Abstract
Normal development of the mammary gland is a multidimensional process that is controlled in part by its mammary microenvironment. The mammary microenvironment is a defined location that encompasses mammary somatic stem cells, neighboring signaling cells, the basement membrane and extracellular matrix, mammary fibroblasts as well as the intercellular signals produced and received by these cells. These dynamic signals take numerous forms including growth factors, steroids, cell-cell or cell-basement membrane physical interactions. Cellular growth and differentiation of the mammary gland throughout the developmental stages are regulated by changes in these signals and interactions. The purpose of this review is to summarize current information and research regarding the role of the mammary microenvironment during normal glandular development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The somatic stem cells of the mammary gland are regulated by their surrounding microenvironment. The microenvironment is a restricted locale that supports the self-renewal of the stem cells and fosters the differentiation of the stem cell progeny or daughter cells. A microenvironment is comprised of stem cells, localized signaling cells, soluble glycoprotein mediators, and the extracellular matrix (ECM) [1]. Within the tissue these microenvironmental factors provide structural constraints and biochemical cues that control cellular behavior [2–4]. Within the mammary gland the localized signaling cells consist of epithelial cells, both luminal and basal, myoepithelial cells, fibroblasts and the cells of the stromal compartment including adipocytes. Endothelial cells of the vasculature and mammary-associated cells of the nervous system may also supply signals that influence the microenvironment. The cells within this specialized microenvironment interpret the myriad of intercellular signals and determine whether the stem cells self-renew, expand via symmetric division, or remain quiescent.
The tissue microenvironment holds many physical, chemical, and mechanical cues that eventually work in concert to allow tissue-specific differentiation of cells. The primary cellular-based components of functional mammary glands, termed acini or alveoli, include luminal epithelial cells, myoepithelial cells, and a basement membrane; in a normal breast, the basement membrane is responsible for separating the epithelium from the connective tissue [5]. A single layer of luminal epithelial cells surrounds the inner lumen of each alveolus. Normal luminal epithelial cells form tight junctions and are polarized [6]. Directly adjacent and basal to the single layer of luminal epithelial cells is a layer of myoepithelial cells. These cells are primarily responsible for the deposition of basement membrane proteins and for maintaining the polarity of luminal epithelial cells [7]. The prefix “myo,” often associated with “muscle,” is appropriate for this cell type because these cells are also contractile in nature; they are responsible for contributing to the milk secretion process during lactation [8]. The proper organization of these cells within the mammary gland is an important regulator of normal glandular development.
There have been many recent discoveries concerning mammary stem cells in both mice and humans regarding their isolation and genetic profiling. However, little has been reported on the surrounding cells within the mammary microenvironment. Without the interactions, including chemical and physical, provided by the surrounding signaling cells the stem cells will not behave normally. The mammary microenvironment can be regarded as the essential functional building block required for the complete development of a functional mammary gland. Understanding the intricate interactions between all the components of the microenvironment is fundamental in the early detection of pathologies and for future tissue engineering technologies in regenerative medicine.
Normal Development
A transplantation model of mouse mammary development, where the mammary fat of a pre-pubescent female is cleared of the endogenous epithelial component, was developed in the late 1950s [9]. Following the removal of the endogenous epithelium, cells or tissues are transplanted and a functional mammary gland is recapitulated. This transplantation model has been used to demonstrate that an entire functional mammary gland can be regenerated by mammary tissue fragments or dissociated cells regardless of donor age or portion of the mammary gland from which tissue fragments or cells are taken, thus proving the existence of somatic mammary stem cells. This transplantation model has proved to be a powerful tool in the understanding of normal mammary development and the influence of the microenvironment. Genetically-modified rodent models that utilize transgenic and gene knockout technologies have been instrumental in efforts to decipher the intricate intercellular signaling within the mammary microenvironment. Knockout models and transgenic models that over express growth factors, receptors, or transcription factors that target either the mammary epithelium or the stroma have proven useful in this endeavor [10]. The use of transgenic tissue in the model developed by DeOme and colleagues has allowed the study of specific proteins to determine their roles in mammary development and disease progression [10].
In both mice and rats there is evidence of a hierarchy of mammary-specific stem cells termed lobule-limited progenitor cells, duct-limited progenitor cells, and unlimited progenitor cells [10–14]. As the names imply, lobule-limited progenitor cell progeny are differentially limited to forming secretory alveoli whereas duct-limited progenitor cell progeny differentiate into mammary ducts only. The unlimited progenitor cells are capable of producing daughter cells that differentiate into either alveolar cells or ductal epithelial cells. Lobule-limited progenitors are unable to produce cap cells that are responsible for the penetration of the growing duct into the surrounding mammary fat pad. Duct-limited progenitors do not produce cells capable of forming and maintaining alveoli during pregnancy [14]. The surrounding signaling cells provide the cues for the progenitor cells to proliferate, differentiate or enter a state of quiescence.
The nuclear steroid receptors estrogen receptor-alpha (ERα) and progesterone receptor (PR) are essential for the normal development of the mammary gland. Loss of ERα in the mammary epithelium results in impaired ductal branching and elongation while expression of PR in the epithelium is required for normal secretory alveolar development [15, 16]. Due to the phenotypic changes observed in these steroid receptor knockout models it is believed that loss of the steroid receptors inhibits the limited mammary progenitor cells. Specifically, loss of ERα affects duct-limited progenitor cells while loss of PR affects the lobule-limited progenitor cells. This effect is due to altered intercellular signaling within the microenvironment. The epidermal growth factor (EGF) family member amphiregulin (AREG) is an essential mediator of ERα action on duct-limited mammary progenitor cells both in vivo and in vitro [16, 17]. Since mammary stem and progenitor cells are ERα-negative and PR-negative [18] the cells positive for these receptors must be the signaling cells within the microenvironment. Mammary stem and progenitor cells respond to estrogen and progesterone, but without the proper receptors these cells receive secondary paracrine signals from neighboring ER-positive or PR-positive cells. Recently it was determined that ovariectomized mice have decreased numbers of mammary stem cells with a diminished capacity for self-renewal [19]. Without these essential ER-positive and PR-positive cells in the microenvironment the mammary stem and progenitor cells do not function properly.
