications for MRI.
Collect clinical information of all the cases and exclude one patient with organic disease in the brain and patients with horizontal head movement exceeding 1 mm and rotational head movement exceeding 1° (8 cases). 56 patients cooperating with examination were divided into three subgroups as follows.
Infrequent episodic migraine (IEM) group: patients met the diagnostic criteria for migraine without aura, and the headache days per month ≤2 days, 19 patients were enrolled (5 males and 14 females, mean age (41.58±2.56) years old);
Frequent episodic migraine (FEM) group
patients met the diagnostic criteria for migraine without aura, 3–14 days of headache per month, 20 patients were enrolled (4male, 16 female, average age (38.00±2.69) years old);
Chronic migraine (CM) group
patients met the diagnostic criteria for chronic migraine (headache days≥15 days/month, lasts longer than 3 months, at least 8 days/month are consistent with migraine characteristics). 17 patients were enrolled (9 males and 8 females, average age (49.59 ± 3.55) years old);
Control group
31 healthy subjects (13 males and 18 females) with an average age (49.77 ± 2.46 years old), who were matched with migraine groups in age and gender.
All subjects signed the written consent form.
Image acquisition
A 3.0 T MRI scanner (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany) was used to perform the resting state functional MRI scanning and T1 structure image scanning with blood oxygenation level-dependent functional magnetic resonance imaging (BLOD-FMR). The subjects were on a supine position, and their heads were fixed with a sponge. When the functional image data were captured, all the subjects were quietly in the examination bed, closed their eyes and relaxed, avoided mental activity, and were prohibited to fall asleep. The relevant scan sequences and parameters were described as follows: Resting state blood oxygenation level-dependent functional magnetic resonance imaging (BOLD-fMRI): Axial position 33 layers, pulse repetition time/echo time (TR/TE) = 2000 ms/ 30 ms, section thickness/ interval = 4 mm/ 0 mm, field of view (FOV) = 256 mm×256 mm, collecting 240 times, and scanning line parallel to the anterior-posterior commissure Plane; The whole brain 3D high-resolution T1WI structure image: 192 layers in sagittal, TR/TE = 2300 ms/900 ms, section thickness/ interval = 1 mm/0 mm, FOV = 256 mm×256 mm.
Data processing
The data from the first 10 time points were eliminated to rule out the effects of magnetic field inhomogeneity and subjects inadaptability at the beginning of scan. The remaining 230 time points were analyzed. Pre-processing: The original resting magnetic resonance data were processed by the DPARSF software based on MATLAB platform, and then the corrected images were normalized and the Full-Width Half Maximum (FWHM) was set as 8 mm.
Statistical analysis
SPM8 was used to perform the independent double-sample t-test analysis for each group of ReHo data. If there were significant differences in the age, gender and education level among migraine groups, the corresponding covariate was introduced in statistical comparison for each group, and analysis of multivariate variance was used to reduce the impact of differences on the results. The threshold was set as P < 0.05 (false discovery rate test correction). Measurement data were expressed as mean±standard deviation.
Results
After screening, 56 MWoA patients were enrolled in the migraine groups, including 19 cases in the IEM group, 20 cases in the FEM group, and 17 cases in the CM group. 31 age-and sex-matched healthy subjects served as control group. The clinical data were shown in Table 1.
-
(1).
Changes in ReHo in brain regions of migraine cases compared with healthy subjects
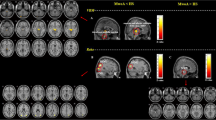
ReHo significantly increased in following brain regions in the IEM group: bilateral thalami, right central anterior gyrus, left central posterior gyrus, right insular lobe and right sacral gyrus; significantly decreased in bilateral prefrontal cortex and left angular gyrus. (See Fig. 1 and Table 2).
ReHo difference between infrequent episodic migraine (IEM) group and health control group. Warm colors showed ReHo increased in MWoA patients; cool colors showed ReHo decreased in MWoA patients; p < 0.05, FDR corrected. Axial images were overlaid on transverse slices of MNI-152 standard anatomical image. The left side of the brain corresponded to the right hemisphere and vice versa. List of structures in Table 2
ReHo significantly increased in following regions in the FEM group: bilateral central posterior gyri, thalami and right middle temporal gyrus; significantly decreased in bilateral anterior cingulate cortex, prefrontal cortex, putamen and right supplementary motor area (See Fig. 2 and Table 3).
