Abstract
Functional Electrical Stimulation (FES) is a technology to generate neural activity in an artificial way to activate muscles. However, as reported by some researchers, the human responses to FES are likely to be affected by several factors, such as spasticity, muscle fatigue, nerve habituation and so forth. Consequently, the function restoration by FES is neither durable, nor stable. In order to realize long-term and stable FES assistance, this study investigated whether and why an Auxiliary Stimulation (AS) to the Gastrocnemius, with current frequency ranged from 2000 to 6000 Hz, could alleviate the symptom of spasticity and muscle fatigue caused by the stimulation to the Tibialis Anterior. We have developed a portable auxiliary stimulator, and performed experiments to verify its effectiveness. The results showed that our approach enabled comparatively stable and durable function restoration assistance. Moreover, for understanding underlying neuromuscular processes elicited by the AS and its qualitative nature, this study also measured the Hoffmann-reflex (H-reflex) in soleus muscle before and after the AS, to interpret the effect of the Auxiliary Stimulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Functional Electrical Stimulation (FES) is an effective way to restore motor function for paralyzed people [1–3]. It can not only improve patients’ cardiopulmonary function, strengthen muscle and recruit partial movement function, but also rebuild patients’ life confidence and help them return to the society [2, 4]. Its therapeutic effects have been proved by numbers of studies [1, 4].
However, the mechanism of FES motor function generally differs from that of the normal motor action. That is, FES motion begins with muscles of Type II (fast fatigue and fast fatigue resistant), and then the small ones (Type I: slow fatigue-resistant) with the increase of muscle output power, while the normal motor action is conducted in a reverse process. Therefore, muscle responses to FES are likely to be affected by the factors such as spasticity [5, 6], abnormal muscle tonus, muscle fatigue, and nerve habituation [7, 8, 9]. More seriously, lower limbs with spasticity will constantly exhibit excessive excitement while conducting FES [10], which aggravates the muscle fatigue, thus reduces the durability and stability of the function restoration. Therefore, in order to realize long-term and stable FES assistance in daily living, it is of great significance to explore appropriate approaches to cope with those factors.
Recently, many treatments, including medical, surgical methods, and electrical stimulation have been employed to relieve the symptoms of spasticity, muscle fatigue, and abnormal muscle tonus [11–13]. Among these treatments, medical, surgical methods will cause muscle weakness [13], whereas the electrical stimulation could reduce muscle tonicity via the reduction of the stretching reflex, causing lower spasticity and allowing a larger range of motion, without weakening the muscles [14–16]. Although much work has been done to date on the electrical stimulation for easing muscular activities, more studies are needed to make clear whether kinds of auxiliary electrical stimulation could be used with FES to improve its stability and durability and understand the underlying neuromuscular processes elicited by electrical stimulation.
The purpose of this study is to ascertain the effectiveness of using a sub-threshold medium-frequency (2–6 KHz) stimulation (called AS in the following sections) to assist FES in restoring walking function. Moreover, in order to understand the underlying neuromuscular processes elicited by electrical stimulation and its qualitative nature, we have measured the Hoffmann-reflex (H-reflex) in human soleus muscle before and after the AS.
Methods and experiments
Auxiliary stimulation and FES devices
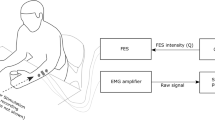
The stimulation device is based on Hitachi’s tiny H8 (H300 core) microprocessor. The device has one output channel. The output signal is generated by using PWM port of the microcontroller. The device is controlled by receiving control commands from a host computer using serial communication at 9600 Bauds. The stimulator is designed to operate on the capacitance channel of the electrodes, limiting its operation to the linear range. In this way, we can warrant reversible operation and avoid detrimental chemical reactions. The stimulator uses commercially available neuroelectrodes, with an area of 32 cm2 for low current density. The stimulation current source and the microprocessor-based controller for wave form generation are optically isolated, and separately powered by one of the two 9v alkaline batteries. Its diagram was shown in Fig. 1. Using biphasic stimulation method, the device requires less energy to provide the same effects as other stimulation methods. By using biphasic stimulation we can keep the current at a low level (less than 10 mA) and avoid accumulation of charge to prevent tissue damage.
The FES device was a product of Compex Company (www.compexsport.jp) [17].
Experiment 1
Subject
One paralyzed subject, female, 42 years old, with major neuro muscular dysfunction and ankle plantar flexor spasticity on the left lower-limb, which caused an asymmetric hemiplegic gait, took part in the experiment. The details of the experiments were explained to the subject until she completely understood the experiments, then an informed consent was signed, and all the experiments were done under the monitoring of a medical doctor.