Numerous reports have suggested that one possible location of the expanding mammary microenvironment during estrogen-mediated ductal elongation in mice is the cap cell region of the terminal end buds (TEBs) (reviewed in 20). The cap cells of the TEBs directly interact with the mammary stroma such that the ECM is degraded, allowing local penetration and expansion of the duct into the stroma. Furthermore, normal mammary epithelium does not grow when transplanted into epidermal growth factor receptor (EGFR)-null fat pads, but EGFR-null epithelium does grow and expand when transplanted into the cleared fat pads of wildtype recipients, indicating an essential role for EGFR in the normal microenvironment [21]. Along these lines, EGFR mRNA is enhanced in stem cell niches [19] and is downstream of ERα-mediated AREG signaling [22].
Secondary side branching in the mammary gland is mediated primarily by the increased circulating levels of progesterone during the estrus cycle. Unlike estrogen and its receptors, progesterone induces proliferation of both PR-positive and PR-negative cells [15]. Key factors that mediate progesterone’s effects include RANKL (receptor activator of NF-κB ligand) [23, 24] and Wnt-4 [25]. RANKL is involved in the proliferation of daughter cells [20] that follows the asymmetric division of stem cells, which is regulated, in part, by Wnt-4 [26–29]. These factors originate in the surrounding microenvironment and function in a paracrine manner.
The mammary gland is considered fully differentiated only during pregnancy or when it is lactating. At all other times the mammary gland is considered to exist in an immature, non-functioning state where the epithelium is embedded in the fat pad which is dominant in these states [30]. During pregnancy the epithelium undergoes massive hormone-induced proliferation and differentiation, building the foundation for lactation. There are no major architectural changes that occur during the lactation phase, however, following lactation the mammary gland undergoes extensive remodeling in a process known as involution where the milk-producing lobuloalveoli are resorbed and the epithelium returns toward a state of immaturity. The process of remodeling the secretory gland toward its immature state requires extensive changes to the ECM, via both proteolysis of existing ECM and the synthesis of new ECM, as well as widespread apoptosis of the secretory epithelial cells [31–34]. These processes are regulated in part by the mammary microenvironment. Genes coding for collagens I, III, IV, V and VI, elastin, fibrillin 1 and microfibrillar-associated protein 5 (reviewed in 30) as well as those for proteases such as matrix metalloproteinase (MMP)-2 and MMP3 are upregulated during involution while MMP9 activity is upregulated without corresponding mRNA production, indicating its post-transcriptional control [35]. These gene products provide the tools necessary for the physical remodeling that occurs in the microenvironment during involution.
Normal Mammary Microenvironment Directs Alien Stem Cells to Adopt a Mammary Phenotype
As mentioned earlier there are required components (stem and signaling cells, basement membrane (BM), ECM, intercellular signals, etc.) for a functional mammary microenvironment. Recent publications have demonstrated that stem cells derived from tissues other than the mammary gland are able to enter reforming microenvironments during glandular regeneration in transplantation models, following removal of the endogenous epithelium where they are directed to act as mammary stem cells. When dissociated stem cells from other organs such as the brain or testes are transplanted alone there is no tissue development [36, 37]. If genetically-labeled stem cells of non-mammary origin are transplanted in concert with normal mammary epithelial cells, the result is full reconstitution of functional mammary glands comprised of progeny of the normal mammary epithelial cells and the progeny of the foreign stem cells [36, 37], thus demonstrating the requirement of the surrounding signaling cells to maintain the normal action of the microenvironment. The genetically-labeled cells react to the signals emanating from the normal mammary microenvironment and differentiate into luminal epithelial cells that express ERα and PR, myoepithelial cells, and secretory epithelial cells that create and secrete milk proteins during pregnancy and lactation.
The normal mammary microenvironment can also redirect tumor-derived cells to participate in the reconstitution of a normal functional mammary gland. Cells derived from mouse mammary tumors that overexpress the EGF family receptor erbB2 as well as NTERA-2cl cells that were derived from a human testicular carcinoma were both able to participate in the regeneration of a normal functional mouse mammary gland [38, 39]. In both cases the tumor-derived cells fully integrated into the regenerated mammary gland, expanded in numbers and self-renewed as demonstrated by their continued presence in second generation transplantation outgrowths. This integration event only occurred when the number of normal cells outnumbered the tumor-derived cells at transplantion. Tumors formed when the tumor-derived cells were transplanted alone or in greater or equal numbers than the normal mammary epithelial cells. Only when the normal cells outnumbered the tumor-derived cells was the reforming mammary microenvironment dominant over the tumor cells. In both cases, i.e. mouse and human derived tumor cells, the normal microenvironment directed the expansion and differentiation of the tumor-derived cells into luminal epithelial cells, myoepithelial cells, and functional secretory cells that produced and secreted milk proteins during pregnancy. Without the signals that originate in the normal microenvironment the foreign stem cells did not acquire a mammary phenotype.
