Abstract
Advances in optical imaging technologies that allow the subcellular resolution of undissected tissue have begun to offer new clues into the biology of development and disease. For cancer, such advances mean that the primary tumor is no longer a black box and that the disease can be studied throughout the metastatic cascade and not just as an endpoint. In this review we examine the advances in multiphoton imaging technology that have been used to define the microenvironment and its role in delineating the invasion and intravasation steps of metastasis inside living mammary tumors. Results show that the tumor microenvironment is a dynamic place where interactions between tumor cells, macrophages, blood vessels, and extracellular matrix fibers define the metastatic phenotype.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One out of three cancers diagnosed among US women is due to breast cancer; 212,920 new invasive breast cancer cases and an additional 61,980 in situ breast cancer cases are expected to be diagnosed in 2006. Around 40,970 women are expected to die from breast cancer in 2006 (American Cancer Society, Breast Cancer Facts and Figures 2006). The metastasis of 10–15% of patients with breast cancer can be aggressive and can take between 3 and 10 years to be manifested after the initial diagnosis [1]. The interest in mechanisms behind invasion and metastasis of breast cancer has lead to the use of animal models with tumors that mimic breast cancer in human patients. Animal models employed to study breast cancer have been used for more than 75 years [2], but only recently have these models been improved to employ genetically encoded fluorescent proteins that allow the optical imaging of cells within living primary tumors. The importance of these animal models is that they allow one to recapitulate the different stages of tumor progression, from hyperplasia to late-stage carcinoma, and define the cellular players that lead to tumor metastasis throughout these stages.
An important advance in the study of tumor biology is the use of optical imaging techniques in visualizing, at subcellular resolution, the intricate steps of invasion and metastasis in vivo. Early studies were limited by two hurdles: the poor penetration of whole tissue by short wavelengths used for excitation of fluorescence and the photo damage of tissue during imaging. The development of multiphoton microscopy has circumvented these limitations and opened fluorescent animal models of cancer to high resolution studies during invasion and metastasis in vivo. In this review, we focus our discussion on the advanced in vivo techniques that are being utilized to study the mechanisms of invasion and metastasis of breast cancer.
Multiphoton Microscopy and Intravital Imaging
The ability to image single cells in vivo has been used to understand both normal development and disease. Metastatic potential has been studied in the liver by both intravital video microscopy [3] and high-frequency ultrasound imaging [4]. The role of platelets and coagulation for tumor cells to adhere and grow in the lung along with the intravascular growth of metastasis has been studied using an intact lung microscopy technique ex vivo [5, 6]. Tumor growth and metastasis was followed in a low resolution whole animal imager that can track single cells in the blood, using GFP-cells injected into nude mice [7]. Angiogenic growth, vessel density and vessel diffusion have been studied using FRAP and multiphoton microscopy in a transparent dwelling chamber in mice [8–10]. While tumor cell motility, invasion and intravasation have been imaged using conventional confocal microscopy [11], only recently, with the advent of multiphoton microscopy, has it been possible to image these events in live undissected tumors [11, 12].
Multiphoton microscopy is unique because it provides the depth of imaging and high temporal and spatial resolution necessary to study the mammary tumor microenvironment and demarcate the importance of the different steps of cancer cell invasion and metastasis [13, 14]. The rational behind multiphoton microscopy dates back to the hypothesis made by Goppert-Mayer in 1931, which won her, with Hans Hensen, a Nobel prize for physics in 1963. This theory predicts that the simultaneous absorption of two “low energy” photons could result in the combination of their energies in order to produce the transition of fluorophores to an excited state [15].
