Abstract
Like other skin appendages, the embryonic mammary gland develops via extensive epithelial–mesenchymal interactions. Early stages in embryonic mammary development strikingly resemble analogous steps in the development of hair follicles and teeth. In each case the first morphological sign of development is a localized thickening in the surface epithelium that subsequently invaginates to form a mammary, hair follicle or tooth bud. Similar sets of intersecting signaling pathways are involved in patterning the mammary, hair follicle and dental epithelium, directing placode formation, and controlling bud invagination. Despite these similarities, subsequent events in the formation of these appendages are diverse. The mammary bud extends to form a sprout that begins to branch upon contact with the mammary fat pad. Hair follicles also extend into the underlying mesenchyme, but instead of branching, hair follicle epithelium folds around a condensation of dermal cells. In contrast, teeth undergo a more complex folding morphogenesis. Here, we review what is known of the molecular and cellular mechanisms controlling early steps in the development of these organs, attempt to unravel both common themes and unique aspects that can begin to explain the diversity of appendage formation, and discuss human genetic diseases that affect appendage morphogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Embryonic surface epithelium appears first as a single layered ectodermal sheet covered by a periderm that is shed towards the end of embryogenesis. While much of the surface ectoderm stratifies to become the epidermis with its protective, cornified outer layer, subsets of surface ectodermal cells undergo complex reciprocal interactions with underlying mesenchymal cells to form a variety of appendages, including hair follicles and teeth as well as mammary glands (Fig. 1). While timing and site of induction for these various appendageal structures differ, early steps in their formation exhibit remarkable similarities at the morphogenetic and molecular levels. In this review, we will compare the cellular and signaling events that shape ectodermal appendages. We will attempt to unravel both common themes and unique aspects in the morphogenesis of diverse appendages that have implications not only for understanding appendage development, but also for elucidating common disease mechanisms and designing strategies for regeneration of certain appendages, such as hair follicles and teeth, in cases of congenital absence or loss through disease.
Overview of Embryonic Mammary Gland Development
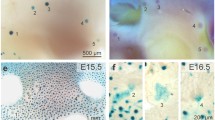
Mammary gland development is confined to two stripes of ventral lateral surface ectoderm, known as the mammary lines, that appear in response to mesenchymal signals [1, 2]. In embryos of some species, such as human and rabbit, mammary lines can be visualized by scanning electron micrography (SEM) as ridges of raised ectoderm. These are not apparent in SEM of mouse embryos; however, they can be identified histologically as lines of thickened surface ectoderm that appear at approximately embryonic day (E) 10.5 and overlie condensed mesenchymal cells [3]. Ectodermal thickening appears to be the result of pseudostratification of the surface ectoderm [3]. Within the next 24 h, the mammary line resolves into discrete lens-shaped thickenings, known as placodes, at sites of future mammary gland development [2] (Fig. 2a). Scanning electron microscopy of rabbit embryos reveals apparently motile cells atop the mammary lines, suggesting that mammary placodes may arise from migration and accumulation of motile cells at defined locations along the lines [4]. Between E11.5 and E12.5 in mice, the placodes invaginate to form bud structures (Fig. 2a; Fig. 3a). At the same time, mesenchymal cells surrounding the buds condense further to form a dense mammary mesenchyme (Fig. 3a) that expresses the androgen receptor, and epithelial cells overlying the bud differentiate into specialized nipple skin. Until this stage the mitotic index in mammary buds is lower than in the surrounding epidermis, consistent with the idea that displacement of epithelial cells, rather than increased proliferative activity, accounts for early morphological changes [1, 5]. However, definitive proof of cell displacement in the mammary region is lacking.
Comparison of the histology of embryonic mammary gland (a), hair follicle (b) and tooth (c) at the bud stage. Note that the mammary bud is shown at a more advanced stage than the hair follicle and tooth buds. All three embryonic buds show invagination of epithelial cells into the underlying mesenchyme, and condensation of adjacent mesenchymal cells.
In male embryos of many mouse strains, the mammary buds become separated from the surface ectoderm between E14.5 and E15.5 under the influence of fetal androgens, and eventually degenerate. In female embryos the buds remain quiescent from E14.5 until approximately E16, when the epithelial cells begin to proliferate and each bud extends through the mesenchyme into the developing fat pad located in the dermis, forming a mammary sprout. Once the sprout reaches the fat pad it starts to branch and subsequently a lumen forms within the epithelium (Fig. 2a). Normal development of the epithelium depends on interactions with the fat pad [6]. At birth the mammary gland is composed of a primary duct and 15–20 secondary branches invested in the mammary fat pad.
Molecular Regulators of Embryonic Mammary Development
Formation of the Mammary Line
The mammary line is marked in mouse embryos at E10.5 by expression of a TOPGAL transgene [7] that is sensitive to activation of the WNT/β-catenin signaling pathway [8]. TOPGAL is activated both in the epithelial line and in underlying mesenchymal cells. Ectopic expression of the secreted WNT inhibitor Dickkopf1 (Dkk1) in the surface ectoderm abolishes TOPGAL expression as well as morphologic and molecular markers of mammary placode development, indicating that WNT/β-catenin signaling plays an essential role in placode formation [7]. Of those Wnt genes whose expression in the mammary region has been examined to date, Wnt10b shows the earliest localized expression, appearing as a series of discontinuous streaks along the mammary line [3, 7]. Ectopic Dkk1 inhibits localized expression of Wnt10b, indicating that Wnt10b upregulation requires an earlier WNT signal.
Fgf10 is expressed in ventral somites prior to appearance of the mammary line, is required for formation of four of the five pairs of mammary placodes in mouse embryos, and is unaffected by surface ectodermal expression of Dkk1 [7, 9]. Fgf10 is required for TOPGAL and Wnt10b expression in the mammary line except for the region encompassing placode 4, whose development is unaffected in Fgf10-null embryos. These observations suggest that Fgf10 signaling acts upstream of WNT activation in most of the mammary line [7, 10]. FGF10 secreted from somitic cells may interact directly with FGFR2B expressed in the surface epithelium, as FGF receptor expression was not detected in somites or dermal mesenchyme, and Fgfr2b-null embryos have mammary phenotypes similar to those of Fgf10-null mutants [2, 10]. Defects in the mammary line are detected in Gli3 and Pax3 mutants that display altered somitic expression of Fgf10 [10].
Another potential regulator of WNT pathway activity in the mammary line is the ERBB4 ligand Neuregulin 3 (NRG3). Aberrant expression of Nrg3 causes altered mammary specification, and exogenous Nrg3 can induce ectopic mammary bud formation [11]. The timing and localization of initial expression of Nrg3 and Erbb4 are similar to that of TOPGAL, and exogenous Nrg3 can enhance expression of another WNT reporter transgene, BAT-gal [12], in the mammary line [11]. It is not yet known whether TOPGAL or BAT-gal activity is affected in Nrg3 mutants, or how Fgf10 signaling impacts on Nrg3 expression.
Development of Mammary Placodes and Buds
Formation of four of the five pairs of mammary placodes in mouse embryos requires the FGF receptor 2b, the WNT pathway transcription factor LEF1, and the T-box transcription factor TBX3, all of which are expressed in placodes (reviewed in [13]). In addition, genes encoding another FGF receptor, FGFR1b, and several FGF ligands are expressed in mammary placodes [13]. Forced activation of WNT/β-catenin signaling in cultured mouse embryos by incubation in LiCl or WNT3A causes accelerated development of expanded placodes; however expression of the TOPGAL WNT reporter transgene is restricted to regions close to the mammary line, underscoring the importance of additional factors that must control competence for response to WNT [7]. WNT inhibition blocks localized expression of both Tbx3 and Lef1 in the mammary region, indicating that these genes lie downstream of an initial WNT signal [7, 14]. Loss of Tbx3 function prevents localized expression of Wnt10b and Lef1 [15], and treatment of cultured embryos with an FGF inhibitor blocks expression of Tbx3 and Lef1 [14].
Another important regulator of mammary placode development is p63, a member of the p53 tumor suppressor gene family. p63 is expressed in the embryonic ectoderm and in self-renewing basal layers of all stratified epithelia in the adult. Two classes of p63 transcripts are produced that either code for, or lack a potent N-terminal transactivation domain (TA and ΔN isoforms, respectively) [16]. Ablation of p63 in mice leads to total lack of multilayered epidermis and its derivatives including teeth, hair follicles, and mammary glands [17, 18]. Localized Lef1 expression is not detected in the mammary region of p63-null embryos suggesting that mammary placodes fail to form [17]. Interestingly, p63 seems to regulate splicing of Fgfr2 transcripts such that the 3b isoform is preferentially produced in p63 mutants [19]. Absence of Fgfr2b may account for failed mammary bud development in p63-null embryos [9]. However, whether the mammary line is properly defined in the absence of p63, and the precise relationship of this factor to WNT activation, are currently unknown. A model of possible interactions between p63, Fgf10, Nrg3, WNT, Tbx3 and Lef1 in mammary placode formation is depicted in Fig. 4a.
