Abstract
The mammary gland undergoes dramatic post-natal growth beginning at puberty, followed by full development occurring during pregnancy and lactation. Following lactation, the alveoli undergo apoptosis, and the mammary gland reverses back to resemble the nonparous gland. This process of growth and regression occurs for multiple pregnancies, suggesting the presence of a hierarchy of stem and progenitor cells that are able to regenerate specialized populations of mammary epithelial cells. Expansion of epithelial cell populations in the mammary gland is regulated by ovarian steroids, in particular estrogen acting through its receptor estrogen receptor alpha (ERα) and progesterone signaling through progesterone receptor (PR). A diverse number of stem and progenitor cells have been identified based on expression of cell surface markers and functional assays. Here we review the current understanding of how estrogen and progesterone act together and separately to regulate stem and progenitor cells within the human and mouse mammary tissues. Better understanding of the hierarchal organization of epithelial cell populations in the mammary gland and how the hormonal milieu affects its regulation may provide important insights into the origins of different subtypes of breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mammary gland undergoes dynamic changes over the lifetime of a woman. Although initial development takes place during embryogenesis, the majority of expansion and differentiation of the epithelium starts during puberty. In humans, the mammary ductal system and immature terminal ductal lobule units (TDLU) lengthen and mature as they grow into the stroma of the breast. This process is under the tight regulation of circulating hormones and localized growth factors. During puberty, epithelial cells of the breast, in particular those in the TDLU, proliferate in response to ovarian steroids, and the mature gland undergoes cyclic proliferation and apoptosis over the course of each subsequent menstrual cycle [1–4]. Similarly, in mice, the mammary gland undergoes ductal elongation during puberty with the development of ductal branches and alveolar budding in the mature gland in response to hormonal changes during the estrus cycle. In both mice and humans, full mammary epithelial maturation occurs during pregnancy, in order to generate complex lobules and specialized epithelial cells in alveoli, which have the ability to synthesize and secrete milk for lactation. Finally, after lactation, evidence suggests that the most mature lobules and differentiated mammary epithelial cells collapse, and the mammary network becomes more similar to the resting state prior to pregnancy [5]. This process of expansion and regression can occur across multiple pregnancies during the reproductive phase of a woman’s lifetime, demonstrating that the epithelial cells of the breast have considerable regenerative abilities.
Epithelial cell hierarchies, where undifferentiated stem cells give rise to more differentiated progenitor cell populations, have been well-characterized in epithelia that are rapidly replaced, such as in the intestines and skin (for review, [6, 7]). Given the extensive proliferation and specialization of the epithelial cells in the breast, a linear hierarchy, with stem cells at its apex, is presumed to exist. Stem cells expand through symmetric and asymmetric divisions in order to prevent exhaustion of linage-restricted progenitors. The mammary gland is exquisitely sensitive to the effects of ovarian steroids, demonstrating well-characterized histological changes over the course of the menstrual cycle [8, 9]. In particular, epithelial cells respond to estrogen acting through its receptor, estrogen receptor alpha (ERα), and progesterone signaling through progesterone receptor (PR). Despite this well-accepted notion, determining the specific effects of each hormone on progenitor and more mature cells has been challenging. This is because in adult mammary tissues of both mice and humans, most ERα+ cells also express PR [10–12], and estrogen has been shown to act through ERα to increase expression of PR [13, 14]. In addition, the full repertoire of progenitor cells is not fully understood, nor is their receptor status well defined. Moreover, the effects of estrogen are modulated by interactions at the PR promoter, which may be cell-type specific [15]. Recent evidence suggests that ERα and PR are also expressed independently within separate cell populations in the breast, and these cell populations may have divergent regulation and growth potential [16–19]. Thus, being able to isolate and study a pure population of cells that are only sensitive to one hormone or the other while only expressing one receptor is difficult.
Identification of progenitor cells and their regulation within the stem cell niche may provide critical insight into the origins of different pathological conditions. For example, transcriptional profiling of breast cancers has revealed intrinsic subtypes, which share similarities to normal epithelial cells of the breast, suggesting that the biology of the normal precursors may play an important role in the underlying phenotype and behavior of the tumor [20–22]. In particular, 70 % of breast cancers express ERα [23–25], and more than half of ERα+ breast cancers also express PR [26, 27]. Therefore, understanding the role of ERα and PR in the epithelial cell hierarchy and its regulation by hormones may lead to a greater understanding about the biology and sensitivity of ERα+ breast cancers. In this review, we attempt to summarize what is currently known in both mice and humans about the identity of progenitor cells in the mammary epithelial hierarchy and focus on those that are regulated by hormones.
Mammary Gland Development
Mammary development begins during embryogenesis with specification of cells from the surface ectoderm that go on to form the nipple and the rudimentary ductal tree. The mammary epithelium is bilayered, composed of luminal cells that line the hollow lumens of the ducts and surrounded by myoepithelial cells that have contractile function. During post-natal development in both mice and humans, mammary ducts grow allometrically into the mammary fat pad until puberty [28–30]. Interestingly during a portion of this developmental period, ERα is readily detectable, while PR expression is low to absent [28, 31]. In humans, ERα is detected in the breast epithelium of the fetus beginning in the third trimester of pregnancy, whereas PR is not detected until 2–3 months after birth [31]. Following birth, 80–90 % of infants continue to produce milk proteins [32, 33], suggesting that the developmental changes in response to the complex maternal hormonal environment of pregnancy exhibit long-lasting effects. Although in mice this lactational response is not seen, the imbalance favoring of ERα expression over PR expression in the early post-natal period is observed [18, 28].
At the start of puberty, estrogen, binding to its receptor ERα, is necessary for rapid growth and expansion of the ducts into the mammary fat pad. In both humans and mice, cells that express ERα however, do not co-localize with markers for proliferation [10–12]. Thus, estrogen’s action through ERα is mediated by a paracrine mechanism to promote the proliferation of surrounding cells [34–36]. In fact, transplanted murine ERα-/- cells are unable to undergo ductal elongation when inoculated alone, but when co-mixed with wild type cells, ERα-/- cells can take part in ductal elongation [34, 35]. These elegant studies illustrated the notion that paracrine factors produced by ERα+ cells are necessary for the proliferation of ERα-/- cells. One paracrine mediator of estrogen is amphiregulin, which is also necessary for ductal elongation [37–39]. However, growth factors of the EGF protein family can also rescue the ERα-/- phenotype [40]. Epidermal growth factor (EGF), transforming growth factor alpha (TGFα), and heregulin are all necessary for pubertal ductal elongation suggesting that activation of EGF family receptors by estrogen is critical for ductal elonagation.
Following puberty, the mouse mammary gland undergoes lateral branching in response to ovarian steroids produced during the estrous cycle. This process is driven primarily by the actions of progesterone. Thus, unlike ERα-/- epithelial cells, PR-/- cells do undergo normal ductal elongation, but the resulting growths lack secondary branches and a complete lack of alveolar development in response to pregnancy [41, 42]. Similar to ERα-/- cells, transplant of PR-/- cells co-mixed with wild type cells also results in normal ductal branching and alveolar development, suggesting that PR also acts through a paracrine mechanism of action on surrounding cells [41].
One paracrine mediator of progesterone activity is RANKL, a member of the tumor necrosis factor superfamily. Ectopic expression of Rankl in PR-/- mice rescues the PR-/- phenotype [43]. Further, transgenic overexpression of Rankl leads to precocious ductal side branching and alveologenesis, similar to the effects of progesterone stimulation [44]. Another downstream target and paracrine mediator of PR is WNT-4, which is upregulated in primary mammary epithelial cells in response to progesterone both in vitro and in vivo [45]. Cyclin D1, which is a critical component of the cell cycle, is also required for progesterone-induced proliferation during mammary gland development. Deletion of PR results in a significant reduction in cyclin D1 expression, and similar to PR-/- mice, mice lacking cyclin D1 do not properly develop alveoli and are unable to nurse their pups [46, 47]. These results suggest that cyclin D1 is important for progesterone-induced proliferation. However, it is not clear whether Rankl, Wnt-4, and cyclin D1 promote proliferation of the same or different cell types in the mammary gland.
Since the human breast exhibits greater anatomical and lobule complexity compared to the mouse mammary gland, it is not surprising that its development is also more complex. Unlike the murine mammary gland, terminal end buds do not emerge and grow into the breast stroma. Rather lobules, separated by connective tissue, develop and are joined to central ducts that range in number from 11 to 48 [48]. Given this difference in early development, it is not clear whether ERα alone mediates this growth. Lobules range in size and have been categorized with regard to their degree of development. Type I lobules are the least developed and have been characterized as having the highest expression of ERα and PR expression [10]. Lobules mature through increasing their size and complexity through pregnancy, with Type IV lobules only present in lactating women [10, 49–51]. Although breast tissue of nulliparous women primarily contains Type I lobules, Type II and Type III lobules are also present [32, 33, 52].
Humans also exhibit important differences in their cycling hormones which also likely affects breast development. In humans, as well as mice, proliferation of mammary epithelial cells is not at its peak during the follicular phase, when circulating estrogens are at their maximum, but rather during the luteal phase, when the ratio of circulating progesterone to estrogen is increased [10, 12]. However, unlike mice, the human corpus luteum secretes estrogen in addition to progesterone [53]. As such, tamoxifen use in women can inhibit breast epithelial proliferation during the luteal phase of the menstrual cycle [54]. This suggests that both estrogen and progesterone regulate proliferation in the human breast. The changes in hormonal activity over the menstrual cycle may also impact the types of lobules observed within the breast, as Type I lobules have been shown to be more abundant during the follicular phase of the menstrual cycle, whereas Type II lobules are more common during the luteal phase [55]. Interestingly, ERα and PR are expressed in different subsets of cells over the course of the menstrual cycle [18], although this response is variable among patient samples, possibly due to differences in parity or history of hormone-based contraceptive use. However, studies to dissect the changes in the specific breast lobules types have met with technical challenges. Lobules have been primarily characterized in human tissues that were fixed and stained as whole mounts or on histological sections, which limited the types of analyses that were performed. Further work to isolate breast lobules for the delineation of the cell populations responsive to estrogen and progesterone may improve our understanding of the complex anatomical development of the human breast.