ReHo difference between frequent episodic migraine (FEM) group and healthy control group. Warm colors showed ReHo increased in MWoA patients; cool colors showed ReHo decreased in MWoA patients; p < 0.05, FDR corrected. Axial images were overlaid on transverse slices of MNI-152 standard anatomical image. The left side of the brain corresponded to the right hemisphere and vice versa. List of structures in Table 3
ReHo significantly increased in following regions in the CM group: the left olfactory cortex, right hippocampus, parahippocampal gyrus, suboccipital gyrus, cuneus, and occipital gyrus; significantly decreased in bilateral prefrontal cortex, precuneus, putamen, and anterior cingulate cortex (see Fig. 3 and Table 4).
-
(2).
Comparison of ReHo among migraine groups
ReHo difference between Chronic migraine (CM) group and normal control group. Warm colors showed ReHo increased in MWoA patients; cool colors showed ReHo decreased in MWoA patients; p < 0.05, FDR corrected. Axial images were overlaid on transverse slices of MNI-152 standard anatomical image. The left side of the brain corresponded to the right hemisphere and vice versa. Full list of structures in Table 4
Compared with the IEM group, the brain regions with increased ReHo in the FEM group were bilateral putamen, left precentral gyrus, left frontal gyrus and right superior frontal gyrus; and the regions with a significantly decreased ReHo were left precuneus and right angular gyrus (see Table 5 and Fig. 4).
ReHo difference between infrequent episodic migraine (IEM) group and frequent episodic migraine (FEM) group. Warm colors showed ReHo increased in MWoA patients; cool colors showed ReHo decreased in MWoA patients, p < 0.05, FDR corrected. Axial images were overlaid on transverse slices of MNI-152 standard anatomical image. The left side of the brain corresponded to the right hemisphere and vice versa. Full list of structures in Table 5
Compared with the FEM group, the brain regions with increased ReHo in the CM group were Left olfactory cortex, paracentral lobule, and left precuneus, and the regions with decreased ReHo were right superior frontal gyrus, right insula, bilateral precentral gyri, postcentral gyri, right middle temporal gyrus, bilateral superior temporal gyri and right thalami (see Table 6 and Fig. 5).
ReHo difference between chronic migraine group(CM) and frequent episodic migraine(FEM) group. Warm colors showed ReHo increased in MWoA patients; cool colors showed ReHo decreased in MWoA patients, p < 0.05, FDR corrected. Axial images were overlaid on transverse slices of MNI-152 standard anatomical image. The left side of the brain corresponded to the right hemisphere and vice versa. List of structures in Table 6
Discussion
So far, the pathogenesis of migraine has not yet been completely clarified. The dominant viewpoints are the trigeminal neurovascular theory and cortical spreading inhibition theory [4].
The ascending projection of the trigeminal neurovascular system was launched from the trigeminal ganglion (TG) and connected the intra- and extra-cranial structures (including blood vessels) with trigeminocervical complex (TCC). The second-order neurons from TCC project to third-order thalamic neurons via spinothalamic tract, and there are also direct and indirect ascending projections from the locus ceruleus (LC), periaqueductal gray (PAG) and hypothalami. The third-order thalamic cortical neurons broadly project synaptic junctions with the cortex, including primary and secondary motor (M1/M2), somatosensory (S1/S2) and visual (V1/V2) cortex. Connection from TCC to superior salivatory nucleus (SUS), which provides parasympathetic innervation to extra- and intracranial structures through the sphenopalatine ganglion (SPG). Trigeminal cervical complex (TCC) is affected by descending pain modulation pathway emitted from PAG and Dorsolateral Pontine Tegmentum (DLPT). There are direct connections between primary somatosensory (S1) and insular cortex (INS), meanwhile indirect projections from S1 are emitted through the hypothalami. The hypothalamic projections form direct TCC-regulated projections, and indirect projections through the locus ceruleus (LC) and the periaqueductal gray (PAG), which can be further transmitted through the rostral ventromedial medulla (RVM). This complex network of direct and indirect pathways provides anti- and pro-nociceptive modulation of trigeminal nociceptive signaling, and its dysfunction is considered to be one of the reasons of migraine attacks.
In recent years, with the development of functional imaging techniques, the pathogenesis of migraine has been given a new content. By investigating the changes of functional brain regions and the high sensitivity of neural networks in migraine patients, we can objectively and scientifically understand the characteristics of changes in brain structure and function during migraine attacks, which would help to explore its pathogenesis and find new therapeutic targets [5].