Invagination of mammary placodes to form buds, and maintenance of buds, appears to require WNT/β-catenin signaling, as embryos lacking Lef1 form four pairs of small placodes that degenerate prior to bud development [13]. The homeodomain-containing transcription factor gene Msx1 is expressed in mammary bud epithelium, and the related gene Msx2 is expressed in both epithelial and mesenchymal cells. While loss of function of either of these genes alone does not affect bud formation, in Msx1-/-; Msx2-/- double mutants mammary development is arrested at the placode stage, revealing an essential role(s) for these factors in bud morphogenesis [13].
Development and Branching of the Mammary Sprout
Elongation of mammary buds to form a sprout requires parathyroid hormone-related protein (PTHrP), expressed in the epithelium, and its receptor, PTH1R, expressed in the mesenchyme [13]. Mammary buds form in embryos lacking either of these proteins, but sprout development, differentiation of specialized mammary mesenchyme, and formation of the nipple do not occur. Remarkably, when PTHrP is overexpressed in the epidermis, the entire ventral surface of the embryo is transformed into nipple skin [20, 21].
WNT/β-catenin signaling is activated in a subset of epithelial cells accompanying initial branch formation [7], but its functional significance is not yet known. Failure of branching morphogenesis in embryonic mammary glands is observed in mice null for the homeodomain transcription factor gene Msx2 [22], or lacking EGF receptor, the EGFR ligand amphiregulin (AREG) or the transmembrane metalloproteinase ADAM17 that releases AREG from its inactive transmembrane form [23]. In addition, mammary buds from RhoGAP p190B-null embryos fail to show ductal outgrowth when transplanted into cleared fat pads, indicating an essential role for this negative regulator of Rho in ductal development [24].
Overview of Hair Follicle Development
Like mammary glands, hair follicles develop via extensive interactions between cells of the surface ectoderm and underlying dermal cells [25–27]. The first morphological sign of hair follicle formation is the development of a regular array of placodes, or local thickenings, in the surface ectoderm. Placode formation is thought to require signals from the dermis [25] (Fig. 2b). Signaling from each placode to the dermis causes the formation of a cluster of mesenchymal cells, known as a dermal condensate (Fig. 3b) [25]. Reciprocal signaling from the dermal condensate to the epithelium results in the proliferation and downgrowth of epithelial cells into the dermis, forming a hair germ. The hair germ elongates, and the epithelial cells surround the dermal condensate, known from this stage on as the dermal papilla. The follicular epithelium differentiates to form several concentric cell layers of the hair shaft and inner root sheath [28]. An outer root sheath that is contiguous with the epidermis surrounds the inner root sheath, and the follicle is bound by a connective tissue sheath. Production of the hair shaft and inner root sheath is dependent on signaling between the dermal papilla and the follicular epithelium [29], and also requires communication between cells in different epithelial layers of the follicle [26].
Molecular Regulators of Hair Follicle Development
The Nature of the “First Dermal Signal” Initiating Hair Follicle Development
The nature of the first dermal signal that initiates hair follicle development is not known. Experiments in the chick suggest that the dermal signal that initiates feather formation is uniform, rather than periodically localized [30]. Further experiments in the chick suggest Fgf10 as a component of the dermal signal, as this gene is expressed in the dermis and a blocking antibody to FGF10 suppresses morphological and molecular indicators of placode formation [31]. Consistent with an early role for FGF signaling in hair follicle morphogenesis, disruption of the mouse Fgfr2b gene causes defective skin development as well as decreased initiation of placode formation [32]. Fgf10-/- embryos do not display this defect, indicating that, if it plays a role in hair follicle initiation, FGF10 must act redundantly with one or more other FGF family members. Activin may also be a component of the dermal signal, based on its mesenchymal site of expression and its ability to induce the placode promoting factor Edar (see below) in embryo skin explants [33]. Nuclear localization of β-catenin, an indication of WNT/β-catenin signaling pathway activity, is detected in the upper dermis 2 days before initiation of feather placode development in the chick, suggesting that this pathway may also play a role in generating the dermal signal [34]. Consistent with this, Lef1 is expressed in the mesenchyme of the mouse vibrissa pad prior to vibrissa follicle development, and initiation of vibrissa follicle development is dependent on mesenchymal Lef1 expression [35].
Development of Hair Follicle Placodes
The initiating dermal signal has been suggested to trigger expression of molecules that either promote or repress follicle fate and compete with one another, resulting in the establishment of a regular array of follicles [36, 37]. Consistent with this model, several positive and negative regulators of hair follicle fate are initially expressed uniformly in the epidermis and subsequently become localized to placodes [26]. In TOPGAL WNT reporter transgenic skin, expression of the reporter is detected in placodes and dermal condensates, providing evidence that the WNT pathway is active in both epithelial and mesenchymal components of developing follicles (reviewed in [26]). Expression of a stabilized form of β-catenin in chick or mouse epidermis causes formation of ectopic follicles, indicating that activation of the WNT signaling pathway in the epithelium is sufficient to direct follicle development [38]. Conversely, loss of function of the β-catenin gene in mouse epidermis results in defective placode development [39], and a null mutation in the mouse gene encoding WNT effector protein LEF1, causes failure of formation of vibrissae and two-thirds of the body hair follicles [35, 40]. The P63 transcription factor is also required for hair follicle placode formation; in mice lacking this factor, hair placodes are not morphologically visible, and localized expression of placodal marker genes is not detected [41].
Ectodysplasin (EDA), a molecule related to tumor necrosis factor, and its receptor ectodysplasin receptor (EDAR) act early in the development of several classes of hair follicle in the mouse, including the earliest developing (primary or guard), and late developing zigzag underfur follicles, but are not required for the formation of awl and most vibrissa (whisker) follicles (reviewed in [26, 27]). Histological changes suggesting pre-placode initiation can be detected transiently in Eda mutant (Tabby) and Edar mutant (downless) embryos, but most placode markers are not expressed [33, 42]. Conversely, over-expression of the A1 isoform of EDA results in formation of hair follicle placodes of increased size, and treatment of embryonic skin with recombinant EDA-A1 in vitro promotes placodal cell fate in a dose-dependent manner [43]. The Eda-null and Edar-null phenotypes are mimicked by ectopic expression of a super-repressor of NF-κB signaling [44], and NF-κB signaling is suppressed in skin epithelium, developing teeth and mammary glands of Eda-null and Edar-null mutants [42] (M. Mikkola, unpublished observations). These findings suggest that Eda and Edar act via NF-κB at early stages of primary and zigzag hair follicle formation, and that alternate signaling pathways control development of awl and whisker follicles. The homeobox-containing genes Msx1 and Msx2 are expressed in placodes [45], and mutant mice lacking both of these genes have reduced numbers of hair follicles [22], indicating that MSX transcription factors also promote placode fate. However, their relationship with other molecular regulators of hair follicle placode formation is not yet clear.
Regulation of Hair Follicle Placode Spacing
The WNT inhibitor Dkk1 is endogenously expressed in dermal cells surrounding developing hair follicles. Endogenous Dkk1 is suppressed by ectopic keratin 14 promoter-driven Dkk1, suggesting that WNT signaling may normally induce Dkk1 expression as part of a negative feedback loop to limit hair follicle growth and perhaps regulate follicular spacing [46]. Members of the bone morphogenetic protein (BMP) family of secreted signaling molecules can also act to inhibit hair follicle formation. Several Bmp family members are endogenously expressed in hair and feather follicle placodes and dermal condensates, and their ectopic expression suppresses feather bud formation [26]. A null mutation in the gene encoding the BMP inhibitor Noggin causes formation of fewer hair follicles than normal and retarded follicular development; conversely, ectopic expression of Noggin in chick or mouse embryonic skin causes enlarged and ectopic follicles [47, 48]. Consistent with an inhibitory role for BMP signaling in embryonic hair follicle development, loss of function of BMP receptor IA in the epithelium leads to accelerated hair follicle formation [49]. A model for possible interactions of WNT, EDA, SHH and BMP factors in hair follicle placode development is depicted in Fig. 4b.