Transcription Factors Regulate Cellular Differentiation
Transcription factors play a central, cell-specific role in lineage selection and cell fate decisions. Some transcription factors have been shown to regulate steroid receptor expression, which in turn can alter the behavior of the surrounding cells through paracrine signaling. For example, Gata-3 is expressed in luminal epithelial cells and has been shown to play a central role in regulation of mammary gland morphogenesis and luminal differentiation during development [56] and in the mature gland [57]. Loss of Gata-3 specifically during lactation results in significantly decreased numbers of differentiated alveolar cells [56]. One mechanism by which Gata-3 regulates luminal differentiation is through the activation of the transcription factor FoxA1 [57]. FoxA1 is important for expression of ERα; FoxA1-deficiency results in significantly reduced ERα expression levels, a block in terminal end bud formation, and an inability of the ducts to properly invade the mammary fat pad [58]. The majority of FoxA1+ cells also express ERα [58]. Therefore, one mechanism through which luminal differentiation is regulated by Gata-3 is likely due to its indirect effects on steroid receptor expression, which in turn alters the behavior of the surrounding cells through paracrine signaling.
The transcription factor C/EBPβ also plays an important role in luminal cell differentiation and the correct patterning of steroid receptors [59, 60]. C/EBPβ-/- mice demonstrate elevated PR expression and increased epithelial proliferation [60]. This results in an increase in differentiated luminal cells but a decrease in cells that secrete milk proteins during lactation [61]. Thus, C/EBPβ may regulate the expansion of luminal cells as well as their ability to differentiate into a population that is able to give rise to alveoli.
While one set of transcription factors regulates cell populations that are responsive to ovarian steroids, another group of transcription factors appears to act downstream of steroid receptors to expand populations of cells that are able to mature into alveoli. One such factor that regulates alveolar cellular proliferation is Stat5a [62]. During pregnancy, Stat5a is required for lobuloalveolar outgrowth and lactogenesis [63, 64], while in nulliparous glands, its active form (p-Stat5a) is necessary for ductal branching and proliferation [65]. Stat5 is regulated by both estrogen and progesterone [66], and p-Stat5a localizes with both ERα and PR expression [66]. Another transcription factor, E74-like factor 5 (Elf5, also known as ESE-2), regulates alveolar differentiation. Elf5 expression is induced by progesterone, although ERα+/PR+ cells do not express Elf5 [67, 68]. Progesterone may regulate Elf5 expression levels through Rankl, since blockade of Rankl signaling prevents progesterone-induced side branching and the expansion of Elf5+ mature luminal cells [68]. During pregnancy, Elf5 is critical for the differentiation of secretory cells and is regulated by both Stat5-mediated and independent mechanisms [62]. Although ERα and PR have been shown to regulate Stat5 and Elf5 in nulliparous glands, delineating the regulation of Stat5 and Elf5 specifically by ERα and PR signaling during pregnancy is complex. This is because both transcription factors are also regulated by the pituitary hormone prolactin, which is critical for alveologenesis during pregnancy and differentiation during lactation [69, 70].
Although gene expression profiling has led to the identification of transcription factors that are expressed in various stem and progenitor cells, identifying their role during lineage commitment and differentiation is an area of great interest [71–73]. It is likely however that transcription factors activated in progenitor cells that drive cell fate decisions may also have continued functions in daughter cells to maintain lineage differentiation [74]. Thus, identification of unique combinations of transcription factors in early progenitor cells compared to more mature progeny may provide insights into how steroid receptors regulate lineage specification and how cell fate decisions in the mammary epithelial cell populations are achieved in response to hormones.
Stem Cell Activity in the Mammary Gland
The discovery that mammary epithelial cells and tissue fragments could regenerate entire mammary tissues led to the notion that stem cells must be present in mammary tissues. Pioneering work by DeOme and colleagues demonstrated the existence of mammary stem cells through transplant experiments. This technique relies on the removal of endogenous mammary tissue fragments prior to puberty and allografting donor epithelium into the cleared fat pads; the epithelium can repopulate and recreate a fully functional mammary ductal tree [75, 76]. These observations were extended using insertional marking of mammary epithelial cells with mouse mammary tumor virus (MMTV) integration, which showed that one putative stem cell could contribute to and account for all cellular renewal of the mammary epithelium over several transplant generations [77]. Similarly, in humans, genetic tracking of cells through inactivation of the same X-chromosome suggested that lobules with contiguous patches of epithelium were derived from the same stem/progenitor cell [78, 79].
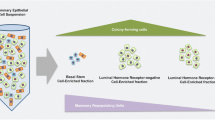
Prospective identification of stem cells was subsequently achieved with the identification of cell surface markers that could separate populations of epithelial cells based on their functional abilities to form colonies in vitro or demonstrate outgrowth potential in vivo. When epithelial cells are dissociated, stained with antibodies to detect CD24 and CD29/β1-integrin, and sorted using fluorescence-activated cell sorting (FACS), a single lacZ+ cell from the CD29hiCD24+ population could generate an entirely lacZ+, functional mammary gland [80]. These data demonstrated that cells from the basal/myoepithelial (ME) lineage are enriched for mammary repopulating units (MRU). Similar results have been observed when epithelial cells were sorted using CD24+CD49f/α6-integrinhi [81].
Although cells in the basal/ME lineage are able to reconstitute an entire mammary tree when transplanted, physiologic lineage tracing models have been less clear as to whether bipotent stem cells exist and are able to reconstitute both lineages in situ. In lineage tracing experiments marking luminal or basal/ME cells, some studies have shown that lineage restricted progenitor cells maintain the separate epithelial lineages after birth [82–84]. Luminal progenitor cells gave rise to mature luminal cells without contribution from a putative stem cell in the basal lineage (Fig. 1a). However, other studies have shown that during pregnancy, the lineage restriction of luminal and basal cells is not maintained, and that cells from the basal lineage can also contribute to the luminal lineage during alveologenesis [85–87] (Fig. 1b). Yet another study has suggested significant contribution of basal/ME stem cell populations to the continued development of the luminal epithelium. Using a stochastic multicolor cre reporter specific to mark either basal or luminal epithelial lineages, cells from the basal lineage significantly contributed to the luminal lineage, even in nulliparous female mice [88]. This contribution of the basal lineage to the luminal lineage has also been observed in other tracing studies, although the occurrence is rare [86, 89] (Fig. 1c). The reasons for the differences among these studies are not clear, but may due to differences in mouse strains or the use of the estrogen antagonist, tamoxifen, to induce lineage tracing. The dosage of tamoxifen has also been shown to have direct effects on mammary gland development, as well as long-lasting effects on progenitor cell activity in the mammary gland and other organs, thus potentially confounding the results of some of the findings [88, 90, 91]. Hence, to reconcile these disparate observations, additional studies are necessary to fully define the contribution of basal/ME cells to progenitor populations in the luminal lineage under physiologic conditions in vivo. A recent review on mammary lineage tracing provides an in-depth discussion of these challenges for determining progenitor contributions to each lineage in the mammary epithelial hierarchy [92].
Models for the contribution of stem cells of basal origin to the luminal lineage in the mammary glands of adult mice. Epithelial cells of basal origin are depicted in red; BP denotes basal progenitor cells. Cells of luminal origin are depicted in green; LP denotes luminal progenitor cells. a In the adult gland, cells of each lineage are restricted. LPs divide in order to expand the luminal cell population, while BPs proliferate in order to generate basal epithelial cells. b In adults, the lineages are restricted until pregnancy, when progenitor cells of the basal lineage contribute to the formation of the luminal alveolar cells. A portion of these cells remain in the gland following involution to expand the alveolar cell population during subsequent pregnancies. c BPs proliferate over the course of the estrus cycle to contribute to the LP population. These cells contribute to formation of the luminal alveolar cells. A portion of these cells remain in the gland following involution to expand the alveolar cell population during subsequent pregnancies. d In humans, cytokeratin (CK) 8, a marker for luminal cells, and CK14, a marker for basal cells, are co-expressed in a subset of cells. Sections of tissue obtained from reduction mammoplasty surgeries were labeled with CK8 (green), CK14 (red), or both (yellow) and counterstained with the nuclear stain, DAPI (blue) using immunofluorescence. White arrows highlight cells that are co-labeled with CK8 and CK14. Image magnification: 200x, 400x
The cellular hierarchy and identity of stem/progenitor cells in the human breast is not as clearly defined as in rodents. This is in part because the expression of luminal and basal markers are not as lineage restricted as they are in mice. In adult mice, mammary luminal cells exclusively express cytokeratins 8 and 18 (CK8, CK18), while basal/ME cells strictly express basal cytokeratins 5 and 14 (CK5, CK14). However in human breast tissue, CK14 and CK8 double-labeled cells are readily observed in both the luminal and basal/ME compartments [52, 93] (Fig. 1d). Type I lobules contain the greatest number of dual-labeled cells, while Type II and Type III lobules exhibit increased lineage restriction of these markers to the luminal and basal epithelial cells [52]. It is possible that these dual-labeled cells reflect a more primitive bipotent state with the ability to differentiate into different lineages. Indeed, microdissection of ducts and lobules in the human breast to isolate cells for functional assays in vitro has shown the presence of enriched progenitor activity in cells that exhibit both luminal CK19 and basal CK14 expression [94]. Further, a population of luminal cells that is enriched for progenitor activity [94, 95] is also enriched for cells that co-express CK8 and CK14 [52, 96, 97]. However, a recent study examining progenitor activity from dissociated primary breast epithelial cells found no significant correlation between reduction mammoplasty samples that exhibit high CK8/14 double labeling and the ability to form colonies in culture [73]. Therefore, whether cells that express CK8/14 are bonafide progenitor cells still remains unclear.
Diversity of Luminal Progenitor Populations
While all reports support the notion that basal/ME epithelial cells are enriched for cells with the most stem/progenitor cell activity, there are conflicting reports regarding stem/progenitor potential of luminal epithelial cells in the same assays [80, 81]. In humans, cells expressing the luminal cell surface marker EpCAM/ESA+ are able to form branching structures reminiscent of TDLUs as well as acinar restricted colonies on Matrigel [75]. On a plastic substrate, EpCAM+ luminal cell populations also generate colonies that express both CK8 and CK14 as well as CK8 only colonies, suggesting that luminal cells have the ability to lose lineage restriction and give rise to basal cells [98–100].
