Abstract
Obesity is associated with increased risk of breast cancer in postmenopausal women and is linked with poor prognosis in pre- and postmenopausal breast cancer patients. The mechanisms underlying the obesity-breast cancer connection are becoming increasingly clear and provide multiple opportunities for primary to tertiary prevention. Several obesity-related host factors can influence breast tumor initiation, progression and/or response to therapy, and these have been implicated as key contributors to the complex effects of obesity on cancer incidence and outcomes. These host factors include components of the secretome, including insulin, insulin-like growth factor-1, leptin, adiponectin, steroid hormones, cytokines, vascular regulators, and inflammation-related molecules, as well as the cellular and structural components of the tumor microenvironment. These secreted and structural host factors are extrinsic to, and interact with, the intrinsic molecular characteristics of breast cancer cells (including breast cancer stem cells), and each will be considered in the context of energy balance and as potential targets for cancer prevention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of obesity, defined by a body mass index (BMI) >30 kg/m2, has more than doubled in the United States over the past 3 decades [1]. Currently, an estimated 36 % and 33 % of American adults are either obese or overweight (defined by BMI 25–29.9), respectively [2]. Between 1960 and 2010, the average height of a US woman between the ages of 20–74 years increased by less than 1 in., while the average weight increased more than 26 lb, and the pace of weight gain in US women has increased to more than 1 lb per year over the past 10 years [1, 3]. Worldwide an estimated 500 million adults are currently obese, and 1.1 billion are overweight [4].
Obesity is an established epidemiologic risk factor for breast cancer in postmenopausal women and also negatively impacts breast cancer prognosis in both pre-and postmenopausal women [5]. Recent epidemiologic studies assessing the obesity-breast cancer relationship in the context of intrinsic breast cancer subtypes suggest the strongest associations between obesity and breast cancer risk are observed with the hormone receptor-positive breast tumors, with inconsistent results for triple-negative, HER2, luminal B and core basal phenotype (CBP) breast cancers [6]. To our knowledge, the association between obesity and the aggressive, breast cancer stem cell (BCSC)-enriched claudin-low subtype of breast cancer [7] has not yet been studied in humans.
Mirroring the human data, animal model studies show the strongest obesity-cancer links with hormone receptor-positive mammary tumors, with additional evidence that basal-like and claudin-low mammary tumor development and/or progression are also enhanced by obesity [8–11]. There is little preclinical support for links between obesity and luminal B or HER-2 breast cancer subtypes [12, 13]. Animal studies also show that calorie restriction (CR), typically accomplished by a 20–40 % reduction in total energy intake relative to a control group fed ad libitum, prevents or reverses obesity and consistently inhibits mammary tumorigenesis, regardless of intrinsic subtype of breast cancer [9, 13, 14].
Given the rising global prevalence of obesity, insights into the molecular targets for mimicking the anticancer effects of CR and breaking the obesity-breast cancer link are urgently needed. To date, potential mechanisms underlying obesity-breast cancer associations have focused on the metabolic perturbations associated with increased adiposity. Obesity often results in a state of metabolic dysregulation referred to as metabolic syndrome and characterized by insulin resistance, hyperinsulinemia and hyperglycemia; dyslipidemias, including hypertriglyceridemia; hypertension; altered systemic coagulation and angiogenic factors such as plasminogen activator inhibitor-1 and vascular endothelial growth factor; altered adipokines, such as increased circulating leptin and hepatocyte growth factor (HGF) and decreased adiponectin; heightened inflammation and elevated local and systemic cytokine levels; and increased serum estradiol and bioavailable insulin-like growth factor (IGF)-1 levels [15]. These extrinsic host factors, most of which are secreted hormones, growth factors, cytokines or other biomolecules, regulate key energy balance-related physiological processes. Thus, it is highly plausible that these energy-responsive components of the secretome (which is defined as the complex set of molecules secreted or shed from living cells; [16]) are important contributors to the obesity-breast cancer link. This review will discuss how energy balance-related hormones and other extrinsic host factors interact with: a) the local microenvironment of the tumor; and b) molecular characteristics of the tumor cells, as well as the breast cancer stem/progenitor cell populations thought to be largely responsible for tumor initiation and maintenance. These interactions will be described in the context of seed and soil, borrowing on the concepts first proposed by Paget in the late 1800s that to grow and spread, cancer cells (the seeds, with intrinsic genetic susceptibilities) require adequate extrinsic factors, such as secreted hormones, nutrients, extracellular matrix, and support cells, in their local microenvironment (the soil) [17].
Extrinsic Factors: The Secretome and the Soil
Proteins, lipid intermediates, and other factors secreted or shed from cells into the extracellular environment are referred to as the secretome and represent an important subset of molecules involved in intracellular communication [16]. Endocrine signaling between distant organ systems and tumor cells, as well as paracrine signaling between tumor and host cells in the local microenvironment, is mediated by an increasingly large roster of obesity-responsive hormones, growth factors, nutrient metabolites, chemokines and cytokines that promote tumor development and/or progression. To date, these factors (summarized below) have been studied primarily by low-throughput methods such as singleplexed or multiplexed immunoassays of serum, plasma or other biologic fluids, but high-throughput proteomic and metabolomic approaches to characterize the secretomes of organisms or cancer cells are being developed to more broadly identify the key factors (and their interactions) underlying disease states such as breast cancer [18].