Formation of Hair Follicle Buds
Induction of the dermal condensate fails in the absence of epithelial β-catenin [39] suggesting a requirement for epithelial WNT signaling in generating secreted signals that direct clustering of mesenchymal cells. In contrast, the secreted signaling factor Sonic hedgehog (SHH) is not required for formation of the dermal condensate, but instead acts within the follicular epithelium via Gli2 and N-myc to control proliferation and downgrowth of follicular cells at the bud stage [50–53]. Expression of Shh and its target/effector Gli1 is absent from the skin of mice ectopically expressing Dkk1, or lacking epithelial β-catenin, Eda or Edar, and is detected upon rescue of Eda-null skin with recombinant EDA, indicating that Shh lies downstream of WNT and EDA/ EDAR signaling [33, 39, 46, 54, 55].
Mice lacking a functional Tgfβ2 gene have reduced numbers of hair follicles and a delay in follicular morphogenesis [56]. Tgfβ2 is required for expression of Snail and down regulation of E-cadherin expression, both of which are correlated with hair follicle bud formation [57]. Down regulation of E-cadherin also requires a combination of Noggin, which activates Lef1 expression, and stabilized beta-catenin, induced by a WNT signal [58]. These observations suggest that several different signals converge to control E-cadherin expression, and provide a possible link between transcription and cell adhesion in the bud.
Differentiation of the Hair Shaft and Inner Root Sheath
As the hair follicle bulb appears, at least seven different epithelial cell layers constituting components of the mature hair follicle are formed, including the three layers of the inner root sheath, as well as the cuticle, cortex and medulla of the hair shaft [28]. Signaling by epithelial BMP receptor 1A is essential for matrix cells in the follicular bulb to cease proliferating and differentiate into any of these structures [49, 59–61]. The transcription factor GATA3 is required for inner root sheath differentiation [62], and Notch signaling is essential for maintenance of this structure [63], while differentiation of the outer root sheath requires the HMG-box-containing gene Sox9 [64]. Other transcription factors required for hair shaft differentiation include a homeobox protein HOXC13 [65], and a winged-helix/forkhead transcription factor FOXN1 (formerly WHN) [66–68], which is mutated in nude mice and regulates expression of an acidic hair keratin gene [69].
Overview of Tooth Development
Tooth morphogenesis is governed by interactions between the oral ectoderm and neural crest-derived ectomesenchyme (for reviews see [70–72]). Initiation of individual teeth is preceded by the formation of dental lamina, a horseshoe shaped stripe of thickened epithelium marking the future dental arch (E11 in mouse embryos). All mammalian teeth bud from the dental lamina, and in this respect dental lamina resembles the mammary line. In mouse, where a toothless diastema region separates the incisor from three molar teeth in each jaw quadrant, the anatomical nature of the dental lamina has been debated [73, 74]. The odontogenic field can, however, be visualized by epithelial molecular markers such as Pitx2 [75, 76], a homeobox transcription factor required for tooth morphogenesis to proceed beyond the placode/early bud stage [77, 78], and Shh [79].
By E12 in mouse embryos, one incisor and one molar placode emerge from the dental lamina. The second molar placode buds from the posterior end of the first molar placode, and the third molar buds off from the second one. Between E12 and E13, dental placodes invaginate further into the mesenchyme which condenses around the nascent tooth bud (Fig. 2c; Fig. 3c). After the bud stage, cells at the tip of the bud form the primary enamel knot, a histologically recognizable aggregate of cells that controls the shape of the tooth crown [70, 80] (Fig. 2c). The enamel knot is a transient signaling center consisting of non-proliferating cells expressing more than ten different growth factors, most of which are also expressed in the nascent dental placodes [81]. The dental epithelium lateral to the enamel knot expands and folds, becoming cap-shaped by E14–E15, and enveloping mesenchymal cells which form the dental papilla and later give rise to dentin-secreting odontoblasts and the tooth pulp. At this stage the epithelial cells differentiate into outer enamel epithelium, stellate reticulum, stratum intermedium, and inner enamel epithelium, lying adjacent to the papilla. By the bell stage (E16 onwards) the enamel knot is removed by apoptosis, and secondary enamel knots form sequentially within the epithelium marking the tips of future cusps and thereby patterning the tooth crown. Dental papilla cells adjacent to the epithelium differentiate into odontoblasts, while cells of the inner enamel epithelium differentiate into enamel-producing ameloblasts [82].
Molecular Regulators of Tooth Development
Establishment of the Dental Field
Classic tissue recombination experiments indicate that before the formation of individual tooth primordia (E9–E11), odontogenic potential resides in the presumptive dental epithelium that can induce tooth formation in neural-crest mesenchyme even from regions normally not participating in tooth development [74, 83]. From the placode stage (E12) onwards this capacity shifts from the epithelium to the mesenchyme. This transition is preceded by a period when expression of many mesenchymal transcription factors such as Pax9 (see below) becomes independent of the epithelium [84–86], and coincides with a shift in expression of Bmp4 from epithelium to mesenchyme [87].
Regional specification of the tooth area is thought to be dependent on antagonistic effects of Fgf8, Fgf9 and Bmp4 expressed in the epithelium [70, 71]. At early stages of development, their spatial distribution is believed to instruct regionalized expression of mesenchymal transcription factors and thereby pattern the jaws into proximal (molar forming) and distal (incisor forming) domains, possibly even prior to dental lamina formation [88–90]. Fgf8 can induce expression of the homeobox gene Pitx2 while BMP4 represses Pitx2 expression [91]. Slightly later, but still prior to morphological signs of odontogenesis, interplay of FGFs and BMPs positions the expression of Pax9 in prospective molar and incisor mesenchyme [84]. Although tooth development is arrested at the bud stage in Pax9 deficient mice, tooth primordia develop in their correct locations implying that other genes are also involved in defining the location of teeth [92].
Development of the Dental Lamina
The cellular mechanisms of dental lamina formation are poorly understood. Interestingly, as seen in the mammary line, it appears that dental lamina formation does not result from local increase in mitotic activity, but rather is associated with changes in orientation of mitotic spindles such that they become perpendicular to oral epithelium instead of the generally parallel orientation [93]. Although this might suggest a stratification mechanism similar to that occurring in the epidermis [94], loss of p63 function prevents epidermal stratification but does not affect formation of the dental lamina [41].
Formation of Dental Placodes and Buds
Although the dental lamina forms in p63 mutants, dental placode formation is abolished in these embryos [17, 18, 41]. p63 is essential for expression of several signaling molecules in the ectoderm, including Fgfr2b, Bmp7, Jagged1, and Notch1, indicating that these lie downstream of p63 and suggesting that p63 activates multiple signaling pathways involved in ectodermal organogenesis [41]. In double knockout mice for transcription factors Msx1 and Msx2, or Dlx1 and Dlx2, tooth development is arrested at the placode-early bud stage (in compound Dlx mutants only upper molars are affected) [95, 96]. Both FGFs and BMPs induce expression of Msx1 and Dlx2; Msx2 is induced by BMP4, and Dlx1 by FGF, highlighting the importance of these signaling pathways in regulating invagination of dental lamina [87, 96, 97]. A loss of function mutation in Pitx2 causes arrest of tooth development at the placode-early bud stage, and is associated with failure to maintain ectodermal Fgf8 expression, with expanded Bmp4 expression [78]. Mice ectopically expressing the secreted WNT inhibitor Dkk1 under control of a keratin 14 promoter also display arrested dental development at the dental lamina-early bud stage, indicating an essential role for WNT/β-catenin signaling in bud development [46]. Mice deficient in Eda do not display altered dental placode development [98, 99]; however forced expression of Eda-A1 promotes survival of an extra dental placode normally found anterior to the first molar in wild type embryos suggesting that this molecule can function to promote dental placode survival [43, 100]. Likewise, in mice deficient for ectodin, a secreted modulator of BMP and WNT pathways, an extra tooth develops from a similar ectopic placode [80]. Whether Eda and ectodin function in the same or in parallel pathways to stimulate placode formation is currently unknown. Interestingly, an extra tooth develops in this location in embryos carrying a hypomorphic mutation in polaris, a protein required for the assembly of cilia [101].
Embryos null for two downstream mediators of Shh, Gli2 and Gli3, show no sign of molar development, revealing an important role for Shh signaling in molar development; however aberrant incisor buds are formed in Gli2/3 double mutants [102]. It is not clear whether formation of incisor buds is due to signaling through Gli1 in these mutants or through Shh-independent signaling pathways. A schematic summary of the roles of signaling and transcription factors in dental lamina and placode development is shown in Fig. 4c.