In 2009, Schwedt et al. [6] pointed out that the abnormality of brain function network in migraine was caused by the cumulative effect of long-term repeated headache attacks, which was not directly related to ictal or interictal period, but to the medical history, degree of pain, frequency of attacks, and accompanying symptoms. The local correlation of blood oxygen level dependent (BOLD) can be measured by ReHo analysis via Kendall coefficient of consistency (KCC), which could be used to study the changes of the brain neuronal activity in a resting state and analyze differences in local brain activity [7].
In this study, we used fMRI technology to investigate migraine patients without aura and explore the visualization of neural mechanisms. Our study displays that changes in brain function of migraineurs are not the result of a single episode but the cumulative effect of repeated attacks, our results confirm with the previous viewpoint.
The changes of fMRI with a resting state in IEM group
In this study, a significantly increased activity has been found in the brain regions of bilateral thalami, right precentral gyrus, left posterior gyrus, right insular lobe and right middle temporal gyrus in IEM patients and decreased activity in bilateral prefrontal cortex and left angular gyrus, which is consistent with previous works [4, 5, 10, 11]. The insular lobe and thalami are critical areas of the pain matrix and play a key role in the development of pain. The insular lobe directly or indirectly participates in the perception, coding, and regulation of pain, and it is related to the emotional responses to pain perception [8]. In addition, there are interactions between the insula and many other brain areas, and the changes of its structure and function may reflect the potential changes of emotional processing in migraineurs. The thalami, as a relay station for sensory conduction, participates in the transmission, projection and emotion-related pain responses [9]. Many previous reports have shown that patients with migraine may have functional changes in insula and thalami during interictal periods [10, 11]. Moulton et al. [12] studied the change of fMRI in interictal migraine patients, which displayed overactivated in the medial temporal lobe when compared with healthy subjects, especially in the anterior temporal pole (TP). TP shows significantly increased functional connectivity in several brain regions relative to pain sensation, pain occurrence, emotional change, pain cognition and regulation, which suggests that TP hyperexcitability may contribute to functional abnormalities in migraine. Recent evidence has suggested that disruption of coordinated activity in brain networks may be involved in the pathogenesis of various neuropsychological disorders. Resting-state functional magnetic resonance imaging (rs-fMRI) studies have suggested that the pathophysiology of migraine is associated with altered functional connectivity (FC) in several brain networks, such as the default mode network (DMN), visual, executive control, attention, and salience networks. These functional disturbances may also relate to the sensory, affective, and cognitive components of pain processing in migraine patients. The medial prefrontal cortex is part of the default mode network (DMN). DMN plays an important role in adaptive behavior, cognitive, emotional, and attentional processes [13]. The prefrontal cortex plays a key role in regulating pain perception through the cognitive control mechanism [14]. Tessitore et al. [15] found that the functional connectivity of the DMN in the prefrontal lobe and temporal lobe was significantly reduced compared with that of healthy subjects, which suggests that DMN dysfunction may be associated with the inadaptability of recurrent stress stimuli, so it may serve as image marker for early migraine. Yu et al. [16] reported a significant decrease in the ReHo values in the prefrontal and orbitofrontal cortex of MwoA patients compared with healthy individuals. Our study shows that the ReHo value in the bilateral prefrontal cortex significantly decreases in all of MwoA patients, which confirms that DMN dysfunction may be related to the stress stimulation due to recurrent headache. Our results show that the brain regions with functional changes in IEM group are mainly associated with the ascending projection in the trigeminovascular system and the pain regulation in the default mode network. These brain regions are directly involved the coding, conduction and regulation of pain signals and may be the neuropathological basis of migraineur susceptible to headache.