Cells within the luminal lineage can be fractionated into two major populations: an EpCAM+CD49fneg mature luminal population in which 55 % of cells express ERα and 71 % express PR or an EpCAM+CD49f+ luminal progenitor population in which 28 % cells express ERα in the absence of PR [95]. When such cells are sorted and transplanted into humanized mammary fat pads of immunocompromised mice, both luminal progenitor and mature luminal cell populations give rise to bi-layered structures that contain both luminal (CK8+) and basal/ME (CK14+ and smooth muscle actin positive (SMA+)) cells. [96]. Thus, luminal cells do have the capacity to exhibit bipotent progenitor activity. In vitro, luminal cells isolated from both populations also form both branching and acinar structures on a collagen substrate [19, 52, 96]. Additionally, it has been shown that aldehyde dehydrogenase (ALDH) activity, which enriches for progenitor activity in multiple cell types [101], is enriched in luminal progenitor cells [102]. These luminal progenitor cells exhibit elevated expression of the ALDH1A3 isoform, suggesting that this isoform of the ALDH family may regulate progenitor activity in the breast [102, 103]. Further, the addition of ErbB3 expression in conjunction with ALDH activity segregates luminal cells into 3 separate populations [104]. However the size and incidence of these populations are not consistent among all reduction mammoplasty samples examined [104]. Improved delineation of luminal cells may be strengthened by clinical information about patients donating breast tissue samples, including parity, history of hormonal contraceptive use, and stage of the menstrual cycle. However, this information is often challenging to obtain.
In mice, multiple luminal epithelial cell populations have also been identified, although some of the populations are overlapping. Fractionation of luminal cells with the surface markers CD61/β3-integrin enriches for progenitor activity in the CD24+ population [56]. With the addition of this marker, the CD24+CD61+ cells have greater progenitor activity with significantly reduced numbers of ERα+ cells, while the CD24+CD61neg cells are enriched for ERα+ cells [56]. Similar to CD61, the use of CD133/prominin-1 separates an ERα/PR enriched population from the CD24+ cells [105]. Stem cell antigen-1 (sca-1) has been associated with stem/progenitor activity in a number of murine cell types (for review, [106]), and initial studies in the mammary gland suggested that sca-1+ epithelial cells exhibit increased progenitor activity compared with sca-1neg cells [107]. In combination with CD49b/α2-integrin, sca-1 separates EpCAM+ luminal cells into three populations: sca-1negCD49b+, sca-1+CD49b+, and sca-1+CD49bneg populations. Sca-1negCD49b+ cells have significant progenitor activity and express detectable CK18/CK5 double labeling, suggesting that they maybe an intermediate cell type [104]. Both sca-1+CD49b+ and sca-1+CD49bneg populations are enriched for ERα, but only the sca-1+CD49b+ population contains functional progenitor activity [104]. Similar to the sca-1neg progenitors, cells isolated using c-kit as a marker exhibit progenitor activity [108]. Together, these studies suggest that many of the cell surface markers used to isolate presumably different luminal progenitor populations identify cells with similar functional activities (Fig. 2). Thus, additional work is necessary to determine whether combining these markers will further refine the cellular populations and identify more pure populations of progenitor cells.
Less clear but equally important are bipotent progenitor cells that appear to exhibit structurally limited outgrowths. Transplantation of murine cells by limiting dilution can produce ductal-limited and secretory alveolar-limited outgrowths [77, 109, 110]. Progenitors that yield these specific outgrowths can be found in CD24hiCD49flo cells when transplanted into pregnant hosts [110]. In humans, breast epithelial cells also have the ability to form ductal-limited or acinar-limited structures when grown on a collagen substrate. Enrichment for ductal-only progenitors can be found in EpCAMnegCD10+ basal cells while acinar-only progenitors are present within the EpCAM+ luminal populations [52, 96]. It is currently unclear if these progenitors within the human breast have similar patterns of growth in vivo. It is also unclear whether these duct-limited or alveolar-limited progenitor cells represent distinct progenitor population or are an intermediate progenitor state that has yet to be described.
Although luminal populations enriched for steroid receptors can exhibit progenitor activity, whether the steroid receptor positive cells themselves have progenitor activity is not clear. This is because cells isolated with the current complement of cell surface markers do not isolate pure steroid receptor positive cells. This leads to the possibility that cells closely associated with steroid receptor positive cells are the source of progenitor activity. Technical challenges of cell sorting may also contribute to this issue; contaminating cells isolated during the sorting process may have measurable progenitor activity, leading to erroneous conclusions. Steroid receptor expression may be measured using immunofluorescence or immunohistochemistry on sorted fractions, which can also lead to challenges for interpretation of the data among different studies. Further, marker differences exist among mouse strains, with some cell surface markers such as c-kit or CD61 having different ranges of expression on progenitor cells in different models [104, 111]. In addition, culture conditions affect lineage restriction as well as progenitor activity in vitro [112]. ERα expression is dependent upon basement membrane components [113], and expression of ERα is rapidly lost in 2D culture making it challenging to directly test ERα progenitor activity in vitro [113, 114]. Similarly, responsiveness to progesterone may depend on other cell-cell interactions in addition to matrix. Cultures of primary cells in matrix do not increase the expression of downstream mediators of progesterone signaling, WNT-4 and RANKL in response to stimulation with progesterone [115], whereas culture of primary epithelial cells in microstructures which retain endogenous cellular interactions do [114]. Even utilizing transplantation assays, which are the gold standard for assessing progenitor activity, has proven challenging. Early studies indicated that luminal cell populations had little ability to generate structures within cleared mammary fat pads [80, 81, 105], however, luminal progenitor populations have the ability to grow in mammary fat pad in the presence of Matrigel [104, 110, 116] or under conditions of pregnancy, suggesting that some populations of progenitor cells may have specific growth requirements.
Together, these observations suggest that the cells in the luminal epithelial lineage have greater progenitor activity than previously appreciated. The progenitor cells in this population may require different stimuli to proliferate, including the hormonal environment of pregnancy. Through the use of multiple assays to detect progenitor activity, such as growth in matrix and treatment with combinations of hormones or injection into pregnant recipients, specific progenitor populations within the luminal lineage may be identified. These assays will also clarify the roles that they play in the mammary hierarchy.
Progesterone Effects on Progenitor Populations
In mice, progesterone signaling through PR has been shown to be necessary during puberty and over the course of subsequent estrous cycles for ductal branching and for full alveolar development during pregnancy. In both mice and humans, proliferation of mammary epithelial cells is at its highest during the luteal phase of the menstrual cycle in women or diestrus in mice, when progesterone peaks. The effects of progesterone on cell proliferation in the mammary gland has been examined both in the physiological period of diestrus as well as following exogenous progesterone administration. During diestrus, luminal cells with the cell surface markers CD24+CD49flo undergo a 3-fold expansion, however the specific luminal progenitor populations that may be affected have not been examined [117]. In addition, stem/basal progenitor cells also undergo a 14-fold increase during diestrus, compared to estrus when estrogen levels are comparatively elevated [117]. To investigate the effects of progesterone on epithelial cell proliferation, mice were treated with exogenous progesterone and proliferating cells were identified using BrdU incorporation. Mammary epithelial cell proliferation occurred in two waves related to PR expression. In the first wave, PR+ cells co-labeled with BrdU, suggesting that PR+ cells themselves proliferate, while in the second wave, PRneg cells incorporated BrdU [43]. Although PR+ progenitor cells have not been identified, progesterone has been shown to regulate the proliferation of neighboring PRneg cells through the secretion of paracrine mediators.
Although PR is expressed only in luminal cells in mice, progesterone coordinates the expansion of both luminal and basal progenitor populations. In luminal cells, progesterone has been shown to increase Rankl, which in a paracrine manner promotes proliferation of neighboring epithelial cells [43, 68, 118]. A paracrine mechanism for PR activity also appears to be conserved in humans. Using a “tissue microstructure” approach to study progesterone action on human epithelial cells, treatment with R5020, a PR agonist, led to a significant increase in RANKL mRNA and its cognate receptor RANK [114]. In addition to RANKL, progesterone induces the secretion of another paracrine acting factor, growth hormone (GH). In mammosphere cultures, primary human epithelial cells treated with progesterone significantly upregulated the expression of GH, and treatment of mammary cells with GH stimulated mammosphere formation in a dose-dependent manner [119]. Staining human breast tissue revealed that growth hormone receptor positive cells were found in the luminal epithelial cell layer and were ERαneg and PRneg [119]. This suggests that GH may also function as a paracrine mediator of progesterone action in luminal epithelial cells. Collectively, these results suggest that luminal PR acts in a paracrine manner to promote cellular proliferation of adjacent luminal PRneg cells (Fig. 3a, b).
Model for progesterone receptor (PR) activity in human and mouse epithelial cell populations. a In murine mammary glands, PR acts through a paracrine mechanism to promote the proliferation of cells in both the luminal and basal lineages. PR expression in basal epithelial cells has not been detected. b In the human breast, PR is expressed in both luminal and basal epithelial cells. Luminal PR acts through a paracrine mechanism to simulate the proliferation of neighboring cells. PR is also expressed in basal epithelial cell populations and promotes the proliferation of basal epithelial cells, however it is currently unclear whether PR activity is mediated through a paracrine or autocrine mechanism
Basal/ME progenitor activity also appears to be regulated by luminal expression of PR paracrine mediators in mice. Luminal epithelial cells express Wnt-4, while axin2 activity, an indicator for canonical Wnt signaling, is restricted to the basal/ME layer [120]. Axin2 expression increased in response to progesterone and is highest during diestrus [120]. Wnt ligand receptors Lrp5 and Lrp6 are also preferentially localized to the basal epithelium [121, 122], suggesting that upregulation of Wnt-4 in response to PR activity acts in a paracrine manner to promote the proliferation of basal epithelial cells (Fig. 3a). Recently, Cxcl12/Sdf-1α was identified using microarray analysis as a novel paracrine mediator of progesterone action on progenitor populations in the mammary gland [123]. Cxcr4, the cognate receptor for Cxcl12, is expressed by both basal and luminal epithelial cells, and inhibition of this signaling pathway led to reduced mammary repopulating ability [124]. This suggests that this pathway also regulates progenitor activity. Together, these observations show that progesterone coordinates the expansion of the luminal and basal epithelial compartments through the secretion of paracrine mediators. The specificity of the signals induced by the paracrine mediators may result from the restricted expression of their receptors on either luminal or basal epithelial cells.
While mouse mammary basal epithelial cells do not express PR and rely on paracrine signals from PR+ luminal cells to be responsive to progesterone, recent studies have revealed that progesterone directly affects basal progenitor epithelial cells in humans. In breast tissue, cells residing in the basal epithelial layer do indeed express PR [17, 52], and co-labeling with basal epithelial cell markers p63 and CK14 revealed that some, but not all, basal/ME cells express PR [17]. However, these PR+ basal/ME cells do not co-express ERα [17]. Interestingly, PR+ basal/ME cells are found primarily within immature Type I lobules, suggesting that these might be primitive basal progenitor cells [52]. Consistent with this, basal cells which are enriched for bipotent colony-forming activity express high PR and low levels of ERα transcripts [16]. Evaluation of cells sorted by FACS has demonstrated that both PR mRNA and protein is robustly detected in the basal EpCAMloCD49f+ fraction of cells compared with the bulk unsorted cells [17, 52]. This suggests that in the human breast, basal/ME progenitor cells do express PR and are likely responsive to progesterone. Indeed, progesterone treatment of primary human epithelial cells in culture increases mammosphere formation [19, 115]. On a collagen substrate, basal/ME EpCAMnegCD10+ cells exhibit enhanced ductal colony growth in response to treatment with progesterone, further indicating that progesterone affects basal progenitor activity [19]. These observations suggest that humans differ from mice in regulation of the basal/ME compartment in response to progesterone. While mice regulate basal progenitors indirectly through luminal-derived paracrine mediators, human basal epithelial cells express PR and respond to progesterone signaling.