Shh acts within the epithelium to regulate proliferation during odontogenesis [103–105], analogous to its role in developing hair follicles. Mutation of Shh or its effector Smoothened causes abnormalities in tooth shape, possibly due to defective epithelial proliferation [104, 105]. Intriguingly, in embryos null for the protein kinase IKKα, incisor and whisker epithelia grow outwards instead of invaginating into the mesenchyme [106], indicating that IKKα regulates the direction of epithelial growth. The mechanism by which this occurs does not involve NFκB signaling, but is not yet understood.
Transition from the Bud to Cap Stage
Folding at the base of the tooth bud and formation of the enamel knot begin at the late bud stage [74, 107]. These processes fail to occur in mice lacking the transcription factors LEF1, MSX1 and PAX9, which are targets of WNT, BMP and FGF pathways, respectively [35, 92, 96]. Interestingly, dental mesenchymal BMP4 expression is lost in Msx1 and Pax9 mutants. Msx1 deficiency can be rescued, at least to the cap stage, by exogenous BMP4 emphasizing the importance of BMP4 for development of the cap stage and induction of the enamel knot [108–110]. Consistent with a role for BMP signaling from the mesenchyme to the epithelium in regulating transition from the bud to the cap stage, epithelial-specific deletion of Bmpr1a causes arrested tooth development at the bud stage [49]. FGF4, a direct transcriptional target of LEF1 in the enamel knot, can overcome developmental arrest in Lef1 null teeth [111]. As LEF1 is required only at a single step in tooth morphogenesis [111] it may act redundantly with other TCF molecules in mediating canonical WNT signaling at other developmental stages. Runx2, encoding a runt domain transcription factor essential for bone development, functions in the mesenchyme and is essential for mediating FGF signaling during the transition from bud to cap stages [112, 113].
Tooth Shape
At the bell stage, induction of secondary, non-proliferating, enamel knots together with proliferation of the rest of the epithelium results in formation of multiple cusps. The species and tooth type specific cusp patterns are thought to be fixed by the positions of secondary enamel knots that express multiple signaling factors [70]. Normal cusp patterning requires the TNF family member Eda acting via the NFκB pathway [98, 106, 114]. In mice lacking the BMP inhibitor ectodin, enamel knots are expanded and cusp patterns irregular, revealing a key role for BMP inhibition in cusp patterning [80].
Common and Unique Strategies in Appendage Development
Initiation of Appendage Development
Induction of the various types of skin appendage is strictly regulated with respect to developmental stage and body region. Thus, mammary gland initiation is confined to two bilateral stripes of cells running from forelimb to hindlimb; teeth develop along the dental laminae of the maxilla and mandible; vibrissae form in a specific region of the snout; and hair follicles are initiated at a later stage and develop all over the body with the exception of footpad/palm regions. As discussed earlier, current evidence suggests that for hair follicle and mammary gland development, initiating signals arise in the dermis, whereas an early epithelial signal is thought to initiate tooth development and can induce formation of dental structures in combination with neural crest derived mesenchyme or aggregated embryonic or adult stem cells [115]. Whether these apparent differences in the site of initiating signal reflect regional differences in signaling capacities of surface epithelium versus mesenchyme, or are due to the use of different embryonic stages in recombination studies, is not entirely clear.
Regional specification of the tooth area is thought to depend on antagonistic effects of epithelial BMP and FGF factors [70, 71]. Less is understood about initiating signals for mammary gland and hair follicle development, although FGF signaling may play a role in both processes [7, 9, 32], and activation of dermal WNT/β-catenin signaling is observed under both mammary line [7] and dental lamina (E. Y. Chu and S. E. Millar, unpublished data).
Do Mammary, Tooth and Hair Follicle Placodes Arise through Similar Mechanisms?
The mammary line and the dental lamina are morphologically similar, and in both cases these thickened epithelial lines resolve to form a series of localized placodes. As discussed above, circumstantial data suggest that in the case of the mammary line, the formation of placodes could involve directed cell movement within the mammary line. Such evidence is not available for the dental lamina, and prevailing models favor the idea that positive and negative regulators of dental placode induction compete to establish final positions for these structures. In contrast, hair follicle development is first apparent in epithelium at the placode stage. Morphologically, the placodes first appear as broad circular thickenings, and gradually become refined to circles of smaller diameter as they begin to invaginate. Gradual restriction of placode fate regulator expression is observed, consistent with the competitive model described above (reviewed in [26, 27]). However, cell displacement from a broad placode into an invaginating bud of smaller diameter may contribute to hair follicle bud formation.
Ectopic expression of the Dkk1 WNT inhibitor under control of a keratin 14 promoter blocks formation of hair follicle and mammary gland placodes, indicating that for these organs, placode development requires WNT signaling [7, 46]. In contrast, tooth development is blocked at the dental lamina-early bud stage in K14-Dkk1 mice [46]. It is currently not clear whether this reflects a relatively later requirement for WNT signaling in dental development, or is due to inefficient inhibition of WNT activity in the oral cavity at the stage when tooth morphogenesis is initiated. FGF signaling is also required at early stages in the formation of mammary, tooth and hair follicle placodes [9, 32, 86], although the specific FGF ligands, or combinations of ligands, involved may differ. p63 is required for development of mammary, hair follicle and dental placodes [17, 18, 41]. It is not yet known whether this is also true for the mammary line. Finally, MSX transcription factors are required for hair follicle, dental and mammary bud formation [22, 96], although how Msx gene expression is controlled has not been investigated in any detail for developing hair follicles and mammary glands. Thus, from what is known to date, several signaling pathways and transcription factors appear to play conserved roles in placode and bud formation of diverse appendages. The effects of mutations in regulatory factors on early stages of embryonic mammary gland, hair follicle and tooth development are summarized in Table 1.
Despite these similarities in molecular regulation of early stages of appendage formation, some striking differences in pathway utilization are noted for each organ type (see Table 1). Sonic hedgehog signaling plays key roles in regulating proliferation in both hair follicle and dental epithelia, and yet is dispensable for development of primordial mammary glands [116]. It is possible that Shh acts redundantly with other hedgehog family members in embryonic mammary development, but this has yet to be demonstrated. Alternatively, distinct requirements for Shh may be in part responsible for differences in the ways that hair follicle, dental and mammary buds proliferate. Indeed, current evidence suggests that Shh causes expansion of a sub-population of epithelial cells in the tooth bud, contributing to the non-uniform shape of molar teeth [105]. EDA/EDAR signaling is necessary for maintenance of primary hair follicle placodes [42] but is not essential for formation or maintenance of awl hair, vibrissa, mammary or dental placodes [37, 98–100]. Forced expression of Eda-A1 in surface ectodermal cells causes formation of an ectopic mammary placode and dental placode within the mammary line and dental lamina regions respectively, eventually producing an ectopic mammary gland and tooth [43, 100]. These data indicate that Eda-A1 is capable of promoting formation of these structures. Eda does not stimulate cell proliferation but rather appears to expand placode cell fate by promoting incorporation of neighboring cells into nascent placodes [43]. It is possible that related TNF and TNF receptor family members (such as Troy and XEDAR) might act redundantly with EDA/EDAR in development of mammary, dental and awl hair follicle placodes. However, expression of an NF-kB super-repressor recapitulates Eda-null and Edar-null phenotypes [44], and NF-kB reporter activity is absent from awl follicle placodes of Eda-null and Edar-null mutant embryos [27], as well as from dental and mammary placodes in these mutants (M. Mikkola, unpublished data). Thus it is more likely that an alternate, parallel signaling pathway is responsible for maintenance and further development of placodes that are unaffected by loss of EDA/EDAR/NFκB signaling.
In addition to these differences in pathway utilization, a number of transcription factors have been found to function differently in the development of each appendage. For instance, Tbx3 is required for formation of mammary but not vibrissa buds [15], and Pitx2-null mice show defects in tooth bud formation [117], but defects in mammary or hair follicle buds have not been described. Further work will be needed to determine whether these phenotypes are due to functional redundancy with other Tbx and Pitx family members, or reflect differences in gene activation that could explain some of the morphogenetic differences between mammary, tooth and hair follicle buds.
Alterations in cell adhesion that allow controlled and limited dissociation and re-association of epithelial cells are likely to be essential for bud development. As discussed above, cell adhesion in hair follicle buds may be regulated in part via control of E-cadherin expression in response to cooperating TGF-β, Noggin and WNT signals [57, 58]. Analysis of conditional mutant mice has revealed that epithelial expression of β1 integrin is required for remodeling the basement membrane and hair follicle downgrowth [118, 119], and that α-catenin is also required for normal follicular morphogenesis [120]. Other studies suggest that remodeling of desmosomal and hemidesmosomal components is associated with both hair follicle and mammary bud formation [121, 122]. Detailed comparative studies of expression and function of cell adhesion molecules in hair follicle, mammary and tooth buds will be important for understanding mechanisms by which signaling molecules effect the morphological changes in epithelium and mesenchyme that are necessary for formation of diverse appendages.