The changes of encephalic region in a resting state fMRI in FEM group
It is observed that the regional homogeneity of bilateral putamen is decreased in the FEM group. The putamen is an important area of basilar ganglia, which may be involved in integrating information among the cortex, thalami region, and three specific pain-processing areas (sensory, affective/cognitive, and endogenous regulation). The basilar ganglia area plays a potential role in the neuropathological mechanism of migraine [1, 17] Zhao et al. [18] analyzed the fMRI changes of migraine patients by stratified analysis and found that the structural abnormalities of the globus pallidus in migraine patients were related to the course of the disease and structural abnormalities of putamen were related to the frequency of attacks. Moulton et al. [11] studied the task-state fMRI of the thermal stimulus and found the basilar ganglia region (caudate nucleus and putamen) became less responsive to pain during a migraine attack. ReHo of the putamen in the FEM group is significantly increased in this study when compared with that of the IEM group, which further validates previous theory. This may be the result of long-term pain, cognitive impairments and emotional disturbances, because increased frequency of migraine attacks can lead to impaired pain-processing and regulation. The anterior cingulate cortex (ACC) is also a key region of the pain matrix. The ACC is related to the insula, thalami, prefrontal cortex, and other subcortical structures, involving emotions, cognition, and pain sensations, which plays a decisive role in regulating pain [18]. Some studies have shown that ACC is a “brain fingerprint” structure in chronic pain. For example, ACC regional dysfunction can be found in Fibromyalgia Syndrome [19], irritable bowel syndrome [20], chronic tension-type headache [21] and migraine patients [22,23,24]. Our study found that compared with IFM group, the changes of the functional brain regions in the FEM group were concentrated on the regions associated with pain regulation, emotional cognition, and pain-related responses, which suggested that these changes may be related to the increased frequency of headache attacks, affective disorders, pain regulation decompensation and so on.
The changes of encephalic regions in resting state fMRI in CM group
Specifically, the activation of BA18 and 19 zones was observed in the CM group. Photophobia is a common accompanying symptom in migraineurs, which refers to a sensory disturbance provoked by light. It is presumed that retinal light signals converge on posterior thalamic neurons via the optic nerve, which also receives noxious inputs from the dura mater via the trigeminal nerve. These inputs are then projected into the sensory cortex and the visual cortex (S1 and S2). Increased cortical excitability in migraine patients leads to sensitivity to light signals, which causes photophobia [4]. Recent finding has shown that in blind and non-blind migraine patients, and a variety of animal models, a novel retino-thalamo-cortical pathway has been found to carry photic signal from melanopsinergic and nonmelanopsinergic retinal ganglion cells (RGCs) to thalamic neurons. The activity of these neurons is driven by migraine attacks and their axonal projections convey signals of headache and light to multiple cortical areas involved in the generation of common migraine symptoms [25]. Since patient was druing interictal, the activation of the primary visual cortex was not observed. But as the secondary visual cortex (V2), the activation of BA 18 and 19 zones associated with visual and attention may also increase the photophobia of migraine. In addition, significant activation in the precuneus (BA7 zone) was observed in the CM group, which had close functional connections with the posterior cingulate cortex and prefrontal cortex. It was thought not only be related to cognition and episodic memory, but also regulate the pain and the attention in migraine and may be mediated by the subcortical structures including the locus coeruleus [26]. The locus coeruleus is an important nociceptive regulation region [27, 28], and it participates in the visual attention network of the precuneus. It may activate the visual cortex via percuneus and enhance the pain discomfort. These theories may seem to explain the photophobia in the attack of migraine. Activation of the olfactory cortex can also be observed in CM. Osmophobia is found to be a common simultaneous symptom of a migraine attack [29, 30]. Its mechanism is unknown, and activation of the olfactory cortex may be associated with the formation of Osmophobia, leading to the over-sensitivity to odors in migraine patients. It is manifested by an abnormal intensity of the odor that is not normally enjoyed and a change of the odor response that can be normally tolerated or even enjoyed.
Since the hippocampus takes part in spatial orientation, memory consolidation, and stress response, Maleki et al. [31] used task-state fMRI to study the functional connectivity of the subcortical brain regions of migraine. The results showed the functional connectivity among the hippocampus, bilateral frontal lobes, supra-marginal gyrus, and insula is significantly reduced in migraine patients with a higher frequency of attacks compared with the infrequent episodic migraine group. Our study shows that the ReHo value of hippocampus in CM patients is increased, which may be long-term stress state in migraine patients, break the original organism homeostasis, result in bidirectional feedback from the brain regions. Thus, the relevant brain function and organism homeostasis are gradually decompensated, resulting in the increasing frequency of migraine attacks, and eventually chronic transformation. Since the olfactory cortex, hippocampus, and parahippocampal gyrus are subordinate to the limbic system, Wilcox et al. [32] studied the fMRI changes caused by emotional stimuli during a migraine attack and found that there was an extensive activation of the limbic system in migraine patients. Maizels et al. [33] proposed a migraine central sensitization model that presented the dysfunction of the limbic system and hyperexcitability of the cortex. Activation of the limbic system makes the migraine patient susceptible to stress and emotional reactions, forming a bi-directional path related to the trigeminal nociceptor, which leads to the migraine attack for hours or even days. The affective disturbance is an important factor associated with the chronic transformation of migraine. In the process of chronic transformation, it can be observed that emotional disorders (anxiety, depression) are increased simultaneously [34, 35]. Therefore, the dysfunction of the limbic system may be an important indicator of chronic migraine. The abnormal function of the limbic system forms a two-way feedback mechanism for the chronic transformation of migraine, that is to say migraine is susceptible to emotional disorders, and emotional disorders exacerbate the headache and promote the chronic transformation. In the CM group, our results showed that more attention was focused on spatial attention and changes in brain regions such as cognition, affective disorder, pain feedback, and so on. This suggests that the mechanisms about pain, emotions and cognitive regulations gradually decompensate. Eventually, the patients develop into anxiety, depression, central sensitization and chronic migraine.