Important Signals in the Mammary Microenvironment
It is well documented that mammary adipose tissue is a source of signaling molecules (Reviewed in [40]). These molecules act directly on the adipose tissue as well as the surrounding endothelial and epithelial cells, thereby influencing the mammary stem cell microenvironment. As mentioned earlier, the presence of ERα-positive and PR-positive epithelial cells is essential for the normal development of the mammary gland. The signals that emanate from ERα+ cells are essential to ductal growth and expansion while PR+ cells provide signals required for secretory alveolar development. The transcription factor Gata3 is also essential for normal mammary luminal epithelial differentiation [41].
Some of the essential stromal-derived signals include transforming growth factor (TGF)-β, fibroblast growth factor (FGF), heregulin, hepatocyte growth factor (HGF), and the insulin-like growth factors (IGF) [42–44]. Mammary stromal fibroblast-derived HGF mediates the proliferation of ER-positive mammary epithelial cells [45].
The TGFβ superfamily and their receptors play critical roles in every phase of normal development (Reviewed in [46–48]). Transcripts of the different TGFβ isoforms are expressed in TEBs and the quiescent non-pregnant ducts where they act as growth-suppressive factors to maintain tissue homeostasis. In mice that overexpress TGFβ1 in the epithelial compartment, ductal formation is delayed with a profound absence of side branches [49]. Secretory development is impaired as a result of early induced apoptosis in mice when TGFβ1 is under control of the pregnancy-specific whey acidic protein promoter [11, 50, 51]. Expression of the TGFβ isoforms increases during pregnancy but declines during lactation. Message levels of TGFβ3 increase within 6 h of the end of lactation; subsequently, message levels of TGFβ1 and TGFβ2 increase during involution, suggesting a role for these growth factors during tissue remodeling associated with involution. TGFβ proteins and the growth factor receptors TGFβRI and TGFβRII are found in both the epithelial and stromal compartments during all stages of mammary development.
Fleming and colleagues examined stroma-derived factors related to the stromal microenvironment in the breast. They found that more than one normal fibroblastic phenotype exists in the breast, and that each has the potential for various epigenetic effects on normal epithelial cells depending on their proximity to the parenchyma. The results of this study showed differences in the expression patterns of several proteins such as collagen type XIV and CD13 and suggested that intralobular fibroblasts are phenotypically distinct from interlobular fibroblasts [52].
The epidermal growth factor (EGF) family of growth factors and erbB receptors are also important signaling molecules in mammary microenvironments throughout normal tissue development. These factors function through autocrine, paracrine and juxtacrine mechanisms, with a number of these signals originating from the surrounding mesenchymal tissue, specifically from cells such as adipocytes as well as neural, lymphoid and endothelial cells. The EGF family members AREG, TGFα, EGF, betacellulin, heparin-binding EGF-like (HB-EGF), epiregulin and neuregulin (NRG)-1 are all expressed during postnatal mammary development with individual expression patterns [53, 54]. Three of the erbB receptors are expressed in the glands of virgin females (EGFR, erbB2 and erbB4) while all four are expressed during pregnancy and lactation [53–55]. AREG is the most abundant EGF family member during pubertal growth in the glands of virgins [53]. As mentioned previously, AREG is required for ER-mediated ductal elongation and TEB formation [22]. However, the expression of AREG is down regulated during and after pregnancy [54, 56]. TGFα mRNA has been detected throughout glandular development, with the highest levels found in mammogenesis and involution [57]. The major role of TGFα is to induce proliferation. This growth factor is found in the proliferating cap cells of the TEBs during ductal expansion and in the developing secretory lobules during early pregnancy (Reviewed in [57]).
Another source of intercellular signals that influence the normal mammary microenvironment is the immune system. Macrophages are essential for normal ductal morphogenesis [58, 59] and support mammary stem cell function [60]. Both macrophages and eosinophils are found around growing TEBs. It is believed that macrophages aid in the removal of apoptotic cells during mammary remodeling. Colony stimulating factor (CSF)-1 and eotaxin are chemoattractants for macrophages and eosinophils respectively and both chemokines are produced by the mammary gland [61]. Overall the functions for macrophages and eosinophils in ductal morphogenesis include supplying trophic factors for mammary epithelial cell growth and matrix remodeling [58].
The paragraphs above outline the mechanisms surrounding influences of the mammary mesenchyme on the mammary epithelium during tissue development. There are also examples of the epithelium influencing the surrounding mesenchyme [62]. Regarding mammary glands from prenatal females, when the mammary mesenchyme that has not been in contact with mammary epithelium (using mesenchyme from other tissues such as pancreas or lung) is placed with mammary epithelium the mesenchyme is induced to respond as normal mammary mesenchyme. The mesenchyme synthesizes tenascin-C, androgen receptors and estrogen receptors. The induction of steroid receptors by mesenchyme is specific to mammary epithelial cells and is not induced by other epithelia (salivary or pancreatic) [62, 63].
Integrins, the major cellular receptors for ECM components, have essential roles during normal cell growth and differentiation. Each integrin is composed of an α-subunit and a β-subunit that form a heterodimeric complex which, in turn, binds to glycoproteins in the BM or interstitial matrix, making the integrins the major environmental receptors of the cell [64, 65]. Integrins are more abundant at the basal surface of the mammary epithelium than in the luminal portion. Not only do integrins bind and react to extracellular stimuli, they also interact with numerous cytosolic mediators that influence numerous integrin-mediated cellular functions including adhesion, migration, polarity, proliferation and apoptosis [64]. All integrins expressed in the mouse mammary gland contain either the β1 or β4 subunit [66]. Prospective mammary stem cells are isolated based on their expression of α6 and β1 integrins (also known as CD49f and CD29) [67, 68] indicating that they are influenced by components of the surrounding microenvironment. The β1 subunit anchors cells to the BM through binding to laminin and Type IV collagen and to the stroma through binding to Type I collagen and fibronectin [69]. β1-integrin is required for full development of secretory alveoli and is essential for the regenerative potential of the mammary gland [70, 71], where its deletion is embryonically-lethal [72]. However, overexpression or conditional deletion of β1-integrin in terminally differentiating luminal epithelial cells during pregnancy and lactation disrupts both ductal growth and alveologenesis [66, 70, 73]. These results indicate β1-integrin is essential for normal functioning of the mammary microenvironment.