Not surprisingly, molecular variations between developing mammary glands, hair follicles and teeth become more striking beyond the bud stage, as these organs undergo clearly distinct patterns of branching or folding morphogenesis and initiate unique programs of differentiation. How differences in signaling and transcription factor expression/activation result in the development of distinct classes of skin appendage will be a fascinating area for future study.
Human Diseases Linked to Appendage Formation
Several inherited and sporadic diseases in humans are caused by loss or gain of function mutations in signaling pathways that regulate appendage formation. These include Gardner’s syndrome, which is associated with loss of function mutation of the WNT pathway antagonist Adenomatous Polyposis Coli (APC), resulting in inappropriate WNT/β-catenin signaling and development of supernumerary teeth and odontomas as well as colon cancer [123, 124]. Loss of function of another WNT antagonist, AXIN2, results in hypodontia as well as colon cancers [125], suggesting that WNT activity must be tightly controlled for normal tooth development. Stabilizing mutations in the β-catenin gene are found in human pilomatricoma, a tumor of hair follicle matrix cells [126], and elevated WNT signaling is also associated with breast cancer in humans and in mouse models [127]. Similarly, deregulation of several other pathways required for embryonic appendage development, including the FGF, EGF and neuregulin signaling pathways, is oncogenic in mammary epithelium [128, 129]. Mutations of human P63 result in a number of dominant syndromes that affect development of the skin and its appendages including hair follicles, teeth and nails. These include ectrodactyly-ectodermal-dysplasia-clefting (EEC), ankyloblepharon-ectodermal dysplasia-clefting or Hay–Wells (AEC), acro-dermato-ungual-lacrimal-tooth (ADULT), and limb-mammary (LMS) syndromes, as well as non-syndromic split-hand/split-foot malformation (SHFM), all of which show a striking genotype-phenotype correlation [130, 131]. Mammary gland abnormalities are a consistent feature of LMS, and are occasionally observed in patients with EEC, AEC and ADULT. The EDA and EDAR genes are mutated in human anhidrotic ectodermal dysplasias, causing a decreased number of hair follicles, defects of the teeth and sweat glands and occasional mammary hypoplasia [132, 133]. Mutations in the SHH pathway target and repressor PTC1 cause Gorlin syndrome, characterized by developmental defects and basal cell carcinoma (BCC) [134–136], a tumor that has several characteristics in common with immature hair follicles, including similar histology, ultrastructure, and patterns of keratin gene expression [136–138]. The majority of sporadic BCCs are associated with PTC1 mutations [139], and inappropriate activation of hedgehog signaling is also associated with odontogenic tumors [140]. Consistent with its functions in the mouse, mutation of human TBX3 causes defects in mammary glands, teeth and limbs, but not hair follicles (Ulnar-mammary Syndrome) [141], and mutation of PITX2 (Rieger Syndrome) causes defects in teeth but is not associated with abnormal breast or hair development [142]. These findings reveal both shared and unique aspects of various human appendage developmental programs, and underscore the relevance of mouse models for dissecting mechanisms underlying human developmental syndromes and appendage tumors including breast cancer.
Abbreviations
- ADULT:
-
acro-dermato-ungual-lacrimal-tooth syndrome
- APC:
-
adenomatous Polyposis Coli
- AREG:
-
Amphiregulin
- AEC:
-
ankyloblepharon-ectodermal dysplasia-clefting syndrome
- BCC:
-
basal cell carcinoma
- BMP:
-
bone morphogenetic protein
- DKK1:
-
Dickkopf 1
- EDA:
-
Ectodysplasin
- EDAR:
-
edctodysplasin receptor
- EEC:
-
ectrodactyly-ectodermal-dysplasia-clefting syndrome
- E:
-
embryonic day
- FGFR:
-
fibroblast growth factor receptor
- LMS:
-
limb-mammary syndrome
- NRG3:
-
Neuregulin 3
- SHFM:
-
non-syndromic split-hand/split-foot malformation
- PTHrP:
-
parathyroid hormone-related protein
- SEM:
-
scanning electron microscopy
- SHH:
-
Sonic hedgehog
References
Sakakura T. Mammary embryogenesis. In: Neville MC, Daniel CW, editors. The mammary gland, development, regulation and function. New York and London: Plenum; 1987. p. 37–66.
Veltmaat JM, Mailleux AA, Thiery JP, Bellusci S. Mouse embryonic mammogenesis as a model for the molecular regulation of pattern formation. Differentiation 2003;71(1):1–17.
Veltmaat JM, Van Veelen W, Thiery JP, Bellusci S. Identification of the mammary line in mouse by Wnt10b expression. Dev Dyn 2004;229(2):349–56.
Propper AY. Wandering epithelial cells in the rabbit embryo milk line. A preliminary scanning electron microscope study. Dev Biol 1978;67(1):225–31.
Balinsky BI. On the pre-natal growth of the mammary gland rudiment in the mouse. J Anat 1950;84:227–35.
Sakakura T, Sakagami Y, Nishizuka Y. Dual origin of mesenchymal tissues participating in mouse mammary gland embryogenesis. Dev Biol 1982;91(1):202–7.
Chu EY, Hens J, Andl T, Kairo A, Yamaguchi TP, Brisken C, et al. Canonical WNT signaling promotes mammary placode development and is essential for initiation of mammary gland morphogenesis. Development 2004;131(19):4819–29.
DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 1999;126(20):4557–68.
Mailleux AA, Spencer-Dene B, Dillon C, Ndiaye D, Savona-Baron C, Itoh N, et al. Role of FGF10/FGFR2b signaling during mammary gland development in the mouse embryo. Development 2002;129(1):53–60.
Veltmaat JM, Relaix F, Le LT, Kratochwil K, Sala FG, van Veelen W, et al. Gli3-mediated somitic Fgf10 expression gradients are required for the induction and patterning of mammary epithelium along the embryonic axes. Development 2006;133(12):2325–35.
Howard B, Panchal H, McCarthy A, Ashworth A. Identification of the scaramanga gene implicates Neuregulin3 in mammary gland specification. Genes Dev 2005;19(17):2078–90.
Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, et al. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci USA 2003;100(6):3299–304.
Hens JR, Wysolmerski JJ. Key stages of mammary gland development: molecular mechanisms involved in the formation of the embryonic mammary gland. Breast Cancer Res 2005;7(5):220–4.
Eblaghie MC, Song SJ, Kim JY, Akita K, Tickle C, Jung HS. Interactions between FGF and Wnt signals and Tbx3 gene expression in mammary gland initiation in mouse embryos. J Anat 2004;205(1):1–13.
Davenport TG, Jerome-Majewska LA, Papaioannou VE. Mammary gland, limb and yolk sac defects in mice lacking Tbx3, the gene mutated in human ulnar mammary syndrome. Development 2003;130(10):2263–73.
Koster MI, Kim S, Mills AA, DeMayo FJ, Roop DR. p63 is the molecular switch for initiation of an epithelial stratification program. Genes Dev 2004;18(2):126–31.
Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature 1999;398(6729):708–13.
Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 1999;398(6729):714–8.
Fomenkov A, Huang YP, Topaloglu O, Brechman A, Osada M, Fomenkova T, et al. P63 alpha mutations lead to aberrant splicing of keratinocyte growth factor receptor in the Hay–Wells syndrome. J Biol Chem 2003;278(26):23906–14.
Foley J, Dann P, Hong J, Cosgrove J, Dreyer B, Rimm D, et al. Parathyroid hormone-related protein maintains mammary epithelial fate and triggers nipple skin differentiation during embryonic breast development. Development 2001;128(4):513–25.
Wysolmerski JJ, Philbrick WM, Dunbar ME, Lanske B, Kronenberg H, Broadus AE. Rescue of the parathyroid hormone-related protein knockout mouse demonstrates that parathyroid hormone-related protein is essential for mammary gland development. Development 1998;125(7):1285–94.
Satokata I, Ma L, Ohshima H, Bei M, Woo I, Nishizawa K, et al. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat Genet 2000;24(4):391–5.
Sternlicht MD, Sunnarborg SW, Kouros-Mehr H, Yu Y, Lee DC, Werb Z. Mammary ductal morphogenesis requires paracrine activation of stromal EGFR via ADAM17-dependent shedding of epithelial amphiregulin. Development 2005;132(17):3923–33.
Chakravarty G, Hadsell D, Buitrago W, Settleman J, Rosen JM. p190-B RhoGAP regulates mammary ductal morphogenesis. Mol Endocrinol 2003;17(6):1054–65.