Although PR has been detected in human basal epithelial cells, how progesterone actually signals to regulate basal cells is not clear. One possibility is that like luminal epithelial cells, PR acts in a paracrine mechanism to induce the proliferation of neighboring PR-negative basal cells. Consistent with this notion, WNT-11 expression is enriched within basal epithelial cells compared to unsorted cells and is upregulated in mammospheres following progesterone stimulation [19]. This suggests that WNT-11 could act as a paracrine mediator of PR activity in the basal compartment. However, other studies have suggested that basal PR+ cells may themselves be induced to proliferate in response to progesterone. This is supported by the expression of both PR and BrdU in rare basal cells in 3D cultures [115]. Isolation and characterization of PR+ cells from the basal epithelium is necessary to determine the mechanism of PR action in regulating this cell population. In total, these observations suggest that in humans, PR acts through a paracrine mechanism in luminal cells to expand luminal populations, while PR localized to basal progenitor cells may coordinate basal cell proliferation in either an autocrine or paracrine manner (Fig. 3b). More experiments are necessary to determine whether PR+ cells directly proliferate in response to progesterone in the basal epithelial cell layer in human tissues.
The underlying biological reason for the difference in PR expression patterns between mice and humans is not clear. In humans, PR signaling within the basal epithelium may have evolved with the formation of complex lobules in the breast. Further analysis of luminal and basal PR signaling and regulation of the human breast epithelial hierarchy may clarify these species differences.
Estrogen and Luminal Progenitor Cells
Estrogen is critical for ductal elongation in mice during puberty, but a specific role for estrogen in the regulation of progenitor activity in the adult mammary gland is still evolving. Treatment of mice with the aromatase inhibitor letrozole significantly diminishes repopulating cell numbers [125]. Letrozole inhibits the conversion of androgen to estrogen leading to a decrease in levels of circulating estrogen. Since loss of estrogen signaling leads to reduced PR expression, letrozole also inhibits PR signaling pathways. Although a role for progesterone in stem cell maintenance in the adult gland has been identified [117], the direct effect of estrogen on adult stem cells has not been characterized. This has led to the suggestion that estrogen functionally enhances stem cell activity indirectly through the upregulation of PR, and progesterone primarily maintains adult stem cells.
However, multiple luminal progenitor populations in mice do express ERα leading to the possibility that estrogen could directly influence progenitor cell activity. For example, in both humans and mice, rare ERα+ cells have been identified that co-label with markers of proliferation, [10–12, 126]. Long-term label-retaining studies, which identify slower cycling stem cells or those that undergo asymmetric division, have also identified ERα+ cells that retain label [60, 84, 127, 128], suggesting the possibility of an ERα+ progenitor cell. Human ERα+ long-term label-retaining cells are also enriched for p21cip, msi-1, and CK19, which have been shown to be putative stem cell markers [128, 129]. Utilizing the cell surface marker c-kit to isolate progenitor cells led to the identification of a subset of c-kit+ cells that are enriched for ERα+ expression and show high proliferative potential [108]. In addition, 6 % of progenitor-enriched CD24+/CD61+ cells express ERα [56], while sca-1+CD49bneg progenitors are also enriched for ERα expression. Although these latter progenitor cells show limited mammary repopulating ability, they do still form small ductal/lobular structures at low frequencies in vivo [104] and exhibit multilineage potential. Interestingly, despite expressing ERα, sca-1+CD49bneg luminal progenitor cells are not affected following ovariectomy, suggesting that these cells are able to survive in a low estrogen and progesterone environment [104]. Together, these observations suggest the existence of a distinct luminal ERα+ progenitor cell type.
The progeny of proliferating ERα+ cells have not been identified, however, a population of dividing ERα+ cells is detected at a 10-fold higher frequency in murine mammary glands during early pregnancy [130, 131]. This suggests that this population is expanding specifically during this time and may be contributing to alveologenesis. Mammary glands from mice expressing a mammary inducible form of Histone H2B fused to eGFP (H2BGFP) have also been used to identify a population of proliferating ERα+ cells [132, 133]. Following label, H2BGFP was co-expressed with ERα, but these cells did not express PR [133]. Gene expression analysis suggested that the H2BGFP+CD24+CD29lo cells show an intermediate transcription profile between mammary stem cells and luminal progenitor cells [133]. Functionally, H2BGFP+CD24+CD29lo cells are able to generate mammary structures capable of differentiation and lactation when transplanted into pregnant recipients, but cannot be serially transplanted [133]. This is consistent with the notion that these cells are likely limited multipotent progenitors rather than true stem cells. Collectively, these studies suggest that ERα+ cells may have bipotent progenitor activity that is regulated by the hormonal environment of pregnancy.
Little is known about the activity of human ERα+ progenitor cells. Using a novel ERE-reporter system, human ERα+ primary mammary epithelial cells were isolated by FACS and characterized [103]. In vitro, these ERα+ cells could form adherent luminal colonies but were unable to form mammospheres [103]. Further, transplant of isolated ERα+ cells into humanized glands of immunocompromised mice resulted in only single layer outgrowths that expressed luminal markers [103], suggesting that in humans, ERα+ cells are luminal restricted progenitor cells. In breast tissues, a population of ERα+ luminal cells has also been identified that expresses p27+ [134]; p27 was shown to regulate stem and progenitor cells in mice [135, 136]. Low but detectable numbers of p27+ cells that also express the proliferation marker Ki67 are observed during early pregnancy [134], although their functional significance is not clear. Further work is necessary to determine whether ERα+p27+ have progenitor activity during pregnancy in the human breast.
Currently, little is known about the regulation of ERα+ progenitor cells. In transgenic mice, Wnt-1 overexpression enhances the proliferation of ERα+ cells [130], suggesting that Wnt family members may play a role in expansion of these progenitors. In vitro, estrogen-treatment of human mammospheres also leads to the upregulation of Wnt ligands (WNT-3A, WNT-4, and WNT-8A), suggesting that estrogen stimulates progenitor activity through the WNT pathway [19]. Human luminal progenitor cells treated with estrogen show increased acinar colony formation, which is dependent on WNT ligand receptor, LRP6 expression [19]. Identification of the specific regulation of ERα+ progenitor cells may provide insight into ERα+ tumors, particularly those that are resistant to treatment with anti-estrogen therapies.
Collectively, these various lines of evidence suggests that 2 pools of ERα-expressing cells reside within mammary tissues: ERα-expressing PR+ cells that regulate progesterone responsiveness, and ERα+ cells that have progenitor activity, which may be enhanced during pregnancy or through WNT signaling (Fig. 4).
Model for estrogen receptor alpha (ERα) activity in human and mouse epithelial cell populations. In luminal epithelial cells, ERα and PR are expressed together and act through a paracrine mechanism to stimulate the proliferation of surrounding cells. A population of ERα+ cells has also been detected that acts through an autocrine mechanism to expand cells of the luminal lineage
Parity and Progenitor Cell Populations
During pregnancy, the expanding lobule-alveoli that become terminally differentiated to produce milk are essentially entirely derived from the progeny of progenitor cells. Pregnancy-induced mammary epithelial cells (PI-MECs) were first shown to be the stem/progenitor cells that contribute to the formation of alveoli. Following involution, these cells remain in the gland to be called upon for alveolar expansion during subsequent pregnancies. Isolated PI-MECs have been shown to contain the potential for both ductal- and lobule- limited outgrowths [137], and the expansion of PI-MECs during pregnancy suggests that these cells may be hormonally regulated. Indeed, ERα may be important for the maintenance or expansion of PI-MECs since a lactation defect was uncovered when exon 3 of ERα was excised during pregnancy [35].
Although PI-MECs indeed serve as the precursor to alveoli during pregnancy, there have been conflicting reports regarding whether they contribute to both luminal and basal/ME lineages. Using a genetic tracking approach in which WAP-cre mice were crossed with Rosa26-lox-Stop-lox-YFP mice, the lineage contribution of PI-MECs during pregnancy was examined. Following involution, YFP-labeled cells were shown to be restricted to the luminal epithelial layer; the majority displayed an ERαneg sca-1loCD49bhi luminal progenitor immunophenotype, although ~6 % of the YFP labeled cells displayed the hormone-sensing ERα-enriched sca-1hiCD49blo phenotype [138]. In contrast, another study also using WAP-cre to genetically label PI-MECs during the second half of pregnancy observed that PI-MECs were primarily localized in the CD24+CD49fhi basal population following involution [139]. The reason for this difference in lineage restriction between the two studies is not clear. One possibility is that WAP is expressed at slightly different time points during pregnancy in each model. If PI-MECs are labeled with YFP late during pregnancy, it is possible that basal progenitor cells have already contributed to the luminal progenitor population earlier in pregnancy and therefore would not have been detected as precursors to alveoli. In fact, contribution of the basal progenitor population to the luminal epithelium during pregnancy is consistent with other lineage tracing studies that demonstrated a basal epithelial cell contribution to alveologenesis [85–87].
The importance of PI-MECs extends beyond serving as the precursors for alveoli during pregnancy. PI-MECs may also serve as the cellular precursors to MMTV-ErbB2 driven tumorigenesis [140–143]. This notion was uncovered by the finding that PI-MECs require cyclin D1 expression for their proliferation. Cyclin D1-/- mice are able to undergo alveologenesis during pregnancy and are particularly resistant to tumor formation in response to overexpression ErbB2 [124, 144]. In addition, transgenic mice that carry a point mutation in cyclin D1, which renders the interactions with cyclin-dependent kinases 4 and 6 catalytically inactive (cyclin D1KE/KE), also exhibit similar phenotypes. Whole mounts of glands from cyclin D1KE/KE mice show a ductal system that is devoid of side branching [110], reminiscent of the phenotype seen in PR-/- mice. In addition, although normal lobuloalveoli develop in cyclin D1KE/KE mice following the first pregnancy, a decline in lobule-alveologenesis is observed in these mice such that by the third pregnancy, lobuloalveoli are absent [145]. Moreover, cyclin D1KE/KE mice are also resistant to MMTV-ErbB2 tumorigenesis [110]. These results suggest that the kinase activity of cyclin D1 is necessary for the development and maintenance of luminal progenitor cell populations, in particular those that are necessary for ductal branching and alveologenesis. Loss of these luminal progenitor cell populations results in the protection from ErbB2-induced mammary tumorigenesis.