In addition to the integrins, mammary cells have other receptors that bind to the ECM including dystroglycan, collagen receptor, and syndecans. Each of these binds specific portions of the ECM where, through their binding, these receptors influence branching morphogenesis [74, 75]. Collagen deposition by cells of the microenvironment alters extracellular tension that affects cellular differentiation (Reviewed in [76]). Indeed, alterations in matrix stiffness promote tumorigenesis by altering integrins and other ECM receptors [77, 78].
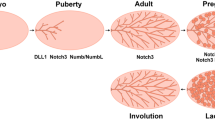
The intercellular signaling discussed here (Summarized in Fig. 1) is a mere fraction of what is known to occur during mammary gland development. The vast and intricate network of biochemical and physical control that occurs locally within the mammary microenvironment and globally throughout the mammary gland is only beginning to be deciphered. The crosstalk between stem cells and signaling cells as well as the physical cues delivered and received by the cells and ECM form the basis of the domineering effects of the normal microenvironment.
Schematic illustrating some of the signals acting in the normal mammary microenvironment. The intercellular communication consists of signals that originate in the luminal, basal and stromal compartments of the mammary gland as well as hormones and signals from the immune system. Abbreviations: AREG-amphiregulin, BM-basement membrane, CSF-1, colony stimulating factor-1, EGFR-epidermal growth factor receptor, ER-estrogen receptor, FGF-fibroblast growth factor, Fib-mamamry fibroblast, HGF-hepatocyte growth factor, IGF-insulin-like growth factor, MΦ-macrophage, MFG-milk fat globule, MSC-mammary stem cell, MYO-myoepithelial cell, PR-progesterone receptor, RANKL-receptor activator of NF-κB ligand, TGF-transforming growth factor
Role of Normal Mammary Microenvironment in Tissue Engineering
The stroma is not a static tissue. The composition and organization of the ECM and cellular components evolve with the developmental stages of the mammary gland [75, 79]. Therefore, it is expected that the variations seen in epithelial-stromal interactions and ratios that occur during the various stages of breast development will influence the components of cellular microenvironment (growth factors, hormones, and ECM) and the nucleus which dictate gene expression that may account for the susceptibility or risk to develop breast cancer [80, 81]. While research has focused on epithelial-stromal interactions and how they influence the proliferation, the differentiation, and, at times, the quiescence of the epithelial cells and their progenitors, there is still much that is unknown. In vitro model systems have been developed, through the use of tissue engineering methods (Reviewed in [82]), in order to better understand the development and structure of the gland parenchyma and how the stroma influences development and even breast cancer initiation and progression.
Tissue engineering, i.e. the construction of tissues using cells and biomaterial “scaffolds” or “matrices” as foundational building blocks, has been of interest for many years for mammary reconstruction following mastectomy or lumpectomy [83, 84], and has been investigated more recently for use in building three-dimensional (3D) mammary tissue models [82]. The intricacies of mammary tissue have provided numerous challenges in tissue engineering. The mammary gland is a complex tissue comprised of epithelial parenchyma embedded in an array of stromal cells that regulate its proliferation, differentiation, and survival [85]. The fibrous connective tissue of the stroma, also known as the ECM, is a 3D network that surrounds the cells and is divided into two zones. The first is the BM, which interacts directly with the epithelium and consists primarily of collagen IV, laminin, entactin/nidogen, and heparan sulfate proteoglycans. The second is the interstitial matrix, which consists of collagen (usually Type I and III) and fibronectin [86]. The latter contributes to the mechanical strength of the tissue [86]. Luminal epithelial cells line the ducts and are surrounded by a layer of myoepithelial cells that attach to the BM [87], which acts as a mechanical barrier between the epithelial-lined ductal structure and the surrounding connective and fat tissue. The ECM, largely through its dynamic chemical and mechanical characteristics, is able to regulate cell shape, proliferation, polarity, differentiation, transcription, synthesis, and secretion for a variety of cell types [81].