The description of experiment 1
The first experiment is to ascertain the effect of using a sub-threshold medium-frequency (2–6 KHz) stimulation to assist FES for restoring walking function.
To inspect the effect of AS, the following three subtests were conducted.
The first subtest was performed to compare the effect of different frequency of auxiliary stimulation. The positions of electrodes for FES were decided empirically, aiming at an assistance of lifting in the early swing phase, as shown in Fig. 2. The simulation through these two pairs of electrodes could lift the leg during walking very well, however, would cause spasticity of gastrocnemius, which results in the drop of overall performance. A sub-threshold auxiliary stimulation was applied between the bellies of two sides of gastrocnemius, and the frequency changes with three values 2000 Hz, 4000 Hz and 6000 Hz. There was one session everyday. Before the auxiliary stimulation (AS), a minimal level of FES was decided by gradually increasing the stimulation level from 0, until a slight leg lifting occurred. Then FES was stopped, the auxiliary stimulation was conducted at point A (Fig. 2) to gastrocnemius for 15–20 min. The FES with the minimal level recorded before the session was conducted at least 10 times, then an ankle raising height was measured. All the experiments were carried out at sitting position.
In the second subtest, the stimulation outputs were the same with the first subtest, but the positions of the electrodes of auxiliary stimulation were different. In this subtest the surface electrodes for AS were posted on the position A and B, as shown in the follow figure (Fig. 2). A is higher than position B, the center of which is around the Chenjin acupoint.
In the third subtest, in order to investigate the combined effect of FES and the auxiliary function of AS on motor function restoration, the AS with 4000 Hz on the position A is performed simultaneously with FES, as shown in Fig. 2.
Experiment 2
In order to explore the underlying neuromuscular processes elicited by auxiliary stimulation, the second experiment was to measure the Hoffmann-reflex (H-reflex) in human soleus muscle of subjects’ dominant leg (Fig. 3), before and after the auxiliary stimulation for 15 min as Fig. 4 shows.
Subject
Fifteen subjects (25 to 35 years old, 172 ± 10 cm high, with weight 72 ± 10 Kg, no distinction on gender, and with no apparent sensory or motor impairment on limbs) participated in the experiment. To all the subjects a full explanation was made about the contents and purpose of this experiment, then an informed consent was written by each subject.
Experiment description
In this experiment a pair of surface electrodes for measurement of H-reflex were placed on the soleus muscle, 5 cm above Achilles. The electrical stimulus was sent to the tibial nerve, which is located in the middle of knee as shown in Fig. 3. Duration of the electrical stimulation activity is 1 msec, one stimulation for every 5 s (output frequency is 1/5 Hz). For measuring the H-reflex, the subjects were required to lie prone, and their lower limbs were fixed to bedside by using a white medical belt [18, 19]. Stimulus intensity begins with just below the threshold intensity of H-wave elicitation voltage, and then was increased with the step of 1 voltage until obtaining the M-wave maximum. The data acquisition and analysis were done using a PC (Panasonic CF-8) where the software of Biopac Student Lab PRO system was installed. To remove noise of EMG and disturbance of electromagnetic, the amplified (500times) H-reflex and M-wave were filtered by a 10 Hz high filter and a 2000 Hz low-filter. A recruitment curve can be obtained by gradually increasing the stimulus intensity as shows in Fig. 5.
Results
Results of experiment 1
Figure 6 shows the ankle raising height of the first subtest. As shown in Fig. 6, 4000 Hz was most long lasting and effective. Compared with that, the raising height by 2000 Hz was not clear, sometimes, even lower than that without AS. 6000 Hz stimulation sometimes led to a high lifting, however, it was unstable in general.
Figure 7 shows the effect of different auxiliary stimulation positions from the second subtest. The comparison of the height indicated that the position A is better, so that it is clear that, the electrode position is important for the auxiliary stimulation.
Usually, in the FES without the auxiliary stimulation, the assistance of FES for walking function could last for 1 h, however, in the third subtest as shown in Fig. 8, with several rest intervals, the experiment lasted for 4 h, and meanwhile, the assistive effectiveness of FES was maintained. The long lasting FES effect was also observed in the other experiment. This shows the possibility that the 4000 Hz auxiliary stimulation has an active effectiveness for alleviating muscle fatigue and spasticity and could enable durable and stable FES assist.