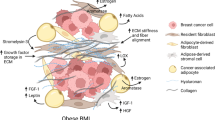
In addition to the secretome, the breast tumor microenvironment is comprised of extracellular matrix components and multiple cell types including adipocytes, epithelial cells, fibroblasts, endothelial cells, macrophages, T-cells, mast cells, and other immune cells [19]. A considerable portion of the breast tumor microenvironment is comprised of adipocytes, which in the obese state are highly active and capable of secreting a vast number of hormones and adipokines [20, 21]. The vasculature also plays a role in the delivery of circulating growth factors, nutrients and cytokines to the tissue [15]. Thus, there is growing interest in the identification and characterization of the key extrinsic factors in the breast microenvironment that represent targets for prevention or treatment strategies for breaking the obesity-breast cancer link. The leading candidates, based on current evidence, are discussed below, and no doubt new candidates willl emerge from high-throughput secretome approaches currently being developed [18].
Growth Factors and their Signals
Insulin is a peptide hormone released by pancreatic beta cells in response to elevated circulating glucose that plays a major role in the regulation of carbohydrate and lipid metabolism. Insulin shares approximately 50 % sequence homology with insulin-like growth factor1 (IGF-1), a peptide growth factor produced primarily by the liver in response to growth hormone. Levels of insulin and circulating free IGF-1 are significantly increased in obese subjects compared with lean subjects [22].
Elevated circulating IGF-1 is an established risk and progression factor for many cancer types including cancers of the breast [23, 24]. Downstream pathways of the insulin receptor (IR) and IGF-1 receptor (IGF-1R) are commonly altered in breast and other epithelial cancers [25]. One of these downstream pathways is the phosphatidylinositol-3 kinase (PI3K)/Akt pathway which integrates intracellular and environmental cues, such as circulating growth factor concentrations and nutrient availability, to regulate cellular survival and proliferation. Akt regulates the mammalian target of rapamycin (mTOR) [26], which is commonly activated in breast tumors and many normal tissues from obese and/or diabetic mice [27], and specific mTOR inhibitors block the tumor-enhancing effects of obesity in mouse models [28, 29].
The adipokine leptin is released by adipocytes in response to insulin, glucocorticoids and tumor necrosis factor-alpha (TNF-α) [30]. Leptin targets the hypothalamus to induce satiety signals and also indirectly modulates immune function, cytokine production, angiogenesis, carcinogenesis and other biological processes [30]. Leptin resistance develops in response to obesity whereby adipose tissue overproduces leptin and the brain no longer responds to the signal. Case-control and prospective studies investigating the relationship between leptin levels and breast cancer risk have found mixed results [31–36], but mechanistic data suggests that alterations in leptin promote tumor growth and development. Specifically, leptin activates intracellular signaling through the janus kinase and signal transducer and activator of transcription (JAK/STAT), extracellular-signal-regulated kinase (ERK1/2), and/or phosphatidylinositide 3-kinases (PI3K) pathways which are often dysregulated in breast cancer [37].
Adiponectin is another adipokine, but in contrast with leptin, it negatively correlates with body adiposity levels. Adiponectin counters the metabolic program associated with obesity and hyperleptinemia by modulating glucose metabolism, increasing fatty acid oxidation and insulin sensitivity and decreasing production of inflammatory cytokines [38]. Case-control [31, 39–41] and prospective studies [42] report a protective effect of plasma adiponectin on the development and growth of breast cancer. Adiponectin likely exerts its anticancer effects by increasing insulin sensitivity and decreasing insulin/IGF-1 and mTOR signaling through activation of 5′ adenosine monophosphate-activated protein kinase (AMPK). Adiponectin also reduces pro-inflammatory cytokine expression by inhibiting nuclear factor kappa-light-chain-enhancer of activated B-cells (NF-kB) [43, 44] which is commonly activated in many tumors and is associated with insulin resistance and elevated circulating levels of leptin, insulin, and/or IGF-1 [45–47]. Associations between the adiponectin-to-leptin ratio and the metabolic syndrome and some cancers have been reported, although further characterization of these links is needed [48–50].
Estrogen
In premenopausal women the ovaries convert large amounts of androgens to estrogens in an enzymatic reaction catalyzed by aromatase. After the loss of ovarian function following menopause, estrogens are produced primarily by aromatization of androgens in adipose tissue, and thus circulating estrogen levels tend to be higher in obese, relative to lean, women due to their excess adipose tissue and associated increased capacity to peripherally aromatize androgens [51]. Several lines of evidence suggest estrogen plays a contributing role in the increased risk of hormone receptor-positive breast cancer in obese postmenopausal women. Estradiol binds to estrogen receptor (ER)-α and enhances cell proliferation and inhibits apoptosis [52]. Estrogens can also enhance angiogenesis via induction of VEGF [53]. In addition to enhancing tumor formation through estrogen receptor α-dependent effects, estrogen can be metabolized into mutagenic DNA reactive metabolites [54].
Chronic Inflammation
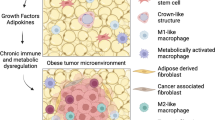
Inflammation is now a recognized hallmark of cancer, and growing evidence continues to indicate that chronic inflammation is associated with increased breast cancer risk [55]. Obesity is considered a low-grade, chronic inflammatory state distinguished by elevated plasma free fatty acids and chemoattraction of immune cells (such as macrophages that also produce inflammatory factors) into the local microenvironment [56–58]. The inflammatory microenvironment exerts tumor-promoting effects specifically via genetic instability and cellular proliferation, survival and angiogenesis [59–61].