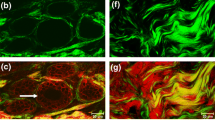
Multiphoton imaging is notable in its ability to image with clarity at greater depth (300 μm) compared to traditional confocal microscopy (<40 μm) [12, 16, 17]. Figure 1 shows that in a living tumor multiphoton microscopy allows for the imaging of many cell layers in a living tumor, while conventional microscopy allows for only the imaging of a single cell layer with any clarity. Multiphoton imaging makes use of non-linear dependence of excitation on photon density in a single plane of focus, while the traditional confocal microscope employs a pinhole aperture to eliminate out-of-focus contributions (and 98% of the light), which renders the multiphoton imaging more efficient than traditional modalities [13, 16]. In addition, the longer wavelengths that are used for excitation in multiphoton imaging are less scattered from the different refractive indices inside live tissues; thus the images generated are less degraded [13, 16].
Multiphoton microscopy allows for greater penetration and less photobleaching during intravital imaging. a and b) intravital imaging of a mammary tumor of the rat resulting from the injection of MTLn3 adenocarcinoma cells into the mammary gland. a Z-series of confocal (left panels) and multiphoton (right panels) shows that the multiphoton microscope gives a superior image to a greater depth in breast tissue. The confocal microscope can only image to approximately 36 μm into a living tumor. The first panel of the multiphoton images (top right panel) begins at 36 μm giving a clearer image than the confocal even at the surface (0 μm) (top left panel). Arrows point to a single cell. Double arrow points to ECM seen only in multiphoton microscopy due to second harmonic generation. b An identical z-series taken 30 mins later in which images were taken every minute shows considerable bleaching in the confocal image (left panels) compared to the multiphoton image (right panels). Scale bar = 25 μm. Taken from [12].
The lack of fluorophore excitation in regions outside the focal plane is an important asset of multiphoton microscopes because it reduces the photobleaching and the generation of toxic by-products produced by the imaging procedure, which can be harmful to the live tissues being imaged [11, 13, 16]. Another important asset of the multiphoton excitation is the ability to image certain alpha helix containing proteins without a fluorophore due to a second harmonic generated (SHG) polarized light [18]. The SHG signal can be excited with wavelengths of 760–960 nm, and can be imaged through filters with a 450–480 nm cutoff [14, 19]. The ECM inside the tumor can be visualized using SHG because SHG is formed by the scattering of polarized light of asymmetrically arranged electron orbitals in amino acids in α-helical proteins like collagen and elastin which are found in the ECM of tumors [13, 20]. Additionally, multiphoton-based-SHG has been used to visualize many structures within different species, such as isolated scales of black tetra fish, tissue layers of mouse ears, extracellular and intracellular structures within mouse leg muscle tissue, C. elegans muscles, and proteins in C. elegans embryos [21].
Furthermore, the advantages brought by using multiphoton microscopy have been important in fields other than tumor biology, for example in studying the role of lung epithelium in tissue remodeling, which can be central to important lung diseases such as interstitial pulmonary fibrosis and bronchial asthma [18, 22]. Multiphoton imaging has also been important in imaging viable T cells, B cells, and APCs in intact lymphoid organs [23].
Animal Models Used for Mutiphoton Imaging
Transgenic and orthotopicaly-injected animals have been used extensively as models for breast cancer invasion and metastasis [24–26]. Figure 2 shows how both models are used in imaging to probe the tumor microenvironment. Transgenic models are well suited for the study of cancer because the growth of tumors in these model animals mimics the growth of tumors in human disease [25]. The interest in understanding the function of certain molecular players in invasion and metastasis has led to the use of specific promoters that can define their expression in a spatial and/or temporal manner. For example, to study mammary tumors, the mouse mammary tumor virus (MMTV) promoter is used because it can limit oncogene expression to mammary epithelium [2]. The MMTV promoter has been used to induce breast tumor formation by targeting the expression of oncogenes like the ERB2 and the polyoma middle T (PyMT) oncogenes, and also GFP expression, to the mammary gland [13]. The use of fluorophores, like GFP, CFP, and YFP as reporters has been indispensable in the imaging of these tumor models because their expression has been shown to have no effect on tumor growth and progression [11]. In addition to the labeling of tumor cells, other transgenic models have been established that allow for imaging other cell types involved in tumor growth, angiogenesis and metastasis. These include the Tie2-GFP mice with fluorescent endothelial cells [27] and the lys-GFP [28], and C-fms-GFP mice [29] that label macrophages and granulocytes [30].