Hardy MH. The secret life of the hair follicle. Trends Genet 1992;8(2):55–60.
Millar SE. Molecular mechanisms regulating hair follicle development. J Invest Dermatol 2002;118(2):216–25.
Schmidt-Ullrich R, Paus R. Molecular principles of hair follicle induction and morphogenesis. BioEssays 2005;27(3):247–61.
Sperling LC. Hair anatomy for the clinician. J Am Acad Dermatol 1991;25(1 Pt 1):1–17.
Oliver RF, Jahoda CA. Dermal–epidermal interactions. Clin Dermatol 1988;6(4):74–82.
Jiang TX, Chuong CM. Mechanism of skin morphogenesis. I. Analyses with antibodies to adhesion molecules tenascin, N-CAM, and integrin. Dev Biol 1992;150(1):82–98.
Mandler M, Neubuser A. FGF signaling is required for initiation of feather placode development. Development 2004;131(14):3333–43.
Petiot A, Conti FJ, Grose R, Revest JM, Hodivala-Dilke KM, Dickson C. A crucial role for Fgfr2-IIIb signalling in epidermal development and hair follicle patterning. Development 2003;130(22):5493–501.
Laurikkala J, Pispa J, Jung HS, Nieminen P, Mikkola M, Wang X, et al. Regulation of hair follicle development by the TNF signal ectodysplasin and its receptor Edar. Development 2002;129(10):2541–53.
Noramly S, Freeman A, Morgan BA. beta-catenin signaling can initiate feather bud development. Development 1999;126(16):3509–21.
Kratochwil K, Dull M, Farinas I, Galceran J, Grosschedl R. Lef1 expression is activated by BMP-4 and regulates inductive tissue interactions in tooth and hair development. Genes Dev 1996;10(11):1382–94.
Slack J. From egg to embryo. Cambridge, UK: Cambridge University Press; 1991.
Barsh G. Of ancient tales and hairless tails. Nat Genet 1999;22(4):315–6.
Gat U, DasGupta R, Degenstein L, Fuchs E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell 1998;95(5):605–14.
Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. Beta-catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 2001;105:533–45.
van Genderen C, Okamura RM, Farinas I, Quo RG, Parslow TG, Bruhn L, et al. Development of several organs that require inductive epithelial–mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev 1994;8(22):2691–703.
Laurikkala J, Mikkola M, James M, Tummers M, Mills AA, Thesleff I. p63 regulates multiple signalling pathways required for ectodermal organogenesis and differentiation. Development 2006;133(8):1553–63.
Schmidt-Ullrich R, Tobin DJ, Lenhard D, Schneider P, Paus R, Scheidereit C. NF-kappaB transmits Eda A1/EdaR signaling to activate Shh and cyclin D1 expression, and controls post-initiation hair placode down-growth. Development 2006;133(6):1045–57.
Mustonen T, Ilmonen M, Pummila M, Kangas AT, Laurikkala J, Jaatinen R, et al. Ectodysplasin A1 promotes placodal cell fate during early morphogenesis of ectodermal appendages. Development 2004;131(20):4907–19.
Schmidt-Ullrich R, Aebischer T, Hulsken J, Birchmeier W, Klemm U, Scheidereit C. Requirement of NF-kappaB/Rel for the development of hair follicles and other epidermal appendices. Development 2001;128(19):3843–53.
Noveen A, Jiang TX, Ting-Berreth SA, Chuong CM. Homeobox genes Msx-1 and Msx-2 are associated with induction and growth of skin appendages. J Invest Dermatol 1995;104(5):711–9.
Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Dev Cell 2002;2(5):643–53.
Botchkarev VA, Botchkareva NV, Roth W, Nakamura M, Chen LH, Herzog W, et al. Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nat Cell Biol 1999;1(3):158–64.
Noramly S, Morgan BA. BMPs mediate lateral inhibition at successive stages in feather tract development. Development 1998;125(19):3775–87.
Andl T, Ahn K, Kairo A, Chu EY, Wine-Lee L, Reddy ST, et al. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development 2004;131(10):2257–68.
St-Jacques B, Dassule HR, Karavanova I, Botchkarev VA, Li J, Danielian PS, et al. Sonic hedgehog signaling is essential for hair development. Curr Biol 1998;8(19):1058–68.
Chiang C, Swan RZ, Grachtchouk M, Bolinger M, Litingtung Y, Robertson EK, et al. Essential role for Sonic hedgehog during hair follicle morphogenesis. Dev Biol 1999;205(1):1–9.
Mill P, Mo R, Fu H, Grachtchouk M, Kim PC, Dlugosz AA, et al. Sonic hedgehog-dependent activation of Gli2 is essential for embryonic hair follicle development. Genes Dev 2003;17(2):282–94.
Mill P, Mo R, Hu MC, Dagnino L, Rosenblum ND, Hui CC. Shh controls epithelial proliferation via independent pathways that converge on N-Myc. Dev Cell 2005;9(2):293–303.
Headon DJ, Overbeek PA. Involvement of a novel Tnf receptor homologue in hair follicle induction. Nat Genet 1999;22(4):370–4.
Headon DJ, Emmal SA, Ferguson BM, Tucker AS, Justice MJ, Sharpe PT, et al. Gene defect in ectodermal dysplasia implicates a death domain adapter in development. Nature 2001;414(6866):913–6.
Foitzik K, Paus R, Doetschman T, Dotto GP. The TGF-beta2 isoform is both a required and sufficient inducer of murine hair follicle morphogenesis. Dev Biol 1999;212(2):278–89.
Jamora C, Lee P, Kocieniewski P, Azhar M, Hosokawa R, Chai Y, et al. A signaling pathway involving TGF-beta2 and snail in hair follicle morphogenesis. PLoS Biol 2005;3(1):e11.
Jamora C, DasGupta R, Kocieniewski P, Fuchs E. Links between signal transduction, transcription and adhesion in epithelial bud development. Nature 2003;422(6929):317–22.
Kobielak K, Pasolli HA, Alonso L, Polak L, Fuchs E. Defining BMP functions in the hair follicle by conditional ablation of BMP receptor IA. J Cell Biol 2003;163(3):609–23.
Yuhki M, Yamada M, Kawano M, Iwasato T, Itohara S, Yoshida H, et al. BMPR1A signaling is necessary for hair follicle cycling and hair shaft differentiation in mice. Development 2004;131(8):1825–33.
Ming Kwan K, Li AG, Wang XJ, Wurst W, Behringer RR. Essential roles of BMPR-IA signaling in differentiation and growth of hair follicles and in skin tumorigenesis. Genesis 2004;39(1):10–25.
Kaufman CK, Zhou P, Pasolli HA, Rendl M, Bolotin D, Lim KC, et al. GATA-3: an unexpected regulator of cell lineage determination in skin. Genes Dev 2003;17(17):2108–22.
Pan Y, Lin MH, Tian X, Cheng HT, Gridley T, Shen J, et al. Gamma-secretase functions through Notch signaling to maintain skin appendages but is not required for their patterning or initial morphogenesis. Dev Cell 2004;7(5):731–43.
Vidal VP, Chaboissier MC, Lutzkendorf S, Cotsarelis G, Mill P, Hui CC, et al. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr Biol 2005;15(15):1340–51.
Godwin AR, Capecchi MR. Hoxc13 mutant mice lack external hair. Genes Dev 1998;12(1):11–20.
Nehls M, Pfeifer D, Schorpp M, Hedrich H, Boehm T. New member of the winged-helix protein family disrupted in mouse and rat nude mutations. Nature 1994;372(6501):103–7.
Segre JA, Nemhauser JL, Taylor BA, Nadeau JH, Lander ES. Positional cloning of the nude locus: genetic, physical, and transcription maps of the region and mutations in the mouse and rat. Genomics 1995;28(3):549–59.
Brissette JL, Li J, Kamimura J, Lee D, Dotto GP. The product of the mouse nude locus, Whn, regulates the balance between epithelial cell growth and differentiation. Genes Dev 1996;10(17):2212–21.
Meier N, Dear TN, Boehm T. Whn and mHa3 are components of the genetic hierarchy controlling hair follicle differentiation. Mech Dev 1999;89(1–2):215–21.
Jernvall J, Thesleff I. Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech Dev 2000;92(1):19–29.
Cobourne MT, Sharpe PT. Tooth and jaw: molecular mechanisms of patterning in the first branchial arch. Arch Oral Biol 2003;48(1):1–14.
Wang X, Thesleff I. Tooth development. In: Unsicker K, Krieglstein K, editors. Cell signalling and growth factors in development. Weinhem: Wiley-VHC; 2006. p.719–754.