Tumor-associated macrophages (TAMs) contribute to the pro-inflammatory tumor microenvironment. Macrophages, which are activated in the obese state, infiltrate tumors and magnify the inflammatory tumor mileu, often through NF-kB-dependent production of cytokines, growth factors and promotors of angiogenesis [47, 62]. Adipocytes also have been shown to transdifferentiate into macrophages, further enhancing the pro-inflammatory cycle that develops in the tumorigenic environment in response to obesity [63]. Additionally, adipocytes can enlarge to the point of rupture thus spilling free fatty acids into the local environment. These necrotic adipocytes are often surrounded by macrophages forming crown-like structures (CLS) in adipose tissue and mammary glands [57]. Quantification of TAM infiltration and CLS formation represent emerging prognostic tools for breast cancer [64].
Increased body adiposity is associated with elevated tumoral cytokine expression [57, 65]. The obesity-associated breast cancer proinflammatory milieu is comprised of cytokines that include the interleukin (IL) family, HGF, chemokine ligands (CCL, CXCL), tumor necrosis factor (TNF)-α and monocyte chemoattractant protein (MCP)-1, free fatty acids and lipid intermediates such as prostaglandins and leukotrienes. Chemokines, which are secreted from adipocytes and TAMs at higher levels in the obese state, stimulate directed chemotaxis in nearby responsive cells causing increased inflammation, tumor progression, and invasion in multiple tumor types [66, 67]. The recruitment of TAMs to the tumor microenvironment is largely dependent on MCP-1, tumoral levels of which are highly correlated with the accumulation of TAMs in breast cancer [68].
Vascular Perturbations
Adipocytes communicate with endothelial cells by producing vascular endothelial growth factor (VEGF), IGF-1, plasminogen activator inhibitor-1 (PAI-1), leptin, HGF, and fibroblast growth factor-2 to facilitate angiogenesis and vascular permeability [69]. In the obese state, these factors stimulate neovascularization in support of the expanding fat mass but these adipose-derived factors may also contribute to obesity-associated enhancement of tumor angiogenesis.
VEGF is a heparin-binding glycoprotein produced by adipocytes and tumor cells that has angiogenic and vascular permeability activity [70]. Circulating levels of VEGF are elevated with increased adiposity and increased tumoral expression of VEGF is associated with poor prognosis for breast cancer [71, 72]. An oxygen deficient microenvironment stimulates, through hypoxia-inducible factor (HIF)-1α and HIF-1β, tumor cell production of VEGF, which then leads to the formation of new blood vessels to nourish the rapidly growing tumor and may also enable the metastatic spread of breast cancer cells [70].
Obese subjects frequently have elevated circulating PAI-1 levels, compared with their lean counterparts [19]. PAI-1 is a serine protease inhibitor produced by endothelial cells, stromal cells and adipocytes that inhibits tissue plasminogen activator (tPA) and urokinase (uPA), thus regulating fibrinolysis and integrity of the extracellular matrix [73]. PAI-1 is also involved in angiogenesis and could potentially promote obesity-driven tumor cell growth, invasion and metastasis [74]. Although weight loss and TNF-α blockers effectively reduce PAI-1 levels in obese individuals [75, 76], the role of PAI-1 in tumorigenesis is not clear at this time and further research will determine if this is an effective target for breast cancer therapies [73].
Intrinsic Factors: The Quality and Quantity of the Seeds
It has been noted that many cancers can acquire similar functional capabilities during tumorigenesis via disparate mechanisms. These capabilities are outlined in the hallmarks of cancer described by Hanahan and Weinberg and are comprised of alterations to many cellular-intrinsic properties including: dysregulated growth signaling, evasion of apoptosis or growth suppression, limitless replicative potential and genomic instability [77, 78]. These properties are controlled by numerous molecular pathways, many of which can be altered by obesity. The remaining sections of this review will summarize the current knowledge of the interactions of obesity with cellular-intrinsic characteristics that are linked to breast cancer development and progression, as well as the emerging role of breast cancer stem cells. The limited nature of this body of knowledge highlights the necessity for further research, especially as the intrinsic properties identified may represent key targets to break the obesity-breast cancer link.
p53 Tumor Suppressor
In response to cellular injury or mutation, p53 is induced to inhibit cell cycle progression and initiate repair of the cell or induce death [79]. The p53 tumor suppressor gene is altered in approximately 30 % of all breast tumors and may be influenced by obesity or may influence adipose tissue deposition. Recent studies suggest that p53 plays a role in adipose tissue differentiation. Specifically, p53 was shown to inhibit white adipose differentiation but to induce differentiation of brown adipose tissue, the more metabolically active adipose tissue depot, which in turn may be able to counter diet induced obesity [80]. Furthermore, we recently reported that obesity decreased p53 protein expression in a murine mammary tumor model and promoted a pathology and proliferative environment in p53+/+ tumors more reflective of p53+/− tumors [20]. Consistent with this observation, Park et al. also demonstrated that a high-fat, high-calorie diet significantly reduced p53 levels in a murine colon cancer model [81]. Together, these data suggest that functional p53 may prevent adipose tissue deposition and the procancer effects of obesity may, in part, be attributed to loss of p53.