Methodological approaches for studying tumor cell behavior inside live animals. Cell graft model: tumor cells stably expressing GFP are injected orthotopically into the mammary gland of either rats or mice. After 3 weeks, the tumors can be imaged while the animals are kept alive under anesthesia. Transgenic model: mammary gland specific promoters like MMTV promote expression of GFP and oncogenes resulting in spontaneous mammary tumors that are fluorescent. This model allows for inspection of stages of progression. Otherwise it is imaged using methods identical to the cell graft model.
Orthotopic models have also been used for in vivo imaging of breast cancer. While their histology is not directly comparable to spontaneous tumors, orthotopic models have the advantage of being faster growing and have a higher throughput in the evaluation of the invasive and metastatic potential of over expression and other mutant tumor cell lines. For example, fluorescently labeled mammary tumor cells, when injected into the mammary glands of rats and mice, generate fluorescent tumors that can be imaged using a multiphoton microscope after 3 weeks of growth [31], compared to 20 weeks for a late stage carcinoma in the PyMT derived mammary tumors. Also, the transfection of target genes allows for direct studies of the effects of these genes on invasion, intravasation and metastasis [32]. Furthermore, co-injection of different cells, alternately labeled by expression of GFP and CFP, into the same mammary fat pad allows for the direct comparison of the behavior of parental cells and cell lines harboring mutations in key genes in different microenvironments of the tumor [19].
The Tumor Microenvironment and its Architecture
The mosaic nature of the mammary tumor microenvironment is very important in defining the fate of cancer cells and in determining the steps in which they participate during invasion and metastasis. The microenvironment is comprised of tumor cells, tumor-associated cells—such as macrophages, fibroblasts, adipocytes, neutrophils, basophils, dendritic cells, mast cells, T cells and B cells—an array of ECM fibers, and blood vessels that nourish the stroma of the tumor [33]. Consequently, assessing the importance of the tumor microenvironment in determining the metastatic outcome involves studying interactions between each of these cell types and with the surrounding ECM network and blood vessels inside the undissected living tumor. Using multiphoton intravital imaging (MPIVI), one can observe these interactions in real time in live tumors. What follows is a description of these interactions, the microenvironments that they define and how they contribute to metastasis.
ECM Fibers Support Blood Vessels and Provide a Scaffolding for Tumor Cell Motility to Vessels
The extracellular matrix inside tumors is important because it enhances cell migration by acting as a substratum for cells [12, 13]. The role of ECM in cell migration depends on the cell type and ECM substrate involved, and can be regulated by integrins which bind to different components of the ECM network, such as fibronection, laminin, collagen, and vitronectin. The degradation of ECM by matrix metalloproteases (MMPs), can aid the migrating cells in their invasion of the matrix by releasing peptides that stimulate cell motility [34]. MMP activity has also been argued to determine the mode of cell migration [35].
Using the second-harmonic generation imaging in the multiphoton microscope, the presence of collagen and other alpha helix containing ECM proteins can be detected. The multiphoton microscope, using an excitation of 860–880 nm, is exquisitely sensitive to collagen, and collagen production and its remodeling by proteolysis can be studied directly in normal mammary development [36] and during invasion and metastasis in mammary tumors [8]. In normal mammary tissue, it has been shown that macrophages play a role in collagen fibrillogenesis that defines the terminal end bud’s 3-dimensional structure (Ingman et al., Developmental Dynamics, in press) and by remodeling the matrix to aid in the epithelial cells’ invasion during morphogenesis [33].
In mammary tumors, collagen fibers become more numerous and support the blood vessels of the tumor as dense arrays of fibers that surround the vessels (Fig. 3). These collagen fibers are co-opted by the carcinoma cells as a highway system to migrate within the tumor and ultimately to reach the blood vessels themselves[13, 26, 31]. On the other hand, the dense ECM that makes up the basement membrane of blood vessels acts as a barrier preventing the movement of tumor cells into blood vessels [13].