The existence of PI-MECs in the human breast is unknown. Analysis of breast tissue from parous and nulliparous women based on cell surface markers has revealed changes in epithelial cell populations. Not surprisingly, compared with nulliparous glands, parous glands exhibit more complexity within the luminal cell populations following pregnancy [52]. These differences in parity-induced epithelial cell populations may contribute to the transcriptional differences observed between the breasts of parous and nulliparous women [146–149]. Following pregnancy, ERα and PR expression is significantly reduced in breasts of parous women compared to nulliparous women [149]. Similarly, mammary glands from parous mice demonstrate significantly reduced levels of ERα and PR compared with age-matched virgin mice, although circulating progesterone levels are similar [150].
One way to differentiate among cell populations that develop during pregnancy may be to identify signaling pathways that are specific to each population. Notch is a highly conserved signaling system that plays important roles in stem cell niches and differentiation processes (for review, [151, 152]). All four Notch receptors are expressed within the mammary gland, and each appears to regulate separate epithelial progenitor niches. Notch1 signaling regulates progenitor cells in the basal lineage [153, 154]. Consistent with this, transgenic mice that overexpress the activated form of Notch1 under control of the MMTV promoter (MMTV/N1IC) demonstrate an expansion of the CD24+CD29hi basal/ME cell cells, which when isolated and grown in vitro exhibit predominantly basal differentiation potential [154]. In addition, transplantation of N1IC epithelial cells into cleared mammary fat pads results in ductal-limited outgrowths, with a loss of secondary and tertiary branches and alveologenesis, which was not observed in transplants of epithelial cells from nontransgenic mice [154].
Other Notch family members appear to only regulate luminal progenitor populations. Notch2 has recently been implicated in patterning of the tertiary branches and formation of alveolar clusters [155]. Given the role of progesterone in tertiary branching and alveologenesis, it would be interesting to determine whether Notch2+ cells are also PR+ or in close proximity to PR+ cells. Notch3 (N3IC) mice that contain an activated intracellular form of Notch exhibit a cyclin D1-dependent expansion of CD24+CD29lo luminal progenitors. Transplants show reduced alveoli in pregnant hosts, with a higher proportion of cells expressing ERα [156], suggesting Notch3 may regulate hormone sensing cell populations. Examination of Notch4 signaling defined yet another type of luminal population. Transgenic mice that overexpress INT3 (Notch4) under control of the WAP promoter fail to undergo secretory alveolar development and are unable to lactate [157]. Mammary epithelial cells of these mice exhibit normal ERα expression, but have significantly reduced numbers of PR+ and Rankl+ epithelial cells [157]. Interestingly, these mice are prone to mammary tumor formation and notably they develop tumors that are strongly ERα+/PRneg [157]. Both Notch1 and Notch4 are significantly upregulated in response to knockdown of Elf-5, a transcription factor necessary for alveologenesis [158], and loss of Elf-5 results in increased luminal progenitor populations during pregnancy [67]. This suggests that Elf-5 may regulate the expression of specific Notch receptors to allow for the maturation of alveolar progenitor cells. Collectively, these studies suggest that the Notch family signaling pathway is a central player in mammary progenitor cell biology and may differentially stimulate hormonally responsive progenitor cell populations that expand during pregnancy.
Studies from mice have suggested that multiple populations of cells emerge in the hormonal milieu of pregnancy. As we continue to delineate specific cells types that are responsive to either estrogen or progesterone or the combination of the two hormones in mice, this may provide critical insight into human counterparts that have not yet been identified experimentally, including the potential cells of origin for ErbB2/HER2+ cancers. This may also have implications for pregnancy-associated breast cancer as well as malignancies that develop in parous aging women.
Stem Cell Populations and Aging
After menopause, serum plasma estrogen and progesterone levels decline sharply, whereas androgen levels, largely testosterone, are unchanged. This leads to regression of the breast epithelium with a specific reduction in the number of Type II and Type III lobules and an increase in the number of Type I lobules [51]. In addition to regression of the lobules, the stroma also regresses with a generalized reduction in density and an increase in adipose tissue [159–161]. While systemic estrogen and progesterone levels dramatically decrease after menopause, local levels of estrogen have been reported to actually elevate due to the increased activity of aromatase, the necessary enzyme in the biosynthesis of estrogen [162].
The elevated levels of localized estrogen likely have an effect on epithelial cells that are present within the breast. This is because in the human breast, the number of ERα+ cells increases after menopause, and in some lobules the expression pattern changes to areas of contiguous expression with over 90 % of epithelial cells expressing ERα [163]. A similar increase in ERα+ cells is also observed in mouse models of menopause generated through ovariectomy [164–166]. Prior to menopause, ERα expressing cells rarely express markers for proliferation [10–12]. However, ERα+ proliferating cells significantly increase with age in the human breast [163], although overall proliferation in the breast is reduced [163, 167].
Besides changes in ERα expression, other age-related changes in epithelial cell phenotypes have been reported. In a study examining a large collection of normal mammary epithelial cell strains derived from primary breast tissue of aging women, a decrease in myoepithelial cells with an increase in luminal progenitor cells that expressed CD49f, CK19, and MUC1/CD227, as well as c-kit+ progenitor cells was observed [168]. In a different study examining 20-month old mice that ceased ovarian cycling, mammary epithelial cells demonstrated diminished colony forming activity in vitro suggesting decreased activity and/or fitness of progenitor cells; however, no other assays were conducted to examine luminal progenitor populations [117].
It is well established that with aging, there is a higher incidence of breast cancer [169]. However, what is frequently not appreciated is that there is also a specific increased incidence of ERα+/PR+ tumors with age which, is different from the incidence of ERα+/PRneg, ERαneg/PR+, or ERαneg/PRneg tumors [170, 171]. The reason for this subtype-specific difference is not clear. Little is known about the complement of mammary gland progenitor cells that are present in the breast and their activity following menopause. While luminal progenitor cells are though to be the precursors to the majority of breast cancers, the mechanism of how they give rise to the different tumor subtypes is not clear. In addition, it is not fully understood whether different types of luminal progenitor cells are the precursors to different type of ERα+ tumors. Since more than 80 % of breast cancers occur in women over 50 years of age [169], understanding the effect of menopause on progenitor populations and their regulation in response to locally produced estrogen may be beneficial for reducing the incidence of ERα+ tumors in aging women.
Conclusions
Diverse types of mammary progenitor cells have been identified, some that are specifically induced during pregnancy, which precluded their identification as progenitor cells in earlier studies. In particular, determining mechanisms for regulation of stem and progenitor cells by estrogen and progesterone has been challenging due to the loss of steroid hormone receptors in vitro and the intertwined nature of ERα and PR regulation. However, as further progress is made with the identification of additional cell surface markers and sophisticated lineage tracing models, more epithelial cell populations in the human breast may be delineated, including those that are specific to parous women. Although many similarities exist between mouse mammary glands and human breasts, the regulation of the epithelial hierarchy as well as its response to estrogen and progesterone in women may be more complex than in mice. Understanding these similarities and differences may lead to improved models to study breast cancer origin and treatment.
Abbreviations
- ALDH:
-
Aldehyde dehydrogenase
- CK:
-
Cytokeratin
- EGF:
-
Epidermal growth factor
- Elf5:
-
E74-like factor
- ERα:
-
Estrogen receptor alpha
- FACS:
-
Fluorescence-activated cell sorting
- GH:
-
Growth hormone
- H2BGFP:
-
Histone H2B fused to eGFP
- ME:
-
Myoepithelial
- MMTV:
-
Mouse mammary tumor virus
- MRU:
-
Mammary repopulating units
- PI-MEC:
-
Pregnancy-induced mammary epithelial cells
- PR:
-
Progesterone receptor
- sca-1:
-
Stem cell antigen-1
- SMA:
-
Smooth muscle actin
- TDLU:
-
Terminal ductal lobule unit
- TGFα:
-
Transforming growth factor alpha
References
Potten CS, Watson RJ, Williams GT, Tickle S, Roberts SA, Harris M, et al. The effect of age and menstrual cycle upon proliferative activity of the normal human breast. Br J Cancer. 1988;58:163–70.
Navarrete MA, Maier CM, Falzoni R, Quadros LG, Lima GR, Baracat EC, et al. Assessment of the proliferative, apoptotic and cellular renovation indices of the human mammary epithelium during the follicular and luteal phases of the menstrual cycle. Breast Cancer Res. 2005;7:R306–13.
Ferguson DJ, Anderson TJ. Morphological evaluation of cell turnover in relation to the menstrual cycle in the “resting” human breast. Br J Cancer. 1981;44:177–81.
Anderson TJ, Ferguson DJ, Raab GM. Cell turnover in the “resting” human breast: influence of parity, contraceptive pill, age and laterality. Br J Cancer. 1982;46:376–82.
Jindal S, Gao D, Bell P, Albrektsen G, Edgerton SM, Ambrosone CB, et al. Postpartum breast involution reveals regression of secretory lobules mediated by tissue-remodeling. Breast Cancer Res. 2014;16:R31.
van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–60.
Perez-Losada J, Balmain A. Stem-cell hierarchy in skin cancer. Nat Rev Cancer. 2003;3:434–43.
Ramakrishnan R, Khan SA, Badve S. Morphological changes in breast tissue with menstrual cycle. Mod Pathol. 2002;15:1348–56.
Vogel PM, Georgiade NG, Fetter BF, Vogel FS, McCarty Jr KS. The correlation of histologic changes in the human breast with the menstrual cycle. Am J Pathol. 1981;104:23–34.
Russo J, Ao X, Grill C, Russo IH. Pattern of distribution of cells positive for estrogen receptor alpha and progesterone receptor in relation to proliferating cells in the mammary gland. Breast Cancer Res Treat. 1999;53:217–27.
Zeps N, Bentel JM, Papadimitriou JM, D’Antuono MF, Dawkins HJ. Estrogen receptor-negative epithelial cells in mouse mammary gland development and growth. Differentiation. 1998;62:221–6.
Clarke RB, Howell A, Potten CS, Anderson E. Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 1997;57:4987–91.
Petz LN, Ziegler YS, Schultz JR, Kim H, Kemper JK, Nardulli AM. Differential regulation of the human progesterone receptor gene through an estrogen response element half site and Sp1 sites. J Steroid Biochem Mol Biol. 2004;88:113–22.