Estrogen Receptor
The growth and progression of breast cancer is also impacted by crosstalk between p53 and the estrogen receptor (ER) [82]. ER is an established breast cancer-related regulator of p53 [83–85], and in turn, p53 regulates ER [86–88]. ERs mediate the physiological effects of serum and tissue estrogens. The hormone receptor complex is activated upon binding of estrogens to the nuclear receptors, ER-α and ER-β, thereby regulating downstream gene transcription. ER-α and ER-β appear to have distinct functions and are even encoded from different chromosomes. ER-α enhances the transcription of genes involved in growth and proliferation while ER-β has been implicated in tumor suppressor activity. The levels of circulating estrogens are impacted by adiposity levels. Serum estrogen levels are highest in overweight and obese postmenopausal women [89] and obesity has been shown to increase mammary aromatase expression thereby increasing localized production of estrogens in the breast tissue [57]. In addition, ER-α-negative breast tumors represent one third of total breast cancers, and confer a worse prognosis than ER-α-positive breast tumors [90] and eliminate possible estrogen ablation therapies. Furthermore, in most ER-α-negative breast tumors, expression of ER-β, the putative tumor suppressor, is also lost [91]. We recently found that obesity accelerated Wnt-1 murine mammary tumor growth and also reduced ER-α protein expression [20].
It is plausible that obesity may reduce ER expression through epigenetic modifications. DNA methylation of the ER promoter has been shown to reduce ER expression and silence its function by prohibiting transcription of downstream gene targets. Although it has not been demonstrated, it is reasonable to believe that obesity, due to elevated levels of circulating growth factors and excess nutrients would enhance DNA methylation of the ER promoter. Emerging data suggests microRNAs may also play an important regulatory role, as discussed below.
MicroRNA Regulation
In normal cells, microRNAs (miRs) are known to regulate the proper functioning of many major cellular processes including growth, differentiation, proliferation, and apoptosis. As dysregulation of these processes has been established as hallmarks of cancer development and progression, it is not surprising that altered miR expression has been linked with many human cancers, including breast cancer [92]. While the oncogenic and tumor-suppressive functions of certain miRs and their expression patterns in breast cancer are becoming more established, the impact of obesity on these miRs is just beginning to be uncovered.
Recently, several studies have demonstrated that increased exposure of the non-transformed human mammary epithelial cell line, MCF-10a, to the pro-inflammatory cytokines MCP-1 and IL-6 affects intrinsic miR expression. As discussed previously, increased macrophage infiltration and cytokine production are well-established mechanisms of obesity’s promotion of breast cancer development and progression. Increased MCP-1 release into the breast microenvironment results in activation of NF-κB signaling, which increases IL-6 production within breast epithelial cells. IL-6 is then able to suppress miR-200c, which ultimately results in further IL-6 expression [93]. This inflammatory feed-forward loop is sufficient to drive MCF-10a transformation and promote tumorigenesis in an MMTV-Neu transgenic mouse model. Not only is the downregulation of miR-200c by IL-6 relevant in the inflammation-mediated initiation of cancer, but this event may also relate to breast cancer metastasis. Specifically, miR-200c, along with other miR-200 family members, target and therefore downregulate key inducers of epithelial-to-mesenchymal transition (EMT), a process which is highly associated with metastasis through increased tumor cell mobility and invasion [92]. Although it has not been directly tested, these insights suggest that obesity’s induction of IL-6 expression may promote breast cancer metastasis through the inhibition of miR-200c expression.
A related study reported that IL-6 overexpression also promotes expression of miR-21 and miR-181b through activation of STAT3, a transcription factor downstream of leptin signaling which binds the promoter regions of these miRs [94]. Not only do these miRs, especially miR-21, possess established oncogenic functions, their overexpression is sufficient to transform MCF-10a cells.
Beyond the involvement of miRs in obesity-associated cancer initiation, another study sheds light on how miR regulation may contribute to obesity-associated tumor progression. Specifically, exposure of the human breast cancer cell line MCF-7 to high levels of IGF-1 (50 ng/mL), which is more bioavailable in the obese state, causes downregulation of several tumor suppressor miRs, including miR-15b, −195, −98, let-7c, and let-7 g [21]. These results suggest that obesity could promote the loss of these miRs in mammary cells through increased IGF-1 signaling. The miR-16 family, which includes miR-15b and miR-195, negatively regulate cellular growth and cell cycle progression, while it has been shown that the let-7 family inhibits the proliferative and metastatic potential of breast tumor initiating cells [34, 92]. One can see how inhibition of these functions could have large effects on the ability of breast cancer cells to proliferate, evade treatment and metastasize.
Cell-Autonomous Alterations of the PI3K Pathway
Kalaany and Sabatini reported that cancer cells with constitutively activated PIK3CA mutations are proliferative in vitro in the absence of insulin or IGF-1 and form CR resistant tumors in vivo [95]. This further illustrates the issue of nature versus nurture in the obesity-breast cancer association, i.e. the contributions of energy balance-responsive host factors in the context of cancer cell-autonomous effects. Similarly, we reported that constitutively activating mTOR in Wnt-1 mammary tumor cells ablates the anticancer effects of CR [29]. Taken together, these findings suggest that cell-autonomous alterations, such as activating PI3K or mTOR, may influence the response of cells to energy balance modulation, although the effects of obesity in the context of such alterations have not yet been characterized. This topic is important given the emerging importance of the mTOR pathway and PIK3CA mutations in breast cancer [96]. Also, although familial breast cancers due to genetic mutations in BRCA1, BCRA2, p53, PTEN, LKB1 or CDH1 [97], are relatively rare, these and other genetic alterations also play a role in many non-familial breast cancers [13, 98], and thus a better understanding of the interactions between obesity and common cancer-associated genetic alterations in breast cells is urgently needed.