Epithelial cell monolayers have traditionally been used to study breast cancer, yet two dimensional (2D) culture does not resemble the structure or the function of the mammary epithelium in vivo [88]. Thus, 3D cultures have been developed in order to better represent the in vivo environment. Much of breast biology and breast cancer research has included mammary epithelial cells or breast cancer cells embedded in natural materials such as collagen Type I, reconstituted basement membrane products (rBM, e.g. Matrigel), or a combination of the two, to represent the naturally-occurring ECM of the breast [88–90]. Dhimolea and colleagues found that flexible Type I collagen matrices supported polarized acini and branching ducts when human breast epithelial (MCF10A) cells and human mammary fibroblasts obtained from reduction mammoplasties (RMF) cells were suspended in the gels in co-culture [91]. Krause and colleagues co-cultured MCF10A and RMF cells embedded in a Type I collagen gel, which resulted in the development of branched ducts, but when rBM was added to the collagen in a 1:1 ratio, branching ducts and alveoli were produced [88]. Muthulekha and coworkers [92] found that the behavior of 3D cultures of normal murine mammary gland (NMuMG) cells, cultured either alone or in combination with mouse mesenchymal stem cells (D1), were dependent on the “matrix” or surrounding biomaterial environment consisting of agarose, collagen, or Matrigel®, alone or in combination. The number of acinar structures was significantly higher in cultures grown in combination matrices of agarose with Matrigel® or collagen I when compared to cultures grown in Matrigel® or collagen I alone. No tubular structures were formed when agarose was included in the matrix, regardless of the combination. Other research groups have used polymeric substrates, such as electrospun polycaprolactone, poly(ethylene glycol) diacrylate hydrogels, and polylactide-co-polyglycolide microspheres or porous disks, to create 3D engineered models of adipose tissue to assess the cytotoxicity of breast cancer drugs [93–97]. Stem cells or preadipocytes have previously been used to produce mature adipose tissue for applications in replacing soft tissue due to trauma, diseases, or congenital abnormalities [95, 99, 100]. However, use of synthetic polymeric matrices seeded with preadipocytes and combined with epithelial cells and fibroblasts (which would not normally be incorporated for tissue reconstruction following breast cancer) embedded in a collagen-rBM gel has the potential to produce engineered 3D models that better represent the architecture and microenvironment of the breast. Although synthetic polymer substrates may fully represent the naturally-occurring ECM, the 3D geometry provides environmental cues for adipogenesis that cannot be obtained in 2D (monolayer) culture [97]. Thus, the substrates provide the necessary biomechanical stability and cell affinity to produce function-specific tissue [98]. Epithelial cells are anchorage-dependent cells that require a substrate to ensure proper cell morphology and functionality. In fact, while synthetic polymeric materials with the appropriate binding sites may be suitable for adipocytes, natural materials generally facilitate polarity in epithelial cell cultures.
The unique mammary gland microenvironment influences mammary tissue homeostasis through hormones, soluble factors, stroma, and physical stress and strain [101]. Tissue engineering and model systems can help better represent the native in vivo conditions of the mammary gland and the surrounding stroma; indeed, the microenvironment and ECM are key components of 3D in vitro mammary systems that can be tailored to study normal epithelial cell-stromal interactions, gland development, and breast cancer.
Conclusions and Future Perspectives
During mammary gland development the microenvironment provides both chemical signaling and physical controls that direct epithelial proliferation and differentiation. Research deciphering the complex signals and cellular interactions is just beginning to shed light on the role of the microenvironment. This review has highlighted select recent discoveries that have begun to scratch the surface of the complexity of the mammary microenvironment. Understanding the mammary microenvironment and its influence on the mammary gland as a whole will not only further our knowledge of basic developmental biology but will also help in understanding disease processes that involve corrupted or mutated signaling to stem cells within the microenvironment. In addition, understanding this complexity will aid in future tissue-engineering treatments that will follow post-traumatic injuries and mastectomy due to disease.
Abbreviations
- 2D:
-
two dimensional
- 3D:
-
three dimensional
- AREG:
-
amphiregulin
- BM:
-
basement membrane
- ECM:
-
extracellular matrix
- EGF:
-
epidermal growth factor
- EGFR:
-
epidermal growth factor receptor
- ERα:
-
estrogen receptor-alpha
- erbB:
-
EGF family receptor
- FGF:
-
fibroblast growth factor
- HB-EGF:
-
heparin-binding EGF-like growth factor
- HGF:
-
hepatocyte growth factor
- IGF:
-
insulin-like growth factor
- MEC:
-
mammary epithelial cell
- MMP:
-
matrix metalloproteinase
- NRG:
-
neuregulin
- PR:
-
progesterone receptor
- RANKL:
-
receptor activator of NF-κB ligand
- RMF:
-
reduction mammoplasty fibroblasts
- TEB:
-
terminal end bud
- TGF:
-
transforming growth factor
References
Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605–31.
Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982;99(1):31–68.
Stoker AW, Streuli CH, Martins-Green M, Bissell MJ. Designer microenvironments for the analysis of cell and tissue function. Curr Opin Cell Biol. 1990;2(5):864–74.
Lin CQ, Bissell MJ. Multi-faceted regulation of cell differentiation by extracellular matrix. FASEB J. 1993;7(9):737–43.
Gudjonsson T, Ronnov-Jessen L, Villadsen R, Bissell MJ, Petersen OW. To create the correct microenvironment: three-dimensional heterotypic collagen assays for human breast epithelial morphogenesis and neoplasia. Methods. 2003;30(3):247–55.
Lodish H, Berk A, Matsudaira P, Kaiser C, Krieger M, Scott M, et al. Molecular Cell Biology, 5th ed. New York: W.H. Freeman and Company, 2004.
Polyak K, Hu M. Do myoepithelial cells hold the key for breast tumor progression? J Mammary Gland Biol Neoplasia. 2005;10(3):231–47.
Schmeichel KL, Bissell MJ. Modeling tissue-specific signaling and organ function in three dimensions. J Cell Sci. 2003;116(Pt 12):2377–88.
DeOme KB, Faulkin Jr LJ, Bern HA, Blair PB. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res. 1959;19(5):515–20.
Smith GH, Medina D. Re-evaluation of mammary stem cell biology based on in vivo transplantation. Breast Cancer Res. 2008;10(1):203.
Smith GH. Experimental mammary epithelial morphogenesis in an in vivo model: evidence for distinct cellular progenitors of the ductal and lobular phenotype. Breast Cancer Res Treat. 1996;39(1):21–31.
Kim ND, Oberley TD, Yasukawa-Barnes J, Clifton KH. Stem cell characteristics of transplanted rat mammary clonogens. Exp Cell Res. 2000;260(1):146–59.
Smith GH, Boulanger CA. Mammary stem cell repertoire: new insights in aging epithelial populations. Mech Ageing Dev. 2002;123(11):1505–19.
Boulanger CA, Smith GH. Reprogramming cell fates in the mammary microenvironment. Cell Cycle. 2009;8(8):1127–32.