Schultz JR, Petz LN, Nardulli AM. Estrogen receptor alpha and Sp1 regulate progesterone receptor gene expression. Mol Cell Endocrinol. 2003;201:165–75.
Schultz JR, Petz LN, Nardulli AM. Cell- and ligand-specific regulation of promoters containing activator protein-1 and Sp1 sites by estrogen receptors alpha and beta. J Biol Chem. 2005;280:347–54.
Hilton HN, Santucci N, Silvestri A, Kantimm S, Huschtscha LI, Graham JD, et al. Progesterone stimulates progenitor cells in normal human breast and breast cancer cells. Breast Cancer Res Treat. 2014;143:423–33.
Hilton HN, Graham JD, Kantimm S, Santucci N, Cloosterman D, Huschtscha LI, et al. Progesterone and estrogen receptors segregate into different cell subpopulations in the normal human breast. Mol Cell Endocrinol. 2012;361:191–201.
Hilton HN, Doan TB, Graham JD, Oakes SR, Silvestri A, Santucci N, et al. Acquired convergence of hormone signaling in breast cancer: ER and PR transition from functionally distinct in normal breast to predictors of metastatic disease. Oncotarget. 2014;5:8651–64.
Arendt LM, St Laurent J, Wronski A, Caballero S, Lyle SR, Naber SP, et al. Human breast progenitor cell numbers are regulated by WNT and TBX3. PLoS One. 2014;9:e111442.
Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–23.
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–74.
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52.
Phipps AI, Malone KE, Porter PL, Daling JR, Li CI. Body size and risk of luminal, HER2-overexpressing, and triple-negative breast cancer in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2008;17:2078–86.
Phipps AI, Buist DS, Malone KE, Barlow WE, Porter PL, Kerlikowske K, et al. Breast density, body mass index, and risk of tumor marker-defined subtypes of breast cancer. Ann Epidemiol. 2012;22:340–8.
Biglia N, Peano E, Sgandurra P, Moggio G, Pecchio S, Maggiorotto F, et al. Body mass index (BMI) and breast cancer: impact on tumor histopathologic features, cancer subtypes and recurrence rate in pre and postmenopausal women. Gynecol Endocrinol. 2013;29:263–7.
McGuire WL. Hormone receptors: their role in predicting prognosis and response to endocrine therapy. Semin Oncol. 1978;5:428–33.
Cui X, Schiff R, Arpino G, Osborne CK, Lee AV. Biology of progesterone receptor loss in breast cancer and its implications for endocrine therapy. J Clin Oncol. 2005;23:7721–35.
Haslam SZ. Acquisition of estrogen-dependent progesterone receptors by normal mouse mammary gland. Ontogeny of mammary progesterone receptors. J Steroid Biochem. 1988;31:9–13.
Hovey RC, Trott JF, Vonderhaar BK. Establishing a framework for the functional mammary gland: from endocrinology to morphology. J Mammary Gland Biol Neoplasia. 2002;7:17–38.
Hennighausen L, Robinson GW. Think globally, act locally: the making of a mouse mammary gland. Genes Dev. 1998;12:449–55.
Keeling JW, Ozer E, King G, Walker F. Oestrogen receptor alpha in female fetal, infant, and child mammary tissue. J Pathol. 2000;191:449–51.
Howard BA, Gusterson BA. Human breast development. J Mammary Gland Biol Neoplasia. 2000;5:119–37.
Russo J, Russo IH. Development of the human breast. Maturitas. 2004;49:2–15.
Mallepell S, Krust A, Chambon P, Brisken C. Paracrine signaling through the epithelial estrogen receptor alpha is required for proliferation and morphogenesis in the mammary gland. Proc Natl Acad Sci U S A. 2006;103:2196–201.
Feng Y, Manka D, Wagner KU, Khan SA. Estrogen receptor-alpha expression in the mammary epithelium is required for ductal and alveolar morphogenesis in mice. Proc Natl Acad Sci U S A. 2007;104:14718–23.
Mueller SO, Clark JA, Myers PH, Korach KS. Mammary gland development in adult mice requires epithelial and stromal estrogen receptor alpha. Endocrinology. 2002;143:2357–65.
Luetteke NC, Qiu TH, Fenton SE, Troyer KL, Riedel RF, Chang A, et al. Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development. 1999;126:2739–50.
Ciarloni L, Mallepell S, Brisken C. Amphiregulin is an essential mediator of estrogen receptor alpha function in mammary gland development. Proc Natl Acad Sci U S A. 2007;104:5455–60.
Kenney NJ, Smith GH, Rosenberg K, Cutler ML, Dickson RB. Induction of ductal morphogenesis and lobular hyperplasia by amphiregulin in the mouse mammary gland. Cell Growth Differ. 1996;7:1769–81.
Kenney NJ, Bowman A, Korach KS, Barrett JC, Salomon DS. Effect of exogenous epidermal-like growth factors on mammary gland development and differentiation in the estrogen receptor-alpha knockout (ERKO) mouse. Breast Cancer Res Treat. 2003;79:161–73.
Brisken C, Park S, Vass T, Lydon JP, O’Malley BW, Weinberg RA. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc Natl Acad Sci U S A. 1998;95:5076–81.
Lydon JP, Demayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery Jr CA, et al. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–78.
Beleut M, Rajaram RD, Caikovski M, Ayyanan A, Germano D, Choi Y, et al. Two distinct mechanisms underlie progesterone-induced proliferation in the mammary gland. Proc Natl Acad Sci U S A. 2010;107:2989–94.
Fernandez-Valdivia R, Mukherjee A, Ying Y, Li J, Paquet M, Demayo FJ, et al. The RANKL signaling axis is sufficient to elicit ductal side-branching and alveologenesis in the mammary gland of the virgin mouse. Dev Biol. 1999;328:127–39.
Brisken C, Heineman A, Chavarria T, Elenbaas B, Tan J, Dey SK, et al. Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes Dev. 2000;14:650–4.
Sicinski P, Donaher JL, Parker SB, Li T, Fazeli A, Gardner H, et al. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell. 1995;82:621–30.
Fantl V, Stamp G, Andrews A, Rosewell I, Dickson C. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev. 1995;9:2364–72.
Going JJ, Moffat DF. Escaping from Flatland: clinical and biological aspects of human mammary duct anatomy in three dimensions. J Pathol. 2004;203:538–44.
Russo J, Lynch H, Russo IH. Mammary gland architecture as a determining factor in the susceptibility of the human breast to cancer. Breast J. 2001;7:278–91.
Russo J, Mills MJ, Moussalli MJ, Russo IH. Influence of human breast development on the growth properties of primary cultures. In Vitro Cell Dev Biol. 1998;25:643–9.
Russo J, Rivera R, Russo IH. Influence of age and parity on the development of the human breast. Breast Cancer Res Treat. 1992;23:211–8.
Arendt LM, Keller PJ, Skibinski A, Goncalves K, Naber SP, Buchsbaum RJ, et al. Anatomical localization of progenitor cells in human breast tissue reveals enrichment of uncommitted cells within immature lobules. Breast Cancer Res. 2014;16:453.
Mishell Jr DR, Nakamura RM, Crosignani PG, Stone S, Kharma K, Nagata Y, et al. Serum gonadotropin and steroid patterns during the normal menstrual cycle. Am J Obstet Gynecol. 1971;111:60–5.
Uehara J, Nazario AC, Rodrigues de Lima G, Simoes MJ, Juliano Y, Gebrim LH. Effects of tamoxifen on the breast in the luteal phase of the menstrual cycle. Int J Gynaecol Obstet. 1998;62:77–82.
Ramakrishnan R, Gann PH, Wiley EL, Khurana KK, Khan SA. Normal breast lobular architecture in breast biopsy samples from breast cancer cases and benign disease controls. Breast Cancer Res Treat. 2004;86:259–68.
Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9:201–9.
Kouros-Mehr H, Slorach EM, Sternlicht MD, Werb Z. GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell. 2006;127:1041–55.
Bernardo GM, Lozada KL, Miedler JD, Harburg G, Hewitt SC, Mosley JD, et al. FOXA1 is an essential determinant of ERalpha expression and mammary ductal morphogenesis. Development. 2010;137:2045–54.
Grimm SL, Seagroves TN, Kabotyanski EB, Hovey RC, Vonderhaar BK, Lydon JP, et al. Disruption of steroid and prolactin receptor patterning in the mammary gland correlates with a block in lobuloalveolar development. Mol Endocrinol. 2002;16:2675–91.
Seagroves TN, Lydon JP, Hovey RC, Vonderhaar BK, Rosen JM. C/EBPbeta (CCAAT/enhancer binding protein) controls cell fate determination during mammary gland development. Mol Endocrinol. 2000;14:359–68.
LaMarca HL, Visbal AP, Creighton CJ, Liu H, Zhang Y, Behbod F, et al. CCAAT/enhancer binding protein beta regulates stem cell activity and specifies luminal cell fate in the mammary gland. Stem Cells. 2010;28:535–44.
Yamaji D, Na R, Feuermann Y, Pechhold S, Chen W, Robinson GW, et al. Development of mammary luminal progenitor cells is controlled by the transcription factor STAT5A. Genes Dev. 2009;23:2382–7.
Miyoshi K, Shillingford JM, Smith GH, Grimm SL, Wagner KU, Oka T, et al. Signal transducer and activator of transcription (Stat) 5 controls the proliferation and differentiation of mammary alveolar epithelium. J Cell Biol. 2001;155:531–42.
Liu X, Robinson GW, Wagner KU, Garrett L, Wynshaw-Boris A, Hennighausen L. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 1997;11:179–86.
Santos SJ, Haslam SZ, Conrad SE. Signal transducer and activator of transcription 5a mediates mammary ductal branching and proliferation in the nulliparous mouse. Endocrinology. 2010;151:2876–85.
Santos SJ, Haslam SZ, Conrad SE. Estrogen and progesterone are critical regulators of Stat5a expression in the mouse mammary gland. Endocrinology. 2008;149:329–38.
Oakes SR, Naylor MJ, Asselin-Labat ML, Blazek KD, Gardiner-Garden M, Hilton HN, et al. The Ets transcription factor Elf5 specifies mammary alveolar cell fate. Genes Dev. 2008;22:581–6.
Lee HJ, Gallego-Ortega D, Ledger A, Schramek D, Joshi P, Szwarc MM, et al. Progesterone drives mammary secretory differentiation via RankL-mediated induction of Elf5 in luminal progenitor cells. Development. 2013;140:1397–401.