Results of experiment 2
H-reflex is a monosynaptic reflex [20, 21]. It can be induced by electrical stimulation of Ia fibers at the intensities over the exciting thresholds [22, 23]. The afferent portion of the H-wave begins at the point of electric stimulation and results in action potentials traveling along afferent fibers. M-wave was known as the efferent arc that produces a response in the EMG. Therefore, Hmax is a measure of maximal reflex activation or, stated differently, is an estimate of the number of motor neurons (MNs) that one is capable of activating in a given state [20]. The Mmax represents activation of the entire MNs pool [20], therefore, maximum muscle activation. The Hmax is an indirect estimate of the number of MNs being recruited and the Mmax represents the entire MN pool, thus, Hmax/Mmax ratio can be interpreted as the proportion of the entire MN pool capable of being recruited [20], in turn, an evaluation for the statues of cell excitability of the spinal cord, thus, used widely in clinic experiments [21]. In this paper Hmax/Mmax was mainly used to evaluate the use of AS, by comparing the index values before and after AS.
Figures 9 and 10 showed Hmax and Mmax values before and after AS. Hmax became lower after AS, but the Mmax almost did not change before and after AS.
Figure 11 showed that the Hmax/Mmax values, as an evaluation of neurologic function and excitability of spinal motor nerve, became lower after AS. Usually, the excess excitability of spinal cord may cause spasticity and fatigue prematurely. Results of the second experiment showed the effect of AS, that is, the comparison of the amplitude before and after AS indicated that AS can alter the value of H-reflex. In other words, AS enables durable and stable FES assist through bringing about a lower excitability of αMN (that is, a lower Hmax/Mmax value).
Discussion
The results of the first experiment (in which motor function restoration was combined with AS) indicated that medium frequency stimulation can provide more durable and stable FES assist. Therefore, it is expected that the negative effect of FES may be reduced or eased by the method. However, the mechanism is still uncertain. It has been reported that, medium-frequency (3–5 KHz) biphasic electrical current applied to peripheral nerves could block conduction of action potentials [24, 25]. This nerve block may be reversible once the stimulation was removed. However, the mechanism of the nerve conduction block induced by medium-frequency biphasic electrical current is unknown. In addition, many researchers also have indicated that the therapeutic electrical stimulation [13, 20] or vibratory stimulation (VS) [21] on antagonist MN Excitability under different frequencies for spastic patients could alter the excitability of spinal motor nerves to improve walking. As shown in Fig. 12, since the muscle activation elicited by FES differs from that of the normal motor contraction, the effect of FES is neither durable nor stable. Thus, in order to realize a long-term and stable FES assist in daily living, it is crucial to decrease the excess excitability of spinal nerves and reduce the muscle fatigue.
As discussed, reducing or decreasing the excess excitability of spinal MNs could enable more durable and stable motor function, so it is very important to find the relation between AS and the excitability of spinal motor nerves. For understanding the underlying neuromuscular processes elicited by the auxiliary stimulation and its qualitative nature, in the second experiment we measured the Hoffmann-reflex (H-reflex) [18] in human soleus muscle, before and after the auxiliary stimulation. The result of the second experiment also showed their Hmax/Mmax, as an evaluation of neurologic function and excitability of spinal motor nerve, became lower after AS, which means lower excitability of spinal action neurons. In addition, as mentioned previously, excessive excitability of spinal motor nerves would cause a serious spasticity or premature fatigue. Thus, using AS to alter the excitability of spinal motor nerve could help FES to support a more durable motor function restoration.
In order to use AS for FES in real time, the influence and function of AS for short term should be also made clear, therefore, in the next stage, the AS experiments should focus on how AS influences the excitability of spinal action neurons in real time.
Moreover, the exact action for excess excitability inhabitation was not made clear. Fig. 13 shows several possible factors [22].
More important, the degree of muscle fatigue and spasticity will be changed with time. Therefore a dynamic method that could measure, model and respond appropriately to the muscle fatigue and spasticity in real time becomes very important.
Conclusion
Through the present study, the effect of the auxiliary stimulation on FES was made clear. With appropriate simulation frequency (4000 Hz for the subject), and suitable stimulation position, the AS could facilitate a durable and stable FES. Regarding underlying mechanism of the AS, measurement of H-Reflex before and after the AS showed that, the AS assists FES through reducing the excessive excitability of Motor Neurons.
In the near future, in order to establish an effective AS, the parameters of AS, such as, stimulation frequency and position should be tested with different walking function impaired subjects. Moreover, the motor neurons and their responses to AS should be further investigated to deepen the understanding of underlying mechanism of AS.
References
Hambrecht, F. T., and Reswick, J. B., Functional Electrical Stimulation Application in Neural Prosthesic. Marcel Dekker, 1977.
Liberson, W. T., Functional electrotherapy stimulation of the peroneal nerve synchronized with the swing phase of the gait of the hemiplegic patients. Arch. Phys. Med. Rehabil. 42:101–105, 1961.