The microenvironment of a mammary tumor can be imaged using multiphoton microscopy. a Tumor cells with GFP-labeled Mena (green) are seen growing as an epithelial layer and have Mena-GFP at the cell periphery. Collagen (purple) is imaged by second harmonic-generated polarized light. b Tumor cells (green) are associated with ECM fibers (purple). ECM fibers not only support the tumor cells and blood vessels but also act as roadways used by the tumor cells to crawl toward blood vessels (arrow). Image is a 60 μm thick z-projection. Scale bar for a and b = 25 μm.
Motility is Imperative in Determining Tumor Cell Fate Inside the Primary Tumor
Tumor cell motility in vivo determines the microenvironment in which the tumor cell is located, and hence its invasion, intravasation and metastatic potential. During movement, the cancer cell generates cycles of actin polymerization which are important to determine cell direction and for each of the consecutive steps of the motility cycle [37]. Tumor cells in mammary tumors are challenged by extracellular stimuli which originate from their immediate microenvironment, such as neighboring cancer cells, macrophages, and blood vessels. This stimulation causes localized actin polymerization and protrusion [37] followed by cell-ECM adhesion, contraction of the cell body creating a tension between the adhesion sites, and subsequently cell translocation [37] resembling an inch worm-like motion (Fig. 4). Migrating cells need to degrade and remodel the ECM in order to invade neighboring tissues and basement membranes that surround blood vessels; this process involves their ability to extend protrusions into the ECM [38–40]. Recently, invadopodia have been characterized as important in the metastasis of cancer cells. These specialized structures contain many actin-regulatory proteins, including N-WASP (WASP in macrophages), and cofilin, adhesion molecules, signaling proteins, and matrix degrading proteins (MMPs) [39].
Tumor cells use ECM fibers for high velocity migration. a In a single optical section, tumor cells (green, white arrows) are seen translocating along ECM fibers (purple) in a living tumor using an inch worm-like movement. The frames are from movies generated from Time-Lapse Z-series of tumor cells in a tumor (ErbB1-MTLn3-GFP) derived from EGFR over expressing MTLn3 cells in mice and are 6 min apart. Scale bar = 25 μm. b Time lapse imaging shown as a z-series projection demonstrates motility of tumor cells in a ErbB1-MTLn3-GFP tumor. GFP-expressing tumor cells (green) are seen crawling on ECM fibers (purple). White arrows track two cells present during the entire sequence; orange arrowheads show their original position. Other cells move into the field of view from a lower plane. Images shown are 20 min apart. Scale bar = 25 μm. Figure 4 b is taken from [45].
Cell Motility Inside Tumors Involves an Array of Different Cell Types Moving in Relation to ECM and to Each Other
Multiphoton-based intravital imaging has shown that cancer cells inside mammary tumors migrate as solitary amoeboid cells, not as a collection of adherent cells, and at much higher speeds than that seen in vitro (∼3.4 vs. 0.45 μm/min, respectively) [12, 26]. The high velocity of tumor cell motility in vivo may be explained by a combination of factors, including the absence of a dense network of ECM in tumor tissue, the presence of linear ECM fibers as a substratum, and the unique gene expression pattern of invasive tumor cells which makes them more motile and chemotactic [12, 13, 26]. Current work has analyzed the motility behaviors inside mammary tumors in vivo, derived either from the injection of tumor cells into the mammary glands or from spontaneous tumors of transgenic animals (Table 1). In both cases cancer cells were readily observed by their GFP fluorescence, while comigrating host cells were observed either as shadows crawling on the GFP expressing cells or directly visualized as macrophages in animals with GFP-labeled macrophages [12, 26]. Tumor cells and macrophages showed motility that is directed by ECM fibers (Fig. 4 and Table 1) and motility that is independent of obvious large ECM fibers (Fig. 5 and Table 1). ECM fiber independent movements included tumor cells and host cells moving on the surface of other tumor cells. Accordingly, macrophage and tumor cell translocation was scored in relation to their movement on ECM, other cells, and in relation to each other. Results show that highly metastatic (MTLn3 cell-derived) mammary tumors have more ECM fiber associated host cell movement than non-metastatic (MTC-derived) mammary tumors (Table 1).