Harris J, Stanford PM, Sutherland K, Oakes SR, Naylor MJ, Robertson FG, et al. Socs2 and elf5 mediate prolactin-induced mammary gland development. Mol Endocrinol. 2006;20:1177–87.
Gallego MI, Binart N, Robinson GW, Okagaki R, Coschigano KT, Perry J, et al. Prolactin, growth hormone, and epidermal growth factor activate Stat5 in different compartments of mammary tissue and exert different and overlapping developmental effects. Dev Biol. 2001;229:163–75.
Kendrick H, Regan JL, Magnay FA, Grigoriadis A, Mitsopoulos C, Zvelebil M, et al. Transcriptome analysis of mammary epithelial subpopulations identifies novel determinants of lineage commitment and cell fate. BMC Genomics. 2008;9:591.
Lim E, Wu D, Pal B, Bouras T, Asselin-Labat ML, Vaillant F, et al. Transcriptome analyses of mouse and human mammary cell subpopulations reveal multiple conserved genes and pathways. Breast Cancer Res. 2010;12:R21.
Raouf A, Zhao Y, To K, Stingl J, Delaney A, Barbara M, et al. Transcriptome analysis of the normal human mammary cell commitment and differentiation process. Cell Stem Cell. 2008;3:109–18.
Soneji S, Huang S, Loose M, Donaldson IJ, Patient R, Gottgens B, et al. Inference, validation, and dynamic modeling of transcription networks in multipotent hematopoietic cells. Ann N Y Acad Sci. 2007;1106:30–40.
DeOme KB, Faulkin Jr LJ, Bern HA, Blair PB. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res. 1959;19:515–20.
Daniel CW, DeOme KB. Growth of mouse mammary glands in vivo after monolayer culture. Science. 1965;149:634–6.
Kordon EC, Smith GH. An entire functional mammary gland may comprise the progeny from a single cell. Development. 1998;125:1921–30.
Lachenmayer A, Alsinet C, Savic R, Cabellos L, Toffanin S, Hoshida Y, et al. Wnt-pathway activation in two molecular classes of hepatocellular carcinoma and experimental modulation by sorafenib. Clin Cancer Res. 2012;18:4997–5007.
Novelli M, Cossu A, Oukrif D, Quaglia A, Lakhani S, Poulsom R, et al. X-inactivation patch size in human female tissue confounds the assessment of tumor clonality. Proc Natl Acad Sci U S A. 2003;100:3311–4.
Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–8.
Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–7.
Van Keymeulen A, Rocha AS, Ousset M, Beck B, Bouvencourt G, Rock J, et al. Distinct stem cells contribute to mammary gland development and maintenance. Nature. 2011;479:189–93.
Prater MD, Petit V, Alasdair RI, Giraddi RR, Shehata M, Menon S, et al. Mammary stem cells have myoepithelial cell properties. Nat Cell Biol. 2014;16:942–7.
Boras-Granic K, Dann P, Wysolmerski JJ. Embryonic cells contribute directly to the quiescent stem cell population in the adult mouse mammary gland. Breast Cancer Res. 2014;16:487.
van Amerongen R, Bowman AN, Nusse R. Developmental stage and time dictate the fate of Wnt/beta-catenin-responsive stem cells in the mammary gland. Cell Stem Cell. 2012;11:387–400.
Wang D, Cai C, Dong X, Yu QC, Zhang XO, Yang L, et al. Identification of multipotent mammary stem cells by protein C receptor expression. Nature. 2015;517:81–4.
Tao L, van Bragt MP, Laudadio E, Li Z. Lineage tracing of mammary epithelial cells using cell-type-specific cre-expressing adenoviruses. Stem Cell Reports. 2014;2:770–9.
Rios AC, Fu NY, Lindeman GJ, Visvader JE. In situ identification of bipotent stem cells in the mammary gland. Nature. 2014;506:322–7.
Pei XH, Bai F, Smith MD, Usary J, Fan C, Pai SY, et al. CDK inhibitor p18(INK4c) is a downstream target of GATA3 and restrains mammary luminal progenitor cell proliferation and tumorigenesis. Cancer Cell. 2009;15:389–401.
Zhu Y, Huang YF, Kek C, Bulavin DV. Apoptosis differently affects lineage tracing of Lgr5 and Bmi1 intestinal stem cell populations. Cell Stem Cell. 2013;12:298–303.
Shehata M, van Amerongen R, Zeeman AL, Giraddi RR, Stingl J. The influence of tamoxifen on normal mouse mammary gland homeostasis. Breast Cancer Res. 2014;16:411.
Sale S, Pavelic K. Mammary lineage tracing: the coming of age. Cell Mol Life Sci. 2015;72:1577–83.
Gusterson BA, Ross DT, Heath VJ, Stein T. Basal cytokeratins and their relationship to the cellular origin and functional classification of breast cancer. Breast Cancer Res. 2005;7:143–8.
Villadsen R, Fridriksdottir AJ, Ronnov-Jessen L, Gudjonsson T, Rank F, LaBarge MA, et al. Evidence for a stem cell hierarchy in the adult human breast. J Cell Biol. 2007;177:87–101.
Lim E, Vaillant F, Wu D, Forrest NC, Pal B, Hart AH, et al. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med. 2009;15:907–13.
Keller PJ, Arendt LM, Skibinski A, Logvinenko T, Klebba I, Dong S, et al. Defining the cellular precursors to human breast cancer. Proc Natl Acad Sci U S A. 2012;109:2772–7.
Proia TA, Keller PJ, Gupta PB, Klebba I, Jones AD, Sedic M, et al. Genetic predisposition directs breast cancer phenotype by dictating progenitor cell fate. Cell Stem Cell. 2011;8:149–63.
Stingl J, Eaves CJ, Kuusk U, Emerman JT. Phenotypic and functional characterization in vitro of a multipotent epithelial cell present in the normal adult human breast. Differentiation. 1998;63:201–13.
O’Hare MJ, Ormerod MG, Monaghan P, Lane EB, Gusterson BA. Characterization in vitro of luminal and myoepithelial cells isolated from the human mammary gland by cell sorting. Differentiation. 1991;46:209–21.
Stingl J, Eaves CJ, Zandieh I, Emerman JT. Characterization of bipotent mammary epithelial progenitor cells in normal adult human breast tissue. Breast Cancer Res Treat. 2001;67:93–109.
Moreb JS. Aldehyde dehydrogenase as a marker for stem cells. Curr Stem Cell Res Ther. 2008;3:237–46.
Eirew P, Kannan N, Knapp DJ, Vaillant F, Emerman JT, Lindeman GJ, et al. Aldehyde dehydrogenase activity is a biomarker of primitive normal human mammary luminal cells. Stem Cells. 2012;30:344–8.
Honeth G, Lombardi S, Ginestier C, Hur M, Marlow R, Buchupalli B, et al. Aldehyde dehydrogenase and estrogen receptor define a hierarchy of cellular differentiation in the normal human mammary epithelium. Breast Cancer Res. 2014;16:R52.
Shehata M, Teschendorff A, Sharp G, Novcic N, Russell A, Avril S, et al. Phenotypic and functional characterization of the luminal cell hierarchy of the mammary gland. Breast Cancer Res. 2012;14:R134.
Sleeman KE, Kendrick H, Robertson D, Isacke CM, Ashworth A, Smalley MJ. Dissociation of estrogen receptor expression and in vivo stem cell activity in the mammary gland. J Cell Biol. 2007;176:19–26.
Holmes C, Stanford WL. Concise review: stem cell antigen-1: expression, function, and enigma. Stem Cells. 2007;25:1339–47.
Welm BE, Tepera SB, Venezia T, Graubert TA, Rosen JM, Goodell MA. Sca-1(pos) cells in the mouse mammary gland represent an enriched progenitor cell population. Dev Biol. 2002;245:42–56.
Regan JL, Kendrick H, Magnay FA, Vafaizadeh V, Groner B, Smalley MJ. c-Kit is required for growth and survival of the cells of origin of Brca1-mutation-associated breast cancer. Oncogene. 2012;31:869–83.
Smith GH. Experimental mammary epithelial morphogenesis in an in vivo model: evidence for distinct cellular progenitors of the ductal and lobular phenotype. Breast Cancer Res Treat. 1996;39:21–31.
Jeselsohn R, Brown NE, Arendt L, Klebba I, Hu MG, Kuperwasser C, et al. Cyclin D1 kinase activity is required for the self-renewal of mammary stem and progenitor cells that are targets of MMTV-ErbB2 tumorigenesis. Cancer Cell. 2010;17:65–76.
Fu N, Lindeman GJ, Visvader JE. The mammary stem cell hierarchy. Curr Top Dev Biol. 2014;107:133–60.
Pechoux C, Gudjonsson T, Ronnov-Jessen L, Bissell MJ, Petersen OW. Human mammary luminal epithelial cells contain progenitors to myoepithelial cells. Dev Biol. 1999;206:88–99.
Novaro V, Roskelley CD, Bissell MJ. Collagen-IV and laminin-1 regulate estrogen receptor alpha expression and function in mouse mammary epithelial cells. J Cell Sci. 2003;116:2975–86.
Tanos T, Sflomos G, Echeverria PC, Ayyanan A, Gutierrez M, Delaloye JF, et al. Progesterone/RANKL is a major regulatory axis in the human breast. Sci Transl Med. 2013;5:182ra55.
Graham JD, Mote PA, Salagame U, van Dijk JH, Balleine RL, Huschtscha LI, et al. DNA replication licensing and progenitor numbers are increased by progesterone in normal human breast. Endocrinology. 2009;150:3318–26.
Vaillant F, Lindeman GJ, Visvader JE. Jekyll or Hyde: does Matrigel provide a more or less physiological environment in mammary repopulating assays? Breast Cancer Res. 2011;13:108.
Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, et al. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465:803–7.
Mulac-Jericevic B, Lydon JP, Demayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci U S A. 2003;100:9744–9.
Lombardi S, Honeth G, Ginestier C, Shinomiya I, Marlow R, Buchupalli B, et al. Growth hormone is secreted by normal breast epithelium upon progesterone stimulation and increases proliferation of stem/progenitor cells. Stem Cell Reports. 2014;2:780–93.
Rajaram RD, Buric D, Caikovski M, Ayyanan A, Rougemont J, Shan J, et al. Progesterone and Wnt4 control mammary stem cells via myoepithelial crosstalk. EMBO J. 2015;34:641–52.
Lindvall C, Zylstra CR, Evans N, West RA, Dykema K, Furge KA, et al. The Wnt co-receptor Lrp6 is required for normal mouse mammary gland development. PLoS One. 2009;4:e5813.