Brisken C, Park S, Vass T, Lydon JP, O’Malley BW, Weinberg RA. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc Natl Acad Sci USA. 1998;95(9):5076–81.
Mallepell S, Krust A, Chambon P, Brisken C. Paracrine signaling through the epithelial estrogen receptor alpha is required for proliferation and morphogenesis in the mammary gland. Proc Natl Acad Sci USA. 2006;103(7):2196–201.
Booth BW, Boulanger CA, Anderson LH, Jimenez-Rojo L, Brisken C, Smith GH. Amphiregulin mediates self-renewal in an immortal mammary epithelial cell line with stem cell characteristics. Exp Cell Res. 2009;316(3):422–32.
Asselin-Labat ML, Shackleton M, Stingl J, Vaillant F, Forrest NC, Eaves CJ, et al. Steroid hormone receptor status of mouse mammary stem cells. J Natl Cancer Inst 2006;98:1011–4.
Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, et al. Control of mammary stem cell function by steroid hormone signalling. Nature 2010;465:798–802.
Brisken C, Duss S. Stem cells and the stem cell niche in the breast: an integrated hormonal and developmental perspective. Stem Cell Rev. 2007;3(2):147–56.
Wiesen JF, Young P, Werb Z, Cunha GR. Signaling through the stromal epidermal growth factor receptor is necessary for mammary ductal development. Development. 1999;126(2):335–44.
Ciarloni L, Mallepell S, Brisken C. Amphiregulin is an essential mediator of estrogen receptor alpha function in mammary gland development. Proc Natl Acad Sci USA. 2007;104(13):5455–60.
Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci USA. 2003;100(17):9744–9.
Brisken C, Ayyannan A, Nguyen C, Heineman A, Reinhardt F, Tan J, et al. IGF-2 is a mediator of prolactin-induced morphogenesis in the breast. Dev Cell 2002;3:877–87.
Brisken C, Heineman A, Chavarria T, Elenbaas B, Tan J, Dey SK, et al. Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes Dev 2000;14:650–4.
Wagner KU, Boulanger CA, Henry MD, Sgagias M, Hennighausen L, Smith GH. An adjunct mammary epithelial cell population in parous females: its role in functional adaptation and tissue renewal. Development. 2002;129(6):1377–86.
Medina D. Mammary developmental fate and breast cancer risk. Endocr Relat Cancer. 2005;12(3):483–95.
Piccirillo SG, Reynolds BA, Zanetti N, Lamorte G, Binda E, Broggi G, et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature 2006;444:761–5.
Matulka LA, Triplett AA, Wagner KU. Parity-induced mammary epithelial cells are multipotent and express cell surface markers associated with stem cells. Dev Biol. 2007;303(1):29–44.
Schedin P, O’Brien J, Rudolph M, Stein T, Borges V. Microenvironment of the involuting mammary gland mediates mammary cancer progression. J Mammary Gland Biol Neoplasia. 2007;12(1):71–82.
Strange R, Li F, Saurer S, Burkhardt A, Friis RR. Apoptotic cell death and tissue remodelling during mouse mammary gland involution. Development. 1992;115(1):49–58.
Lund LR, Romer J, Thomasset N, Solberg H, Pyke C, Bissell MJ, et al. Two distinct phases of apoptosis in mammary gland involution: proteinase-independent and -dependent pathways. Development 1996;122:181–93.
Schedin PJ, Thackray LB, Malone P, Fontaine SC, Friis RR, Strange R. Programmed cell death and mammary neoplasia. Cancer Treat Res. 1996;83:3–22.
Masso-Welch PA, Darcy KM, Stangle-Castor NC, Ip MM. A developmental atlas of rat mammary gland histology. J Mammary Gland Biol Neoplasia. 2000;5(2):165–85.
Schedin P, Mitrenga T, McDaniel S, Kaeck M. Mammary ECM composition and function are altered by reproductive state. Mol Carcinog. 2004;41(4):207–20.
Boulanger CA, Mack DL, Booth BW, Smith GH. Interaction with the mammary microenvironment redirects spermatogenic cell fate in vivo. Proc Natl Acad Sci USA. 2007;104(10):3871–6.
Booth BW, Mack DL, Androutsellis-Theotokis A, McKay RD, Boulanger CA, Smith GH. The mammary microenvironment alters the differentiation repertoire of neural stem cells. Proc Natl Acad Sci USA. 2008;105(39):14891–6.
Bussard KM, Boulanger CA, Booth BW, Bruno RD, Smith GH. Reprogramming human cancer cells in the mouse mammary gland. Cancer Res. 2010;70(15):6336–43.
Booth BW, Boulanger CA, Anderson LH, Smith GH. The normal mammary microenvironment suppresses the tumorigenic phenotype of MMTV-neu transformed mammary tumor cells. Oncogene 2010 (Accepted for publication).
Hovey RC, Aimo L. Diverse and active roles for adipocytes during mammary gland growth and function. J Mammary Gland Biol Neoplasia 2010.
Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol 2007;9:201–9.
Lu P, Ewald AJ, Martin GR, Werb Z. Genetic mosaic analysis reveals FGF receptor 2 function in terminal end buds during mammary gland branching morphogenesis. Dev Biol. 2008;321(1):77–87.
Soriano JV, Pepper MS, Orci L, Montesano R. Roles of hepatocyte growth factor/scatter factor and transforming growth factor-beta1 in mammary gland ductal morphogenesis. J Mammary Gland Biol Neoplasia. 1998;3(2):133–50.
Stull MA, Rowzee AM, Loladze AV, Wood TL. Growth factor regulation of cell cycle progression in mammary epithelial cells. J Mammary Gland Biol Neoplasia. 2004;9(1):15–26.