Breast Cancer Stem Cells (BCSCs)
The cancer stem cell hypothesis postulates that tumors originate through dysregulation of the normal self-renewal process resulting in altered replication and differentiation characteristic of many breast tumors [99]. In addition to being responsible for initiating primary breast tumorigenesis, BCSCs may underlie resistance to chemotherapeutic agents and thus contribute to tumor recurrence and metastasis [100]. Strategies aimed at limiting enrichment and/or proliferation of BCSCs may therefore be useful for primary and secondary breast cancer prevention, as well as treatment.
Although this area has not been well studied to date, we and others have observed possible links between obesity and BCSCs. Orthotopically transplanted basal-like mammary tumor cells derived from murine mammary tumor virus-Wnt-1 transgenic mice are dependent on leptin for growth [101]. Specifically, transplanted Wnt-1 tumor growth in genetically obese, leptin receptor deficient (db/db) mice, which have very high systemic leptin concentrations, was ~8-fold higher than tumor growth in wild-type mice. In contrast, tumor growth in genetically obese but leptin-deficient (ob/ob mice) was almost undetectable. The residual tumors in ob/ob mice were found to have fewer cells with breast cancer stem cell markers. When sorted by LepRb expression and further evaluated, these cells exhibited several stem cell properties, including tumorsphere formation and wound healing in vitro, and their survival was regulated by leptin.
Dunlap, et al. showed that a mesenchymal Wnt-1 mammary tumor cell line (named M-Wnt and isolated from a spontaneous MMTV-Wnt-1 tumor) profile with human claudin-low breast tumors, highly express EMT markers, are stably enriched in breast cancer stem cell markers, and exhibit stem cell properties [9]. In addition, M-Wnt cells orthotopically injected into C57BL/B6 mice rapidly form claudin-low tumors that are highly responsive to the tumor enhancing effects of obesity, as well as the anticancer effects of CR. In contrast, E-Wnt cells (also isolated from a MMTV-Wnt-1 tumor, have an epithelial morphology, minimal expression of EMT and BCSC markers, and (when orthotopically transplanted into C57BL/7 mice) form basal-like tumors that are less responsive to obesity or CR relative to M-Wnt tumors. These findings indicate a mechanistic link between energy balance, the epithelial-to-mesenchymal transition (EMT) and BCSCs in breast cancer progression. Using our analogy of seed and soil, one can speculate that obesity prepares fertile soil (the tumor microenvironment), including intratumoral adipocytes, and local and systemic hormones, growth factors, and cytokines, for enhanced tumor progression, and that determinants of growth in this fertile soil include the plant variety (intrinsic breast cancer subtype and the genetic alterations and differentiation state of the BCSCs initiating the tumor) and/or the seed density (the extent of BCSC enrichment). In contrast, CR may discourage tumor development and progression by acting on the soil antithetically to obesity, including reduced intratumoral adipocyte infiltration, and decreased systemic hormones, growth factors and cytokines. CR also decreases BCSC enrichment and induces a mesenchymal-to-epithelial transition [9]. Future studies are warranted to determine whether the tumor-enhancing effects of obesity (and the anticancer effects of CR) are dependent on mechanisms related to EMT and the BCSC compartment in different subtypes of breast cancer.
Conclusions
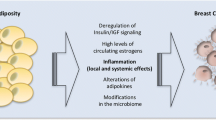
As summarized in Fig. 1, this review considers lessons learned from obesity and breast cancer research to discuss promising extrinsic and cellular intrinsic targets for breast cancer prevention, particularly for breaking the obesity-breast cancer link. Potential cell-extrinsic targets include components of the secretome, including systemic and locally produced hormones, growth factors, adipokines, inflammatory mediators, and vascular regulators, as well as structural and cellular components of the host microenvironment. Currently, more is known about the effects of obesity on the extrinsic pathways compared with cellular intrinsic properties of breast cancer. Obesity-responsive host characteristics likely interact with several breast cancer cell-intrinsic factors, including alterations in the p53 tumor suppressor, estrogen receptor, tumor suppressive and oncogenic miRs, components of the PI3K/Akt/mTOR pathway, and intrinsic characteristics related to stemness. Thus, given the complexity of the obesity-breast cancer relationship, and the expanding global epidemic of obesity, new precision medicine-based approaches that target extrinsic and cell-intrinsic interactions discussed in this review are urgently needed to break the obesity-breast cancer link.