Badders NM, Goel S, Clark RJ, Klos KS, Kim S, Bafico A, et al. The Wnt receptor, Lrp5, is expressed by mouse mammary stem cells and is required to maintain the basal lineage. PLoS One. 2009;4:e6594.
Shiah YJ, Tharmapalan P, Casey AE, Joshi PA, McKee TD, Jackson HW, et al. A progesterone-CXCR4 axis controls mammary progenitor cell fate in the adult gland CXCR4 function in mammary progenitors. Stem Cell Reports. 2015;4:313–22.
Gauthier ML, Berman HK, Miller C, Kozakeiwicz K, Chew K, Moore D, et al. Abrogated response to cellular stress identifies DCIS associated with subsequent tumor events and defines basal-like breast tumors. Cancer Cell. 2007;12:479–91.
Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, et al. Control of mammary stem cell function by steroid hormone signalling. Nature. 2010;465:798–802.
Clarke RB. Ovarian steroids and the human breast: regulation of stem cells and cell proliferation. Maturitas. 2006;54:327–34.
Booth BW, Smith GH. Estrogen receptor-alpha and progesterone receptor are expressed in label-retaining mammary epithelial cells that divide asymmetrically and retain their template DNA strands. Breast Cancer Res. 2006;8:R49.
Clarke RB, Spence K, Anderson E, Howell A, Okano H, Potten CS. A putative human breast stem cell population is enriched for steroid receptor-positive cells. Dev Biol. 2005;277:443–56.
Clarke RB, Anderson E, Howell A, Potten CS. Regulation of human breast epithelial stem cells. Cell Prolif. 2003;36:45–58.
Mastroianni M, Kim S, Kim YC, Esch A, Wagner C, Alexander CM. Wnt signaling can substitute for estrogen to induce division of ERalpha-positive cells in a mouse mammary tumor model. Cancer Lett. 2010;289:23–31.
de Silva D, Kunasegaran K, Ghosh S, Pietersen AM. Transcriptome analysis of the hormone-sensing cells in mammary epithelial reveals dynamic changes in early pregnancy. BMC Dev Biol. 2015;15:7.
dos Santos CO, Rebbeck C, Rozhkova E, Valentine A, Samuels A, Kadiri LR, et al. Molecular hierarchy of mammary differentiation yields refined markers of mammary stem cells. Proc Natl Acad Sci U S A. 2013;110:7123–30.
Kaanta AS, Virtanen C, Selfors LM, Brugge JS, Neel BG. Evidence for a multipotent mammary progenitor with pregnancy-specific activity. Breast Cancer Res. 2013;15:R65.
Choudhury S, Almendro V, Merino VF, Wu Z, Maruyama R, Su Y, et al. Molecular profiling of human mammary gland links breast cancer risk to a p27(+) cell population with progenitor characteristics. Cell Stem Cell. 2013;13:117–30.
Menchon C, Edel MJ, Izpisua Belmonte JC. The cell cycle inhibitor p27Kip(1) controls self-renewal and pluripotency of human embryonic stem cells by regulating the cell cycle, Brachyury and Twist. Cell Cycle. 2011;10:1435–47.
Cheng T, Rodrigues N, Dombkowski D, Stier S, Scadden DT. Stem cell repopulation efficiency but not pool size is governed by p27(kip1). Nat Med. 2000;6:1235–40.
Booth BW, Boulanger CA, Smith GH. Alveolar progenitor cells develop in mouse mammary glands independent of pregnancy and lactation. J Cell Physiol. 2007;212:729–36.
Chang TH, Kunasegaran K, Tarulli GA, de Silva D, Voorhoeve PM, Pietersen AM. New insights into lineage restriction of mammary gland epithelium using parity-identified mammary epithelial cells. Breast Cancer Res. 2014;16:R1.
Matulka LA, Triplett AA, Wagner KU. Parity-induced mammary epithelial cells are multipotent and express cell surface markers associated with stem cells. Dev Biol. 2007;303:29–44.
Boulanger CA, Wagner KU, Smith GH. Parity-induced mouse mammary epithelial cells are pluripotent, self-renewing and sensitive to TGF-beta1 expression. Oncogene. 2005;24:552–60.
Henry MD, Triplett AA, Oh KB, Smith GH, Wagner KU. Parity-induced mammary epithelial cells facilitate tumorigenesis in MMTV-neu transgenic mice. Oncogene. 2004;23:6980–5.
Smith GH, Medina D. Re-evaluation of mammary stem cell biology based on in vivo transplantation. Breast Cancer Res. 2008;10:203.
Wagner KU, Boulanger CA, Henry MD, Sgagias M, Hennighausen L, Smith GH. An adjunct mammary epithelial cell population in parous females: its role in functional adaptation and tissue renewal. Development. 2002;129:1377–86.
Yu Q, Geng Y, Sicinski P. Specific protection against breast cancers by cyclin D1 ablation. Nature. 2001;411:1017–21.
Landis MW, Pawlyk BS, Li T, Sicinski P, Hinds PW. Cyclin D1-dependent kinase activity in murine development and mammary tumorigenesis. Cancer Cell. 2006;9:13–22.
Russo J, Balogh GA, Russo IH. Full-term pregnancy induces a specific genomic signature in the human breast. Cancer Epidemiol Biomarkers Prev. 2008;17:51–66.
Balogh GA, Heulings R, Mailo DA, Russo PA, Sheriff F, Russo IH, et al. Genomic signature induced by pregnancy in the human breast. Int J Oncol. 2006;28:399–410.
Verlinden I, Gungor N, Wouters K, Janssens J, Raus J, Michiels L. Parity-induced changes in global gene expression in the human mammary gland. Eur J Cancer Prev. 2005;14:129–37.
Asztalos S, Gann PH, Hayes MK, Nonn L, Beam CA, Dai Y, et al. Gene expression patterns in the human breast after pregnancy. Cancer Prev Res (Phila). 2010;3:301–11.
Meier-Abt F, Milani E, Roloff T, Brinkhaus H, Duss S, Meyer DS, et al. Parity induces differentiation and reduces Wnt/Notch signaling ratio and proliferation potential of basal stem/progenitor cells isolated from mouse mammary epithelium. Breast Cancer Res. 2013;15:R36.
Liu J, Sato C, Cerletti M, Wagers A. Notch signaling in the regulation of stem cell self-renewal and differentiation. Curr Top Dev Biol. 2010;92:367–409.
Koch U, Lehal R, Radtke F. Stem cells living with a Notch. Development. 2013;140:689–704.
Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–70.
Ling H, Sylvestre JR, Jolicoeur P. Notch1-induced mammary tumor development is cyclin D1-dependent and correlates with expansion of pre-malignant multipotent duct-limited progenitors. Oncogene. 2010;29:4543–54.
Sale S, Lafkas D, Artavanis-Tsakonas S. Notch2 genetic fate mapping reveals two previously unrecognized mammary epithelial lineages. Nat Cell Biol. 2013;15:451–60.
Ling H, Sylvestre JR, Jolicoeur P. Cyclin D1-dependent induction of luminal inflammatory breast tumors by activated notch3. Cancer Res. 2013;73:5963–73.
Bruno RD, Boulanger CA, Smith GH. Notch-induced mammary tumorigenesis does not involve the lobule-limited epithelial progenitor. Oncogene. 2012;31:60–7.
Chakrabarti R, Wei Y, Romano RA, DeCoste C, Kang Y, Sinha S. Elf5 regulates mammary gland stem/progenitor cell fate by influencing notch signaling. Stem Cells. 2012;30:1496–508.
Milanese TR, Hartmann LC, Sellers TA, Frost MH, Vierkant RA, Maloney SD, et al. Age-related lobular involution and risk of breast cancer. J Natl Cancer Inst. 2006;98:1600–7.
McCormack VA, Perry NM, Vinnicombe SJ, dos Santos SI. Changes and tracking of mammographic density in relation to Pike’s model of breast tissue aging: a UK longitudinal study. Int J Cancer. 2010;127:452–61.
Well D, Yang H, Houseni M, Iruvuri S, Alzeair S, Sansovini M, et al. Age-related structural and metabolic changes in the pelvic reproductive end organs. Semin Nucl Med. 2007;37:173–84.
Cleland WH, Mendelson CR, Simpson ER. Effects of aging and obesity on aromatase activity of human adipose cells. J Clin Endocrinol Metab. 1985;60:174–7.
Shoker BS, Jarvis C, Sibson DR, Walker C, Sloane JP. Oestrogen receptor expression in the normal and pre-cancerous breast. J Pathol. 1999;188:237–44.
Shyamala G, Chou YC, Louie SG, Guzman RC, Smith GH, Nandi S. Cellular expression of estrogen and progesterone receptors in mammary glands: regulation by hormones, development and aging. J Steroid Biochem Mol Biol. 2002;80:137–48.
Arendt LM, Evans LC, Rugowski DE, Garcia-Barchino MJ, Rui H, Schuler LA. Ovarian hormones are not required for PRL-induced mammary tumorigenesis, but estrogen enhances neoplastic processes. J Endocrinol. 2009;203:99–110.
Arendt LM, Grafwallner-Huseth TL, Schuler LA. Prolactin-growth factor crosstalk reduces mammary estrogen responsiveness despite elevated ERalpha expression. Am J Pathol. 2009;174:1065–74.
Christov K, Chew KL, Ljung BM, Waldman FM, Duarte LA, Goodson III WH, et al. Proliferation of normal breast epithelial cells as shown by in vivo labeling with bromodeoxyuridine. Am J Pathol. 1991;138:1371–7.
Garbe JC, Pepin F, Pelissier FA, Sputova K, Fridriksdottir AJ, Guo DE, et al. Accumulation of multipotent progenitors with a basal differentiation bias during aging of human mammary epithelia. Cancer Res. 2012;72:3687–701.
Walker RA, Martin CV. The aged breast. J Pathol. 2007;11:232–40.
Yasui Y, Potter JD. The shape of age-incidence curves of female breast cancer by hormone-receptor status. Cancer Causes Control. 1999;10:431–7.
Tarone RE, Chu KC. The greater impact of menopause on ER- than ER+ breast cancer incidence: a possible explanation (United States). Cancer Causes Control. 2002;13:7–14.
Acknowledgments
The authors would like to thank Drs. Jerrica Breindel and Ania Wronski and John Hinds for a critical review of the manuscript. NIH/NICHD HD073035, NIH/NICHD CA170851 and The Breast Cancer Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arendt, L.M., Kuperwasser, C. Form and Function: how Estrogen and Progesterone Regulate the Mammary Epithelial Hierarchy. J Mammary Gland Biol Neoplasia 20, 9–25 (2015). https://doi.org/10.1007/s10911-015-9337-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10911-015-9337-0