Tumor and host cells migrate when not associated with large ECM fibers. a A time-lapse movie of tumor cells in a ErbB1-MTLn3-GFP tumor shows a cell moving across other cells in a living tumor. Arrow in first panel shows the starting position of the cell. The white line delineates the cell boundary in the other panels. Frames are 5 min apart. b A host cell (black shadow, white arrows) is seen moving independently of large ECM fibers. Frames are 3 min apart. ECM fibers are purple in both a and b. Scale bars for a and b = 25 μm.
EGF Receptor Over Expression Enhances Cell Motility Inside Tumors
The epidermal growth factor family of receptors form subclass I of the receptor tyrosine kinase family, which includes EGFR (also known as ErbB1), ErbB2, ErbB3, and ErbB4 [41]. The ErbB receptors are important for normal physiological processes, but elevated expression status of ErbB1 or ErbB2 in tumors is an indicator of poor prognosis [42, 43]. Though many drugs have been designed to inhibit ErbB receptors, clinical trials have shown limited success in inhibiting the growth of tumors. The effect of these inhibitors on invasion and metastasis has not been widely studied. Thus the need for more thorough understanding of the role of the ErbB receptors in cancer progression, and their role in metastasis, in particular, is essential.
Animal models in which ErbB family members are specifically expressed are valuable because they mimic human breast cancers of poor prognosis [25, 44]. Of particular note, mammary tumors derived from ErbB1 over expressing adenocarcinoma cells show an increase in intravasation and lung metastasis [45]. Ligands for the EGF receptor, such as EGF, produced by tumor-associated macrophages, and the presence of hydrolysable EGF-like repeats in some components of the ECM fibers, have been shown to play a role in the chemotaxis of carcinoma cells in metastatic tumors [12, 13, 26]. Interestingly, pathways involved in invadopod production can also be stimulated through the EGF receptor in mammary epithelia-derived cancer cells [39, 40]. The involvement of EGF and its receptor is not unexpected because it is a physiological stimulus that can induce chemotaxis and invasion of cancer cells, and is also involved in cell motility [41, 46]. Furthermore, invasive MTLn3 cells collected from mammary tumors express high levels of EGFR when compared to non-invasive cells from the same tumor [32]. In addition, non-metastatic MTC cells show increased metastatic ability when EGFR is exogenously expressed in them [47–49].
To understand the relationship between EGF-receptor expression and breast tumor metastasis, tumors derived from MTLn3 cells over expressing the ErbB1 receptor (ErbB1-MTLn3) were imaged using multiphoton microscopy. Results show that cells over expressing the EGF receptor have a higher frequency of invasive cell movements and higher percentage of cell orientation toward blood vessels (Fig. 6 and Table 1). ErbB1-MTLn3 cells exhibit an increase in both host and tumor cell movements on ECM fibers when compared to the tumors derived from cells expressing the plasmid only (PLXSN-GFP), 11 and 5 fold, respectively, [45] and also an increase in movement of both tumor and host cells that is not along ECM fibers, 33 and 4.6 fold, respectively (Table 1). In addition, ErbB1-MTLn3 tumors contained cells that showed an eight fold increase in the protrusive behavior over those in parental cell derived tumors (Table 1).
EGFR-overexpressing MTLn3 (ErbB1-MTLn3-GFP) cells show higher frequency of motility in vivo. Time lapse images of a tumor derived from GFP-MTLn3 cells (green) co-injected with ErbB1-MTLn3-GFP (EGFR over expressing) cells (white). One GFP-MTln3 cell has moved into the invasive front of the tumor (*), while numerous ErbB1-MTLn-3-GFP cells have moved over the same interval (numbered 1–5). ECM is purple in images. Numbers and * in left panel show starting positions. White lines delineate distance cells have moved. Time between images is 8 min. Scale bar = 25 μm.