Extrinsic and Cell-Intrinsic Factors Involved in the Obesity-Breast Cancer Link. Obesity dysregulates multiple aspects of the host microenvironment, including increasing circulating levels of hormones and growth factors, such as insulin, leptin:adiponectin, hepatocyte growth factor (HGF), estrogen and insulin-like growth factor (IGF)-1); vascular regulators such as plasminogen activated inhibitor (PAI)-1 and vascular endothelial growth factor (VEGF); inflammatory factors such as cytokines, chemokines, prostaglandins and free fatty acids. Obesity also enriches and/or activates several cell types in the microenvironment, including adipocytes, macrophages, T-cells, and fibroblasts. These extrinsic host factors interact with cell-intrinsic characteristics of mammary epithelial cells, including estrogen receptor (ER); p53; numerous oncogenic or suppressive microRNAs (miRNA); components of the phosphatidyl inositol-3 kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway; and characteristics associated with breast cancer stem cells, including the epithelial-to-mesenchymal transition (EMT)
Abbreviations
- mTOR:
-
mammalian target of rapamycin
- IGF-1:
-
Insulin-like growth factor-1
- ER:
-
estrogen receptor
- miRs:
-
microRNAs
References
Finucane MM et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377(9765):557–67.
Flegal KM et al. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–7.
Prevention, C.f.D.C.a. 2012; Available from: http://www.cdc.gov/nchs/fastats/bodymeas.htm.
Available from: http://www.iaso.org/resources/obesity-data-portal/resources/charts/.
Reeves GK et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335(7630):1134.
Yang XR et al. Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium studies. J Natl Cancer Inst. 2011;103(3):250–63.
Prat A et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res: BCR. 2010;12(5):R68.
Dogan S et al. Effects of high-fat diet and/or body weight on mammary tumor leptin and apoptosis signaling pathways in MMTV-TGF-alpha mice. Breast Cancer Res. 2007;9(6):R91.
Dunlap SM et al. Dietary energy balance modulates epithelial-to-mesenchymal transition and tumor progression in murine claudin-low and basal-like mammary tumor models. Cancer Prev Res. 2012;5(7):930–42.
Giles ED et al. Obesity and overfeeding affecting both tumor and systemic metabolism activates the progesterone receptor to contribute to postmenopausal breast cancer. Cancer Res. 2012;72(24):6490–501.
Hakkak R et al. Obesity increases the incidence of 7,12-dimethylbenz(a)anthracene-induced mammary tumors in an ovariectomized Zucker rat model. Int J Oncol. 2007;30(3):557–63.
Cleary MP et al. Diet-induced obesity and mammary tumor development in MMTV-neu female mice. Nutr Cancer. 2004;50(2):174–80.
Ford NA et al. IGF1 dependence of dietary energy balance effects on murine Met1 mammary tumor progression, epithelial-to-mesenchymal transition, and chemokine expression. Endocr-Relat Cancer. 2013;20(1):39–51.
Mizuno NK et al. Combination of intermittent calorie restriction and eicosapentaenoic acid for inhibition of mammary tumors. Cancer Prev Res. 2013;6(6):540–7.
Hursting SD, Hursting MJ. Growth signals, inflammation, and vascular perturbations: mechanistic links between obesity, metabolic syndrome, and cancer. Arterioscler Thromb Vasc Biol. 2012;32(8):1766–70.
Makridakis M, Vlahou A. Secretome proteomics for discovery of cancer biomarkers. J Proteome. 2010;73(12):2291–305.
Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;1:571–3.
Brown KJ et al., The human secretome atlas initiative: Implications in health and disease conditions. Biochimica et Biophysica Acta, 2013.
Place AE, Jin Huh S, Polyak K. The microenvironment in breast cancer progression: biology and implications for treatment. Breast Cancer Res. 2011;13(6):227.
Deng Y, Scherer PE. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann N Y Acad Sci. 2010;1212:E1–19.
Karastergiou K, Mohamed-Ali V. The autocrine and paracrine roles of adipokines. Mol Cell Endocrinol. 2010;318(1–2):69–78.
Nam SY et al. Effect of obesity on total and free insulin-like growth factor (IGF)-1, and their relationship to IGF-binding protein (BP)-1, IGFBP-2, IGFBP-3, insulin, and growth hormone. Int J Obes Relat Metab Disord. 1997;21(5):355–9.
Hursting SD et al., Calories and cancer: the role of insulin-like growth factor-1, in The IGF system and cancer, D. Leroith, Editor 2011, Springer, NY. p. 231–243.
Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8(12):915–28.
Wong KK, Engelman JA, Cantley LC. Targeting the PI3K signaling pathway in cancer. Curr Opin Genet Dev. 2010;20(1):87–90.
Memmott RM, Dennis PA. Akt-dependent and -independent mechanisms of mTOR regulation in cancer. Cell Signal. 2009;21(5):656–64.
Moore T et al. Dietary energy balance modulates signaling through the Akt/mammalian target of rapamycin pathways in multiple epithelial tissues. Cancer Prev Res (Phila). 2008;1(1):65–76.
De Angel RE et al. The enhancing effects of obesity on mammary tumor growth and Akt/mTOR pathway activation persist after weight loss and are reversed by RAD001. Mol Carcinog. 2013;52(6):446–58.
Nogueira LM et al. Calorie restriction and rapamycin inhibit MMTV-Wnt-1 mammary tumor growth in a mouse model of postmenopausal obesity. Endocr Relat Cancer. 2012;19(1):57–68.
Gautron L, Elmquist JK. Sixteen years and counting: an update on leptin in energy balance. J Clin Invest. 2011;121(6):2087–93.
Gross A et al., Adipocytokines, inflammation, and breast cancer risk in postmenopausal women: a prospective study. Cancer Epidemiol Biomarkers Prev, 2013.