McNeal, D. R., Analysis of a model for excitation of my elinated nerve. IEEE7kms. Biomd. Eng. 23:329–337, 1976.
Handa, Y., and Hoshimiya, N., Functional electrical stimulation for the control of the upper extremities. Med. Pro. Technology 12:51–63, 1980.
Lance, J. W., Symposium synopsis. In: Feldman, R. G., Young, R. R., and Koella, W. P. (Eds.), Spasticity: Disordered Motor Control. Year Book Medical, St Louis, pp. 485–494, 1980.
Katz, R. T., and Rymer, W. Z., Spastic hypertonia: Mechanisms and measurement. Arch. Phys. Med. Rehab. 70:144–155, 1989.
Kinya, F., Feasibility of physiome-based functional electrical stimulation, IEICE technical report. ME and Bio Cybernetics. 102(728):17–20, 2003.
Matsunaga, T., Shimada, Y., and Sato, N., Muscle fatigue from intermittent stimulation with low and high frequency, electrical pulses. Arch. Phys. Med. Rehabil. 80:48–53, 1999.
Thomas, F., Jochen, Q., Robert, R., and Günther, S., Walking with walk! IEEE Eng. Med. Biol. Mag. 10:38–48, 2008.
Makoto, M., Relationship between AFO and Neurological Organization in Spastic Hemiplegia. Jpn. Phys. Ther. Assoc. 27(5):145–150, 2000.
Bogataj, U., Gros, N., Kljajic, M., Acimovic, R., and Malezic, M., A comparison between conventional therapy and multichannel functional stimulation therapy, rehabilitation of gait in patients with hemiplegia. Phys. Ther. 75:490–502, 1995.
Albright, A. L., Neurosurgical treatment of spasticityand other pediatric movement disorders. J. Child Neurol. 18(1):67–78, 2003.
Bakhtiary, A. H., and Fatemy, E., Does electrical stimulation reduce spasticity afterstroke? A randomized controlled study. Clin. Rehabil. 418–425, 2008.
Hazlewood, M. E., Brown, J. K., Rowe, P. J., and Sao, F., Therapeutic electrical stimulation in the treatment of hemiplegic cerebral palsy. Dev. Med. Child Neurol. 36(8):661–673, 1994.
Kralj, T., and Grobelnik, B., T-10 paraplegic patient walking in parallel bars, 1979
Masuda, M., Kiyoshige, Y., and IHASHI, K., Influence of therapeutic electrical stimulation on contractile properties of human paralyzed muscles. Jpn. Phys. Ther. Assoc. 25(3):121–127, 1998.
Hoffmann, P., Beitrag zur Kenntnis der menschlichen Reflexe mit besonderer Berucksichtigung der elektrischen Erscheinungen. Arch. Anat. Physiol. 1:223–246, 1910.
Magladery, J. W., and McDougal, D. B., Electrophysiological studies of nerve and reflex activity in normal man, I: Identification of certain reflexes in the electromyogram and the conduction velocity of peripheral nerve fibers. Bull. Johns Hopkins 86:265–289, 1950.
Riann, M. P., Christopher, D. I., and Mark, A. H., The Hoffmann reflex: Methodologic considerations and applications for use in sports medicine and athletic training research. J. Athl. Train. 39(3):268–277, 2004.
Iwatsuki, H., Effects of vibratory stimulation on antagonist motoneuron excitability under different frequencies. J. Jpn. Phys. Ther. Assoc. 18(1):41–44, 1991.
Sköld, C., Lönn, L., Harms-Ringdahl, K., Hultling, C., Levi, R., Nash, M., and Seiger, A., Effects of functional electrical stimulation training for six months on body composition and spasticity in motor complete tetraplegic spinal cord-injured individuals. J. Rehabil. Med. 34:25–32, 2002.
Levin, M. F., and Hui-Chan, C., Are H and stretch reflexes in hemiparesis reproducible and correlated with spasticity? J. Neurol. 240:63–71, 1993.
Reboul, J., and Rosenblueth, A., The action of alternating currentsupon the electrical excitability of nerve. Am. J. Physiol. 125:205–215, 1939.
Bowman, B. R., and McNeal, D. R., Response of single alpha motoneurons to high-frequency pulse train: Firing behavior and conduction block phenomenon. Appl. Neurophysiol. 49:121–138, 1986.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yuan, B., Sun, G., Gomez, J. et al. The Effect of an Auxiliary Stimulation on Motor Function Restoration by FES. J Med Syst 35, 855–861 (2011). https://doi.org/10.1007/s10916-010-9517-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10916-010-9517-9