Zhang HZ, Bennett JM, Smith KT, Sunil N, Haslam SZ. Estrogen mediates mammary epithelial cell proliferation in serum-free culture indirectly via mammary stroma-derived hepatocyte growth factor. Endocrinology. 2002;143(9):3427–34.
Daniel CW, Robinson S, Silberstein GB. The transforming growth factors beta in development and functional differentiation of the mouse mammary gland. Adv Exp Med Biol. 2001;501:61–70.
Serra R, Crowley MR. TGF-b in mammary gland development and breast cancer. Breast Dis. 2004;18:61–73.
Serra R, Crowley MR. Mouse models of transforming growth factor b impact in breast development and cancer. Endocr-Relat Cancer. 2005;12:749–60.
Pierce DF, Jr., Johnson MD, Matsui Y, Robinson SD, Gold LI, Purchio AF, et al. Inhibition of mammary duct development but not alveolar outgrowth during pregnancy in transgenic mice expressing active TGF-beta 1. Genes Dev 1993;7:2308–17.
Jhappan C, Geiser AG, Kordon EC, Bagheri D, Hennighausen L, Roberts AB, et al. Targeting expression of a transforming growth factor beta 1 transgene to the pregnant mammary gland inhibits alveolar development and lactation. EMBO J 1993;12:1835–45.
Kordon EC, McKnight RA, Jhappan C, Hennighausen L, Merlino G, Smith GH. Ectopic TGF beta 1 expression in the secretory mammary epithelium induces early senescence of the epithelial stem cell population. Dev Biol. 1995;168(1):47–61.
Fleming JM, Long EL, Ginsburg E, Gerscovich D, Meltzer PS, Vonderhaar BK. Interlobular and intralobular mammary stroma: genotype may not reflect phenotype. BMC Cell Biol. 2008;9:46.
Kenney NJ, Huang RP, Johnson GR, Wu JX, Okamura D, Matheny W, et al. Detection and location of amphiregulin and Cripto-1 expression in the developing postnatal mouse mammary gland. Mol Reprod Dev 1995;41:277–86.
Schroeder JA, Lee DC. Dynamic expression and activation of ERBB receptors in the developing mouse mammary gland. Cell Growth Differ. 1998;9(6):451–64.
Sebastian J, Richards RG, Walker MP, Wiesen JF, Werb Z, Derynck R, et al. Activation and function of the epidermal growth factor receptor and erbB-2 during mammary gland morphogenesis. Cell Growth Differ 1998;9:777–85.
D'Cruz CM, Moody SE, Master SR, Hartman JL, Keiper EA, Imielinski MB, et al. Persistent parity-induced changes in growth factors, TGF-beta3, and differentiation in the rodent mammary gland. Mol Endocrinol 2002;16:2034–51.
Booth BW, Smith GH. Roles of transforming growth factor-alpha in mammary development and disease. Growth Factors. 2007;25(4):227–35.
Gouon-Evans V, Lin EY, Pollard JW. Requirement of macrophages and eosinophils and their cytokines/chemokines for mammary gland development. Breast Cancer Res. 2002;4:155–64.
Schwertfeger KL, Rosen JM, Cohen DA. Mammary gland macrophages: pleiotropic functions in mammary development. J Mammary Gland Biol Neoplasia. 2006;11:229–38.
Gyorki DE, Asselin-Labat ML, van Rooijen N, Lindeman GJ, Visvader JE. Resident macrophages influence stem cell activity in the mammary gland. Breast Cancer Res. 2009;11:R62.
Gouon-Evans V, Rothenberg ME, Pollard JW. Postnatal mammary gland development requires macrophages and eosinophils. Development. 2000;127:2269–82.
Robinson GW, Karpf AB, Kratochwil K. Regulation of mammary gland development by tissue interaction. J Mammary Gland Biol Neoplasia. 1999;4(1):9–19.
Durnberger H, Heuberger B, Schwartz P, Wasner G, Kratochwil K. Mesenchyme-mediated effect of testosterone on embryonic mammary epithelium. Cancer Res. 1978;38(11 Pt 2):4066–70.
Giancotti FG, Tarone G. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu Rev Cell Dev Biol. 2003;19:173–206.
Pontier SM, Muller WJ. Integrins in mammary-stem-cell biology and breast-cancer progression—a role in cancer stem cells? J Cell Sci. 2009;122(Pt 2):207–14.
Taddei I, Faraldo MM, Teuliere J, Deugnier MA, Thiery JP, Glukhova MA. Integrins in mammary gland development and differentiation of mammary epithelium. J Mammary Gland Biol Neoplasia. 2003;8(4):383–94.
Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, et al. Generation of a functional mammary gland from a single stem cell. Nature 2006;439:84–8.
Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, et al. Purification and unique properties of mammary epithelial stem cells. Nature 2006;439:993–7.
Woodward TL, Mienaltowski AS, Modi RR, Bennett JM, Haslam SZ. Fibronectin and the alpha(5)beta(1) integrin are under developmental and ovarian steroid regulation in the normal mouse mammary gland. Endocrinology. 2001;142(7):3214–22.
Naylor MJ, Li N, Cheung J, Lowe ET, Lambert E, Marlow R, et al. Ablation of beta1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J Cell Biol 2005;171:717–28.
Taddei I, Deugnier MA, Faraldo MM, Petit V, Bouvard D, Medina D. Beta1 integrin deletion from the basal compartment of the mammary epithelium affects stem cells. Nat Cell Biol 2008;10:716–22.
Fassler R, Meyer M. Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 1995;9(15):1896–908.