A Paracrine Loop is Required for Tumor Cell Migration and Intravasation in Mammary Tumors
The tumor microenvironment is essential for the survival, growth, invasion and intravasation of cancer cells into the tumor blood vessels. Several reports have shown that stromal cells are important in defining signaling pathways that tumor cells use for the various steps of metastasis. Tumor-associated macrophages (TAMs) have been identified as major players in invasion, angiogenesis, immunosuppression, and metastasis of tumors [33, 50]. Detailed studies have shown that TAMs are distinct from macrophages in healthy tissues, which suggests an alteration in their immunological functions [50, 51].
The expression in mammary tumors of EGFR and related family members is known to be a poor prognostic factor for human breast cancer patients [52]. A possible link of this correlation to mechanism has been recently discovered as a paracrine loop between cancer cells and macrophages involving EGFR [14] (Fig. 7). In this paracrine loop, cancer cells in the primary tumor express the EGF receptor and CSF-1 ligand, which is stimulatory and chemotactic for macrophages, and macrophages in turn express the CSF-1 receptor and EGF, which is stimulatory and chemotactic to cancer cells thereby completing the paracrine loop [14, 53]. Results show that this paracrine loop is also important for the co-migration of both macrophages and tumor cells on the collagen I matrix that is elaborated in mammary tumors [53]. Using multiphoton microscopy with either cell injection or PyMT derived mammary tumors, the significance of the tumor cell-macrophage paracrine loop was examined in determining if it is required for intravasation. In order to image the interaction between tumor cells and macrophages, intravenous injections of texas red-dextran were used to specifically label macrophages by phagocytosis. Results show that perivascular tumor cell motility is linked to the presence of neighboring macrophages and that perivascular macrophage clusters in mammary tumors are sites of tumor cell intravasation (Wyckoff et al., Cancer Research, in press). These observations demonstrate that the paracrine loop between macrophages and tumor cells creates a microenvironment that is involved in both the invasion and intravasation of cancer cells in mammary tumors [33].
A paracrine loop exists between macrophages and tumor cells. a Images from a time lapse sequence, taken at 8 min intervals, of a tumor cell (green) moving into a perivascular macrophage cluster (red) in a live animal with a WAP-Cre/CAG-CAT-EGF/MMTV-PyMT generated tumor. The first panel illustrates the vessel (white lines-oval marks the lumen of the vessel), the green tumor cell (arrow), and the red macrophages (arrowheads). Asterisks mark the starting point of the tumor cell. Arrow in last panel shows endpoint of the tumor cell. Scale bar = 25 um. b Model for the cooperative function of invadopodia and podosomes in tumor invasion. Tumor-associated macrophages secrete EGF and express the CSF-1 receptor, while carcinoma cells secrete CSF-1 and express the EGF receptor to establish an EGF/CSF-1 paracrine loop. This paracrine loop enhances chemotactic cell migration and matrix remodeling required for tumor invasion through the coupled formation of invasive membrane protrusions, podosomes and invadopodia. WASP and N-WASP each play an important role in the regulation of podosome and invadopodium formation in macrophages and tumor cells, respectively, and are, therefore, critical in the development of tumor invasion and metastasis. Taken from [59].
Tumor Cell Intravasation Predicts the Metastatic Potential of Mammary Tumors
Tumors need a functional blood flow for growth, intravasation and metastasis. Since angiogenesis has been used as a target for many cancer therapies, understanding how cells get to and enter the vascular system is crucial. Therefore, new techniques of imaging the tumor’s vasculature and its dynamics have been developed. Intravital imaging again aids in the ability to follow angiogenesis and vascular density within a tumor, along with the ability to follow blood flow and determine diffusion within and the leakiness of vessels. To study the microvasculature density inside a tumor, multiphoton microscopy has been used to assemble 2D and 3D reconstructions of the complex vasculature of a xenograft tumor placed in a viewing chamber in a living mouse [10]. The diffusion coefficients for transport within a tumor were calculated using multiphoton FRAP [8].