Wu MH et al. Circulating levels of leptin, adiposity and breast cancer risk. Br J Cancer. 2009;100(4):578–82.
Mantzoros CS et al. Leptin in relation to carcinoma in situ of the breast: a study of pre-menopausal cases and controls. Int J Cancer. 1999;80(4):523–6.
Petridou E et al. Leptin and insulin growth factor I in relation to breast cancer (Greece). Cancer Causes Control. 2000;11(5):383–8.
Stattin P et al. Plasma leptin and breast cancer risk: a prospective study in northern Sweden. Breast Cancer Res Treat. 2004;86(3):191–6.
Harris HR et al. Plasma leptin levels and risk of breast cancer in premenopausal women. Cancer Prev Res (Phila). 2011;4(9):1449–56.
Cirillo D et al. Leptin signaling in breast cancer: an overview. J Cell Biochem. 2008;105(4):956–64.
Vaiopoulos AG et al. The role of adiponectin in human vascular physiology. Int J Cardiol. 2012;155(2):188–93.
Miyoshi Y et al. Association of serum adiponectin levels with breast cancer risk. Clin Cancer Res. 2003;9(15):5699–704.
Mantzoros C et al. Adiponectin and breast cancer risk. J Clin Endocrinol Metab. 2004;89(3):1102–7.
Tian YF et al. Anthropometric measures, plasma adiponectin, and breast cancer risk. Endocr Relat Cancer. 2007;14(3):669–77.
Tworoger SS et al. Plasma adiponectin concentrations and risk of incident breast cancer. J Clin Endocrinol Metab. 2007;92(4):1510–6.
Barb D et al. Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence. Am J Clin Nutr. 2007;86(3):s858–66.
Stofkova A. Leptin and adiponectin: from energy and metabolic dysbalance to inflammation and autoimmunity. Endocr Regul. 2009;43(4):157–68.
Renehan AG, Roberts DL, Dive C. Obesity and cancer: pathophysiological and biological mechanisms. Arch Physiol Biochem. 2008;114(1):71–83.
Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441(7092):431–6.
Del Prete A et al. Molecular pathways in cancer-related inflammation. Biochem Med (Zagreb). 2011;21(3):264–75.
Chen DC et al. Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer Lett. 2006;237(1):109–14.
Ashizawa N et al. Serum leptin-adiponectin ratio and endometrial cancer risk in postmenopausal female subjects. Gynecol Oncol. 2010;119(1):65–9.
Cleary MP et al. Targeting the adiponectin: leptin ratio for postmenopausal breast cancer prevention. Front Biosci (Schol Ed). 2009;1:329–57.
Key TJ et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95(16):1218–26.
Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354(3):270–82.
Pequeux C et al. Stromal estrogen receptor-alpha promotes tumor growth by normalizing an increased angiogenesis. Cancer Res. 2012;72(12):3010–9.
Brodie AM, Marsh D, Brodie HJ. Aromatase inhibitors–IV. Regression of hormone-dependent, mammary tumors in the rat with 4-acetoxy-4-androstene-3,17-dione. J Steroid Biochem. 1979;10(4):423–9.
Basu S et al. Eicosanoids and adipokines in breast cancer: from molecular mechanisms to clinical considerations. Antioxid Redox Signal. 2013;18(3):323–60.
Harvey AE, Lashinger LM, Hursting SD. The growing challenge of obesity and cancer: an inflammatory issue. Ann N Y Acad Sci. 2011;1229:45–52.
Subbaramaiah K et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila). 2011;4(3):329–46.
Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–46.
Ono M. Molecular links between tumor angiogenesis and inflammation: inflammatory stimuli of macrophages and cancer cells as targets for therapeutic strategy. Cancer Sci. 2008;99(8):1501–6.
Aggarwal BB, Gehlot P. Inflammation and cancer: how friendly is the relationship for cancer patients? Curr Opin Pharmacol. 2009;9(4):351–69.
Kundu JK, Surh YJ. Inflammation: gearing the journey to cancer. Mutat Res. 2008;659(1–2):15–30.
Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7.
Charriere G et al. Preadipocyte conversion to macrophage. Evidence of plasticity. J Biol Chem. 2003;278(11):9850–5.
Allavena P et al. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66(1):1–9.
Calabro P, Yeh ET. Obesity, inflammation, and vascular disease: the role of the adipose tissue as an endocrine organ. Subcell Biochem. 2007;42:63–91.
Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4(7):540–50.
Kulbe H et al. The chemokine network in cancer–much more than directing cell movement. Int J Dev Biol. 2004;48(5–6):489–96.
Allavena P et al. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev. 2008;222:155–61.
Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest. 2007;117(9):2362–8.
Byrne AM, Bouchier-Hayes DJ, Harmey JH. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF). J Cell Mol Med. 2005;9(4):777–94.
Liu Y et al. The association between vascular endothelial growth factor expression in invasive breast cancer and survival varies with intrinsic subtypes and use of adjuvant systemic therapy: results from the Nurses’ Health Study. Breast Cancer Res Treat. 2011;129(1):175–84.
Chen CT et al. Targeting the IKKbeta/mTOR/VEGF signaling pathway as a potential therapeutic strategy for obesity-related breast cancer. Mol Cancer Ther. 2012;11(10):2212–21.
Iwaki T, Urano T, Umemura K. PAI-1, progress in understanding the clinical problem and its aetiology. Br J Haematol. 2012;157(3):291–8.