Conclusion
In this paper, the ReHo method is used to compare the difference in the resting state brain function between MwoA patients and healthy subjects. The highlight is that patients with migraine headaches are grouped according to the headache days per month and the characteristics of functional brain regions in each group of patients are compared. Our purpose is to explore the changes of brain function in different regions with the increasing frequency of headache and chronic transformation. It will help to reveal the mechanism of occurrence, development and chronic transformation of migraine to some extent. However, our study still has some limitations. First, the number of enrolled cases is relatively small, so selection bias is unavoidable. Our results should be verified in a larger group. Second, our study is limited to the migraine without aura, and functional changes in corresponding brain regions in other type of migraine cases need to be further explored.
References
Lanteri-Minet, M., Economic and costs of chronic migraine[J]. Cur pain, headache R 18(1):385, 2014.
Global Burden of Disease Study, Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013[J]. Lancet. 386(9995):743–800, 2015.
Yalın, OIEM Groupan et al., Phenotypic features of chronic migraine[J]. J Headache Pain. 17(1):17–26, 2016.
Goadsby, P. J., Holland, P. R., Martins-Oliveira, M. et al., Pathophysiology of Migraine: A Disorder of Sensory Processing[J]. Physiol Rev. 97(2):553–622, 2017.
Schwedt, T. J., Chiang, C. C., Chong, C. D. et al., Functional MRI of migraine[J]. Lancet Neurol 14(1):81–91, 2015.
Schwedt, T. J., and Dodick, D. W., Advanced neuroimaging of migraine. Lancet Neurol 8(6):560–568, 2009.
Zhang, J., Su, J., Wang, M. et al., The sensorimotor network dysfunction in migraineurs without aura: a resting-state fMRI study. J Neurol. 264(4):654–663, 2017.
Lee, J., Lin, R. L., Garcia, R. G. et al., Reduced insula habituation associated with amplification of trigeminal brainstem input in migraine[J]. Cephalalgia. 37(11):1026–1038, 2017.
Niddam, D. M., Lai, K. L., Tsai, S. Y. et al., Neurochemical changes in the medial wall of the brain in chronic migraine[J]. Brain. 11:1–14, 2017.
Chai, S. C., Kung, J. C., and Shyu, B. C., Roles of the anterior cinglate cortex and medial thalami in short-term and long-term aversive information processing[J]. Mol Pain. 6:42–51, 2010.
Liang, F., Qin, W., Gong, J. et al., Alterations in regional homogeneity assessed by FMRI in patients with migraine without aura stratified by disease duration[J]. J Headache Pain. 14(1):1–9, 2013.
Moulton, E. A., Becerral, L., Maleki, N. et al., Painful heat reveals hyperexcitability of the temporal pole in interictal and inctal migraine states[J]. Cereb Cortex. 21(2):435–448, 2011.
Coppola, G., Renzo, A. D., Tinelli, E. et al., Evidence for brain morphometric changes during the migraine cycle: A magnetic resonance-based morphometry study[J]. Cephalalgia. 35(9):783–791, 2015.
Lorenz, J., Minoshima, S., and Casey, K. L., Keeping pain out of mind: the role of the dorsolateral precentral gyrus in pain modulation[J]. Brain. 126(5):1079–1091, 2003.
Tessitore, A., Russo, A., Giordano, A. et al., Disrupted default mode network connectivity in migraine without aura[J]. J Headache Pain. 14:89, 2013.