Li N, Zhang Y, Naylor MJ, Schatzmann F, Maurer F, Wintermantel T, et al. Beta1 integrins regulate mammary gland proliferation and maintain the integrity of mammary alveoli. EMBO J 2005;24:1942–53.
Fata JE, Werb Z, Bissell MJ. Regulation of mammary gland branching morphogenesis by the extracellular matrix and its remodeling enzymes. Breast Cancer Res. 2004;6(1):1–11.
Kass L, Erler JT, Dembo M, Weaver VM. Mammary epithelial cell: influence of extracellular matrix composition and organization during development and tumorigenesis. Int J Biochem Cell Biol. 2007;39(11):1987–94.
Paszek MJ, Weaver VM. The tension mounts: mechanics meets morphogenesis and malignancy. J Mammary Gland Biol Neoplasia. 2004;9(4):325–42.
Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol 1997;137:231–45.
Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005;8:241–54.
Martins-Green M, Bissell MJ. Cell-ECM interactions in development. Semin Dev Biol. 1995;6(2):149–59.
Russo J, Lynch H, Russo IH. Mammary gland architecture as a determining factor in the susceptibility of the human breast to cancer. Breast J. 2001;7(5):278–91.
Ghajar CM, Bissell MJ. Extracellular matrix control of mammary gland morphogenesis and tumorigenesis: insights from imaging. Histochem Cell Biol. 2008;130(6):1105–18.
Burg T, Cass CA, Groff R, Pepper M, Burg KJ. Building off-the-shelf tissue-engineered composites. Philos Transact A Royal Soc. 2010;368(1917):1839–62.
Shekhar MP, Pauley R, Heppner G. Host microenvironment in breast cancer development: extracellular matrix-stromal cell contribution to neoplastic phenotype of epithelial cells in the breast. Breast Cancer Res. 2003;5:130–5.
Hu M, Polyak K. Molecular characterisation of the tumour microenvironment in breast cancer. Eur J Cancer. 2008;44:2760–5.
Arendt LM, Rudnick JA, Keller PJ, Kuperwasser C. Stroma in breast development and disease. Semin Cell Dev Biol. 2010;21(1):11–8.
Erler JT, Weaver VM. Three-dimensional context regulation of metastasis. Clin Exp Metastasis. 2009;26(1):35–49.
Alcaraz J, Nelson CM, Bissell MJ. Biomechanical approaches for studying integration of tissue structure and function in mammary epithelia. J Mammary Gland Biol Neoplasia. 2004;9(4):361–74.
Krause S, Maffini MV, Soto AM, Sonnenschein C. A novel 3D in vitro culture model to study stromal-epithelial interactions in the mammary gland. Tissue Eng Part C Methods. 2008;14(3):261–71.
Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, Briand P, et al. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci USA 1998;95:14821–6.
Lee GY, Kenny PA, Lee EH, Bissell MJ. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods. 2007;4(4):359–65.
Dhimolea E, Maffini MV, Soto AM, Sonnenschein C. The role of collagen reorganization on mammary epithelial morphogenesis in a 3D culture model. Biomaterials. 2010;31(13):3622–30.
Muthulekha S, Eddy JM, Burg KJL, Dreau D. Matrix compositions in the development of breast acini and ducts in 3D cultures. Matrix Biol 2010; (In Press).
Patrick Jr CW, Chauvin PB, Hobley J, Reece GP. Preadipocyte seeded PLGA scaffolds for adipose tissue engineering. Tissue Eng. 1999;5(2):139–51.
Halbleib M, Skurk T, de Luca C, von Heimburg D, Hauner H. Tissue engineering of white adipose tissue using hyaluronic acid-based scaffolds. I: in vitro differentiation of human adipocyte precursor cells on scaffolds. Biomaterials. 2003;24(18):3125–32.
Alhadlaq A, Tang M, Mao JJ. Engineered adipose tissue from human mesenchymal stem cells maintains predefined shape and dimension: implications in soft tissue augmentation and reconstruction. Tissue Eng. 2005;11(3–4):556–66.
Choi YS, Park SN, Suh H. Adipose tissue engineering using mesenchymal stem cells attached to injectable PLGA spheres. Biomaterials. 2005;26(29):5855–63.
Kang X, Xie Y, Powell HM, James Lee L, Belury MA, Lannutti JJ, et al. Adipogenesis of murine embryonic stem cells in a three-dimensional culture system using electrospun polymer scaffolds. Biomaterials 2007;28:450–8.
Horning JL, Sahoo SK, Vijayaraghavalu S, Dimitrijevic S, Vasir JK, Jain TK, et al. 3-D tumor model for in vitro evaluation of anticancer drugs. Mol Pharm 2008;5:849–62.
Gomillion CT, Burg KJ. Stem cells and adipose tissue engineering. Biomaterials. 2006;27(36):6052–63.
Gomillion CT, Parzel CA, White RL, Burg KJL. Tissue engineering: breast. Encyclopedia of biomaterials and biomedical engineering. New York: Informa Healthcare, Taylor & Francis; 2007.
Hebner C, Weaver VM, Debnath J. Modeling morphogenesis and oncogenesis in three-dimensional breast epithelial cultures. Annu Rev Pathol. 2008;3:313–39.
Acknowledgements
The authors wish to acknowledge Eve E. Kingsley Booth for her illustration. This work was supported by the NSF Emerging Frontiers in Research and Innovation (#CBE0736007) and the Institute for Biological Interfaces of Engineering.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McCave, E.J., Cass, C.A.P., Burg, K.J.L. et al. The Normal Microenvironment Directs Mammary Gland Development. J Mammary Gland Biol Neoplasia 15, 291–299 (2010). https://doi.org/10.1007/s10911-010-9190-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10911-010-9190-0