Peterkova R. The common developmental origin and phylogenetic aspects of teeth, rugae palatinae, and fornix vestibuli oris in the mouse. J Craniofac Genet Dev Biol 1985;5(1):89–104.
Mina M, Kollar EJ. The induction of odontogenesis in non-dental mesenchyme combined with early murine mandibular arch epithelium. Arch Oral Biol 1987;32(2):123–7.
Mucchielli ML, Mitsiadis TA, Raffo S, Brunet JF, Proust JP, Goridis C. Mouse Otlx2/RIEG expression in the odontogenic epithelium precedes tooth initiation and requires mesenchyme-derived signals for its maintenance. Dev Biol 1997;189(2):275–84.
Keranen SV, Kettunen P, Aberg T, Thesleff I, Jernvall J. Gene expression patterns associated with suppression of odontogenesis in mouse and vole diastema regions. Dev Genes Evol 1999;209(8):495–506.
Lu MF, Pressman C, Dyer R, Johnson RL, Martin JF. Function of Rieger syndrome gene in left–right asymmetry and craniofacial development. Nature 1999;401(6750):276–8.
Liu W, Selever J, Lu MF, Martin JF. Genetic dissection of Pitx2 in craniofacial development uncovers new functions in branchial arch morphogenesis, late aspects of tooth morphogenesis and cell migration. Development 2003;130(25):6375–85.
Sarkar L, Cobourne M, Naylor S, Smalley M, Dale T, Sharpe PT. Wnt/Shh interactions regulate ectodermal boundary formation during mammalian tooth development. Proc Natl Acad Sci U S A 2000;97(9):4520–4.
Kassai Y, Munne P, Hotta Y, Penttila E, Kavanagh K, Ohbayashi N, et al. Regulation of mammalian tooth cusp patterning by ectodin. Science 2005;309(5743):2067–70.
Pispa J, Mikkola ML, Mustonen T, Thesleff I. Ectodysplasin, Edar and TNFRSF19 are expressed in complementary and overlapping patterns during mouse embryogenesis. Gene Expr Patterns 2003;3(5):675–9.
Thesleff I, Hurmerinta K. Tissue interactions in tooth development. Differentiation 1981;18(2):75–88.
Lumsden AG. Spatial organization of the epithelium and the role of neural crest cells in the initiation of the mammalian tooth germ. Development 1988;103(Suppl):155–69.
Neubuser A, Peters H, Balling R, Martin GR. Antagonistic interactions between FGF and BMP signaling pathways: a mechanism for positioning the sites of tooth formation. Cell 1997;90(2):247–55.
Ferguson CA, Tucker AS, Heikinheimo K, Nomura M, Oh P, Li E, et al. The role of effectors of the activin signalling pathway, activin receptors IIA and IIB, and Smad2, in patterning of tooth development. Development 2001;128(22):4605–13.
Mandler M, Neubuser A. FGF signaling is necessary for the specification of the odontogenic mesenchyme. Dev Biol 2001;240(2):548–59.
Vainio S, Karavanova I, Jowett A, Thesleff I. Identification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell 1993;75(1):45–58.
Tucker AS, Matthews KL, Sharpe PT. Transformation of tooth type induced by inhibition of BMP signaling. Science 1998;282(5391):1136–8.
Trumpp A, Depew MJ, Rubenstein JL, Bishop JM, Martin GR. Cre-mediated gene inactivation demonstrates that FGF8 is required for cell survival and patterning of the first branchial arch. Genes Dev 1999;13(23):3136–48.
Ferguson CA, Tucker AS, Sharpe PT. Temporospatial cell interactions regulating mandibular and maxillary arch patterning. Development 2000;127(2):403–12.
St Amand TR, Zhang Y, Semina EV, Zhao X, Hu Y, Nguyen L, et al. Antagonistic signals between BMP4 and FGF8 define the expression of Pitx1 and Pitx2 in mouse tooth-forming anlage. Dev Biol 2000;217(2):323–32.
Peters H, Neubuser A, Kratochwil K, Balling R. Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit craniofacial and limb abnormalities. Genes Dev 1998;12(17):2735–47.
Ruch JV, Lesot H, Karcher-Djuricic V, Meyer JM, Mark M. Epithelial–mesenchymal interactions in tooth germs: mechanisms of differentiation. J Biol Buccale 1983;11(3):173–93.
Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 2005.
Thomas BL, Tucker AS, Qui M, Ferguson CA, Hardcastle Z, Rubenstein JL, et al. Role of Dlx-1 and Dlx-2 genes in patterning of the murine dentition. Development 1997;124(23):4811–8.
Bei M, Maas R. FGFs and BMP4 induce both Msx1-independent and Msx1-dependent signaling pathways in early tooth development. Development 1998;125(21):4325–33.
Kettunen P, Karavanova I, Thesleff I. Responsiveness of developing dental tissues to fibroblast growth factors: expression of splicing alternatives of FGFR1, -2, -3, and of FGFR4; and stimulation of cell proliferation by FGF-2, -4, -8, and -9. Dev Genet 1998;22(4):374–85.
Pispa J, Jung HS, Jernvall J, Kettunen P, Mustonen T, Tabata MJ, et al. Cusp patterning defect in Tabby mouse teeth and its partial rescue by FGF. Dev Biol 1999;216(2):521–34.
Kangas AT, Evans AR, Thesleff I, Jernvall J. Nonindependence of mammalian dental characters. Nature 2004;432(7014):211–4.
Mustonen T, Pispa J, Mikkola ML, Pummila M, Kangas AT, Pakkasjarvi L, et al. Stimulation of ectodermal organ development by Ectodysplasin-A1. Dev Biol 2003;259(1):123–36.
Zhang Q, Murcia NS, Chittenden LR, Richards WG, Michaud EJ, Woychik RP, et al. Loss of the Tg737 protein results in skeletal patterning defects. Dev Dyn 2003;227(1):78–90.
Hardcastle Z, Mo R, Hui CC, Sharpe PT. The Shh signalling pathway in tooth development: defects in Gli2 and Gli3 mutants. Development 1998;125(15):2803–11.
Cobourne MT, Hardcastle Z, Sharpe PT. Sonic hedgehog regulates epithelial proliferation and cell survival in the developing tooth germ. J Dent Res 2001;80(11):1974–9.
Dassule HR, Lewis P, Bei M, Maas R, McMahon AP. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development 2000;127(22):4775–85.
Gritli-Linde A, Bei M, Maas R, Zhang XM, Linde A, McMahon AP. Shh signaling within the dental epithelium is necessary for cell proliferation, growth and polarization. Development 2002;129(23):5323–37.
Ohazama A, Hu Y, Schmidt-Ullrich R, Cao Y, Scheidereit C, Karin M, et al. A dual role for ikkalpha in tooth development. Dev Cell 2004;6(2):219–27.
Vaahtokari A, Aberg T, Jernvall J, Keranen S, Thesleff I. The enamel knot as a signaling center in the developing mouse tooth. Mech Dev 1996;54(1):39–43.
Chen Y, Bei M, Woo I, Satokata I, Maas R. Msx1 controls inductive signaling in mammalian tooth morphogenesis. Development 1996;122(10):3035–44.
Bei M, Kratochwil K, Maas RL. BMP4 rescues a non-cell-autonomous function of Msx1 in tooth development. Development 2000;127(21):4711–8.
Jernvall J, Aberg T, Kettunen P, Keranen S, Thesleff I. The life history of an embryonic signaling center: BMP-4 induces p21 and is associated with apoptosis in the mouse tooth enamel knot. Development 1998;125(2):161–9.
Kratochwil K, Galceran J, Tontsch S, Roth W, Grosschedl R. FGF4, a direct target of LEF1 and Wnt signaling, can rescue the arrest of tooth organogenesis in Lef1(-/-) mice. Genes Dev 2003;16(24):3173–85.
D’Souza RN, Aberg T, Gaikwad J, Cavender A, Owen M, Karsenty G, et al. Cbfa1 is required for epithelial–mesenchymal interactions regulating tooth development in mice. Development 1999;126(13):2911–20.
Aberg T, Wang XP, Kim JH, Yamashiro T, Bei M, Rice R, et al. Runx2 mediates FGF signaling from epithelium to mesenchyme during tooth morphogenesis. Dev Biol 2004;270(1):76–93.
Jernvall J, Kettunen P, Karavanova I, Martin LB, Thesleff I. Evidence for the role of the enamel knot as a control center in mammalian tooth cusp formation: non-dividing cells express growth stimulating Fgf-4 gene. Int J Dev Biol 1994;38(3):463–9.