Yu, D., Yuan, K., Zhao, L. et al., Regional homogeneity abnormalities in patients with interictal migraine without aura[J]: a resting-state study. NMR Biomed. 25(5):806–812, 2012.
Nicole, S., Faiza, A. B., Arkink, E. B. et al., Attack Frequency and Disease Duration as Indicators for Brain Damage in Migraine[J]. Headache. 48(7):1044–1055, 2008.
Zhao, L., Liu, J., Dong, X. et al., Alterations in regional homogeneity assessed by FMRI in patients with migraine without aura stratified by disease duration[J]. J Headache Pain. 14:85, 2013.
Kuchinad, A., Schweinhardt, P., Seminowicz, D. A. et al., Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain[J]. J Neurosci. 27(15):4004–4007, 2007.
Davis, K. D., Pope, G., Chen, J. et al., Corticalthinning in IBS: implications for homeostatic, attention, and pain processing[J]. Neurology. 70(2):153–154, 2008.
Schmidt, W. T., Leinisch, E., Straube, A. et al., Gray matter decrease in patients with chronic tension type headache[J]. Neurology. 65(9):1483–1486, 2005.
Valfre, W., Rainero, I., Bergui, M. et al., Voxel-based morphometry reveals gray matter abnormalities in migraine[J]. Headache. 48(1):109–117, 2008.
Kim, J. H., Kim, S., Suh, S. I. et al., Interictal meta bolicchanges in episodic migraine: a voxel-based FDG-PET study[J]. Cephalalgia. 30(1):53–61, 2010.
Prescot, A., Becerra, L., Pendse, G. et al., Excitatory neurotranIEM Groupitters in brain regions ininterictal migraine patients[J]. Mol Pain. 5:34, 2009.
Burstein, R., Noseda, R., Fulton, A. B. et al., Neurobiology of Photophobia. J Neuroophthalmol[J]. 39(1):94–102, 2019.
Géraud, G., Trotter, Y., Fabre, N. et al., Photophobia in migraine: an interictal PET study of cortical hyperexcitability and its modulation by pain[J]. J Neurol Neurosurg Psychiatry 81:978–984, 2010.
Matsutani, K., Tsuruoka, M., Shinya, A. et al., Stimulation of the locus coeruleus suppresses trigeminal sensorimotor function in the rat[J]. Brain Res Bull. 53:827–832, 2000.
Tsuruoka, M., Matsutani, K., Maeda, M. et al., Coeruleotrigeminal inhibition of nociceptive processing in the rat trigeminal subnucleus caudalis[J]. Brain Res. 993(1–2):146–153, 2003.
Rocha-Filho, P., Marques, K. S., Torres, R. et al., Osmophobia and Headaches in Primary Care: Prevalence, Associated Factors, and Importance in Diagnosing Migraine[J]. J Headache Pain. 55(6):840–845, 2015.
Jingjing, Q., Gang, Y., Xijing, M. et al., Non-headache symptoms in migraine attack[J]. Chinese Journal of Neuromedicine 11(2):173–176, 2012.
Maleki, N., Becerra, L., Brawn, J. et al., Common hippocampal structural and functional changes in migraine[J]. Brain Struct Funct. 218(4):903–912, 2013.
Wilcox, S. L., Veggeberg, R., Lemme, J. et al., Increased functional activation of limbic brain regions during negative emotional processing in migraine[J]. Frontiers in Human Neuroscience. 10:1–10, 2016.
Maizels, M., Aurora, S., and Heinricher, M., Beyond neurovascular: migraine as a dysfunctional neurolimbic pain matrix[J]. Headache. 52:1553–1565, 2012.
IEM Groupitherman, T. A., Rains, J. C., and Penzien, D. B., Psychiatric comorbidities and migraine chronification. Curr. Pain Headache Rep. 13(4):326–331, 2009.
Zhang, Q., Shao, A., Jiang, Z. et al., The exploration of mechanisms of comorbidity between migraine and depression. J. Cell. Mol. Med[J]. 00:1–9, 2019.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Patient Facing Systems
Can Chen and Manyun Yan are co-first authors
Rights and permissions
About this article
Cite this article
Chen*, C., Yan*, M., Yu, Y. et al. Alterations in Regional Homogeneity Assessed by fMRI in Patients with Migraine Without Aura. J Med Syst 43, 298 (2019). https://doi.org/10.1007/s10916-019-1425-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10916-019-1425-z