Carter JC, Church FC. Obesity and breast cancer: the roles of peroxisome proliferator-activated receptor-gamma and plasminogen activator inhibitor-1. PPAR Res. 2009;2009:345320.
Skurk T, Hauner H. Obesity and impaired fibrinolysis: role of adipose production of plasminogen activator inhibitor-1. Int J Obes Relat Metab Disord. 2004;28(11):1357–64.
Muldowney 3rd JA et al. Pentoxifylline lowers plasminogen activator inhibitor 1 levels in obese individuals: a pilot study. Angiology. 2012;63(6):429–34.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70.
Hollstein M et al. p53 mutations in human cancers. Science. 1991;253(5015):49–53.
Molchadsky A et al. p53 is required for brown adipogenic differentiation and has a protective role against diet-induced obesity. Cell Death Differ. 2013;20(5):774–83.
Park H et al. A high-fat diet increases angiogenesis, solid tumor growth, and lung metastasis of CT26 colon cancer cells in obesity-resistant BALB/c mice. Mol Carcinog. 2012;51(11):869–80.
Dhasarathy A, Kajita M, Wade PA. The transcription factor snail mediates epithelial to mesenchymal transitions by repression of estrogen receptor-alpha. Mol Endocrinol. 2007;21(12):2907–18.
Liu G, Schwartz JA, Brooks SC. Estrogen receptor protects p53 from deactivation by human double minute-2. Cancer Res. 2000;60(7):1810–4.
Hurd C et al. Estrogen-dependent and independent activation of the P1 promoter of the p53 gene in transiently transfected breast cancer cells. Oncogene. 1999;18(4):1067–72.
Konduri SD et al. Mechanisms of estrogen receptor antagonism toward p53 and its implications in breast cancer therapeutic response and stem cell regulation. Proc Natl Acad Sci U S A. 2010;107(34):15081–6.
Shirley SH et al. Transcriptional regulation of estrogen receptor-alpha by p53 in human breast cancer cells. Cancer Res. 2009;69(8):3405–14.
Zhang X et al. Estrogen receptor positivity in mammary tumors of Wnt-1 transgenic mice is influenced by collaborating oncogenic mutations. Oncogene. 2005;24(26):4220–31.
Fuchs-Young R et al. P53 genotype as a determinant of ER expression and tamoxifen response in the MMTV-Wnt-1 model of mammary carcinogenesis. Breast Cancer Res Treat. 2011;130(2):399–408.
Freeman EW et al. Obesity and reproductive hormone levels in the transition to menopause. Menopause. 2010;17(4):718–26.
Wolff AC et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131(1):18–43.
Skliris GP et al. Reduced expression of oestrogen receptor beta in invasive breast cancer and its re-expression using DNA methyl transferase inhibitors in a cell line model. J Pathol. 2003;201(2):213–20.
Luo J et al. A comparison of batch effect removal methods for enhancement of prediction performance using MAQC-II microarray gene expression data. Pharmacogenomics J. 2010;10(4):278–91.
Wang ZV et al. Identification and characterization of a promoter cassette conferring adipocyte-specific gene expression. Endocrinology. 2010;151(6):2933–9.
Karastergiou K et al. Epicardial adipokines in obesity and coronary artery disease induce atherogenic changes in monocytes and endothelial cells. Arterioscler Thromb Vasc Biol. 2010;30(7):1340–6.
Kalaany NY, Sabatini DM. Tumours with PI3K activation are resistant to dietary restriction. Nature. 2009;458(7239):725–31.
Elkabets M et al. mTORC1 inhibition is required for sensitivity to PI3K p110alpha Inhibitors in PIK3CA-mutant breast cancer. Sci Trans Med. 2013;5(196):196ra99.
Njiaju UO, Olopade OI. Genetic determinants of breast cancer risk: a review of current literature and issues pertaining to clinical application. Breast J. 2012;18(5):436–42.
Ford NA et al. Obesity, independent of p53 gene dosage, promotes mammary tumor progression and upregulates the p53 regulator MicroRNA-504. PLoS One. 2013;8(6):e68089.
Gupta PB, Chaffer CL, Weinberg RA. Cancer stem cells: mirage or reality? Nat Med. 2009;15(9):1010–2.
Velasco-Velazquez MA et al. Breast cancer stem cells. Int J Biochem Cell Biol. 2012;44(4):573–7.
Zheng Q et al. Leptin deficiency suppresses MMTV-Wnt-1 mammary tumor growth in obese mice and abrogates tumor initiating cell survival. Endocr Relat Cancer. 2011;18(4):491–503.
Acknowledgments
SDH is funded, in part, by grants from the National Cancer Institute (R01CA129409 and R01CA135306), the Breast Cancer Research Foundation (UTA09-001068), and the National Institute of Environmental Health Sciences (P30ES007784). NAF was supported by an American Institute for Cancer Research Postdoctoral Fellowship and KLD was supported by a predoctoral fellowship from the National Institute of Environmental Health Sciences (T32ES07247).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ford, N.A., Devlin, K.L., Lashinger, L.M. et al. Deconvoluting the Obesity and Breast Cancer Link: Secretome, Soil and Seed Interactions. J Mammary Gland Biol Neoplasia 18, 267–275 (2013). https://doi.org/10.1007/s10911-013-9301-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10911-013-9301-9