Ohazama A, Modino SA, Miletich I, Sharpe PT. Stem-cell-based tissue engineering of murine teeth. J Dent Res 2004;83(7):518–22.
Gallego MI, Beachy PA, Hennighausen L, Robinson GW. Differential requirements for shh in mammary tissue and hair follicle morphogenesis. Dev Biol 2002;249(1):131–9.
Lin CR, Kioussi C, O’Connell S, Briata P, Szeto D, Liu F, et al. Pitx2 regulates lung asymmetry, cardiac positioning and pituitary and tooth morphogenesis. Nature 1999;401(6750):279–82.
Brakebusch C, Grose R, Quondamatteo F, Ramirez A, Jorcano JL, Pirro A, et al. Skin and hair follicle integrity is crucially dependent on beta 1 integrin expression on keratinocytes. EMBO J 2000;19(15):3990–4003.
Raghavan S, Bauer C, Mundschau G, Li Q, Fuchs E. Conditional ablation of beta1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J Cell Biol 2000;150(5):1149–60.
Vasioukhin V, Bauer C, Degenstein L, Wise B, Fuchs E. Hyperproliferation and defects in epithelial polarity upon conditional ablation of alpha-catenin in skin. Cell 2001;104(4):605–17.
Nanba D, Hieda Y, Nakanishi Y. Remodeling of desmosomal and hemidesmosomal adhesion systems during early morphogenesis of mouse pelage hair follicles. J Invest Dermatol 2000;114(1):171–7.
Nanba D, Nakanishi Y, Hieda Y. Changes in adhesive properties of epithelial cells during early morphogenesis of the mammary gland. Dev Growth Differ 2001;43(5):535–44.
Ida M, Nakamura T, Utsunomiya J. Osteomatous changes and tooth abnormalities found in the jaw of patients with adenomatosis coli. Oral Surg Oral Med Oral Pathol 1981;52(1):2–11.
Nishisho I, Nakamura Y, Miyoshi Y, Miki Y, Ando H, Horii A, et al. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science 1991;253(5020):665–9.
Lammi L, Arte S, Somer M, Jarvinen H, Lahermo P, Thesleff I, et al. Mutations in AXIN2 cause familial tooth agenesis and predispose to colorectal cancer. Am J Hum Genet 2004;74(5):1043–50.
Chan EF, Gat U, McNiff JM, Fuchs E. A common human skin tumour is caused by activating mutations in beta- catenin. Nat Genet 1999;21(4):410–3.
Brennan KR, Brown AM. Wnt proteins in mammary development and cancer. J Mammary Gland Biol Neoplasia 2004;9(2):119–31.
Dillon C, Spencer-Dene B, Dickson C. A crucial role for fibroblast growth factor signaling in embryonic mammary gland development. J Mammary Gland Biol Neoplasia 2004;9(2):207–15.
Sternlicht MD. Key stages in mammary gland development: the cues that regulate ductal branching morphogenesis. Breast Cancer Res 2006;8(1):201.
van Bokhoven H, Hamel BC, Bamshad M, Sangiorgi E, Gurrieri F, Duijf PH, et al. p63 Gene mutations in eec syndrome, limb-mammary syndrome, and isolated split hand–split foot malformation suggest a genotype–phenotype correlation. Am J Hum Genet 2001;69(3):481–92.
van Bokhoven H, McKeon F. Mutations in the p53 homolog p63: allele-specific developmental syndromes in humans. Trends Mol Med 2002;8(3):133–9.
Kere J, Srivastava AK, Montonen O, Zonana J, Thomas N, Ferguson B, et al. X-linked anhidrotic (hypohidrotic) ectodermal dysplasia is caused by mutation in a novel transmembrane protein. Nat Genet 1996;13(4):409–16.
Monreal AW, Ferguson BM, Headon DJ, Street SL, Overbeek PA, Zonana J. Mutations in the human homologue of mouse dl cause autosomal recessive and dominant hypohidrotic ectodermal dysplasia. Nat Genet 1999;22(4):366–9.
Hahn H, Wicking C, Zaphiropoulous PG, Gailani MR, Shanley S, Chidambaram A, et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell 1996;85(6):841–51.
Johnson RL, Rothman AL, Xie J, Goodrich LV, Bare JW, Bonifas JM, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science 1996;272(5268):1668–71.
Unden AB, Holmberg E, Lundh-Rozell B, Stahle-Backdahl M, Zaphiropoulos PG, Toftgard R, et al. Mutations in the human homologue of Drosophila patched (PTCH) in basal cell carcinomas and the Gorlin syndrome: different in vivo mechanisms of PTCH inactivation. Cancer Res 1996;56(20):4562–5.
Markey AC, Lane EB, Macdonald DM, Leigh IM. Keratin expression in basal cell carcinomas. Br J Dermatol 1992;126(2):154–60.
Jih DM, Lyle S, Elenitsas R, Elder DE, Cotsarelis G. Cytokeratin 15 expression in trichoepitheliomas and a subset of basal cell carcinomas suggests they originate from hair follicle stem cells. J Cutan Pathol 1999;26(3):113–8.
Gailani MR, Stahle-Backdahl M, Leffell DJ, Glynn M, Zaphiropoulos PG, Pressman C, et al. The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat Genet 1996;14(1):78–81.
Zhang L, Chen XM, Sun ZJ, Bian Z, Fan MW, Chen Z. Epithelial expression of SHH signaling pathway in odontogenic tumors. Oral Oncol;2005.
Bamshad M, Lin RC, Law DJ, Watkins WC, Krakowiak PA, Moore ME, et al. Mutations in human TBX3 alter limb, apocrine and genital development in ulnar-mammary syndrome. Nat Genet 1997;16(3):311–5.
Idrees F, Bloch-Zupan A, Free SL, Vaideanu D, Thompson PJ, Ashley P, et al. A novel homeobox mutation in the PITX2 gene in a family with Axenfeld–Rieger syndrome associated with brain, ocular, and dental phenotypes. Am J Med Genet B Neuropsychiatr Genet;2006.
Harada H, Toyono T, Toyoshima K, Yamasaki M, Itoh N, Kato S, et al. FGF10 maintains stem cell compartment in developing mouse incisors. Development 2002;129(6):1533–41.
Satokata I, Maas R. Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nat Genet 1994;6(4):348–56.
Ma L, Liu J, Wu T, Plikus M, Jiang TX, Bi Q, et al. ‘Cyclic alopecia’ in Msx2 mutants: defects in hair cycling and hair shaft differentiation. Development 2003;130(2):379–89.
Bei M, Stowell S, Maas R. Msx2 controls ameloblast terminal differentiation. Dev Dyn 2004;231(4):758–65.
Hansen LA, Alexander N, Hogan ME, Sundberg JP, Dlugosz A, Threadgill DW, et al. Genetically null mice reveal a central role for epidermal growth factor receptor in the differentiation of the hair follicle and normal hair development. Am J Pathol 1997;150(6):1959–75.
Luetteke NC, Phillips HK, Qiu TH, Copeland NG, Earp HS, Jenkins NA, et al. The mouse waved-2 phenotype results from a point mutation in the EGF receptor tyrosine kinase. Genes Dev 1994;8(4):399–413.
Matzuk MM, Kumar TR, Vassalli A, Bickenbach JR, Roop DR, Jaenisch R, et al. Functional analysis of activins during mammalian development. Nature 1995;374(6520):354–6.
Ferguson CA, Tucker AS, Christensen L, Lau AL, Matzuk MM, Sharpe PT. Activin is an essential early mesenchymal signal in tooth development that is required for patterning of the murine dentition. Genes Dev 1998;12(16):2636–49.
Wang XP, Suomalainen M, Jorgez CJ, Matzuk MM, Werner S, Thesleff I. Follistatin regulates enamel patterning in mouse incisors by asymmetrically inhibiting BMP signaling and ameloblast differentiation. Dev Cell 2004;7(5):719–30.
Wang XP, Suomalainen M, Jorgez CJ, Matzuk MM, Wankell M, Werner S, et al. Modulation of activin/bone morphogenetic protein signaling by follistatin is required for the morphogenesis of mouse molar teeth. Dev Dyn 2004;231(1):98–108.
Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet 2005;1(4):e53.
Acknowledgements
Research in Sarah Millar’s laboratory is supported by NIH grants R01-AR47709 and R01- DE015342.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mikkola, M.L., Millar, S.E. The Mammary Bud as a Skin Appendage: Unique and Shared Aspects of Development. J Mammary Gland Biol Neoplasia 11, 187–203 (2006). https://doi.org/10.1007/s10911-006-9029-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10911-006-9029-x