Mammary tumors derived from both metastatic MTLn3 cells and PyMT oncogene expression show rounded and non-polarized cancer cells, except near blood vessels, where cancer cells are highly polarized, while the tumors derived from non-metastatic MTC cells contain cancer cells that are elongated to form tight sheets, where their polarity is unrelated to the position of the blood vessels [11, 12, 31, 54]. Using multiphoton microscopy as a tool shows that the organization of cancer cells inside mammary tumors and their relation to ECM and blood vessels is an important factor in the fate of these cells (Fig. 8). Current observations indicate that cell orientation toward blood vessels and the ability to cross the vessel wall as an intact cell are related properties and predict the likelihood of cell fragmentation during intravasation, which is inversely related to metastatic efficiency and metastatic potential [31]. The polarity of metastatic cells toward blood vessels is due to vessel-mediated attraction for the tumor cells, believed to involve chemotaxis. In this view, the polarity of tumor cells toward vessels results from their attraction to an extrinsic signal emanating from the vessel and/or its associated cells [12, 14, 31].
Blood vessels as sites of intravasation can be imaged using multiphoton-based intravital imaging. a Tumor cells (arrowheads) are seen entering blood vessels (dark spaces) inside a living tumor of a WAPCre-GFP-PyMT transgenic mouse. Scale bar = 25 μm. b Tumor cells (arrowheads) in a WAPCre-GFP-PyMT tumor are seen inside a blood vessel (dark space) after intravasation. Scale bar = 25 μm. c Macrophages phagocytically loaded with Texas Red-dextran are seen lining a blood vessel (delineated by dashed lines) in a living tumor. Scale bar = 25 μm.
The Future of Novel Approaches for Imaging and Analyzing the Mammary Tumor Microenvironment
The great advance of using multiphoton microscopes in visualizing live tissues is the ability to detect cell behavior in vivo in undissected mammary tumors. One of the few limitations in the use of these microscopes is the limited availability of fluorophores that can be used to detect cells or proteins of interest at sub-cellular resolution within the power spectrum of commercially available lasers. Fluorophores like GFP and CFP which can be genetically encoded in imaged cells, for example, have been used to analyze the behavior of cancer and host cells inside tumors [12, 13, 19]. While RFP has been imaged along with GFP in conventional microscopy and whole body imaging [7], the high intensity laser used in multiphoton imaging produces light only in the 720–980 nm range, which makes it impossible to excite fluorophores like RFP using multiphoton methods [19]. Additionally, using different variants of RFP, tetrameric and dimeric, experiments have shown that they are not suitable for live cell imaging mainly because they cause aggregates inside cells, which ultimately can cause loss of cell viability [19]. In addition, the YFP fluorophore is not a successful candidate because it is poorly excited by the wavelength used for excitation in multiphoton microscopes. Thus the challenge is to find fluorophores that can be simultaneously used to image more than two colors in vivo in the multiphoton microscope.
Newly developed variants of mRFP that shift the excitation toward the orange and yellow wavelengths show promise in multi-color multiphoton imaging. Since the challenge is to find a fluorophore that is excitable by wavelengths around 960–980 nm, but that do not crosstalk into GFP filters, proteins such as mOrange and tdTomato hold the most promise, as both have excitations within the power spectrum of conventional 2-photon lasers [55].
The use of a GRIN (gradient index) lens will greatly enhance the ability to image even deeper into the mouse. GRIN lenses use a negative gradient to focus light and can be up to 16 mm long with varying numerical apertures. In combination with the multiphoton microscope, in vivo imaging of organ systems anywhere in the living mouse can be achieved. A recent example of this procedure is the use of GRIN lens in deep brain imaging [56]. While the field size of GRIN lens is greatly reduced, cell–cell interactions can be imaged. This approach will lead to better understanding of cell morphology, protein localization, and the microenvironments in many types of tissues without dissection of the subject animal.
Recently, there is a growing awareness of the importance of defining the microenvironment of metastasis. Thus there is a need for new techniques, such as multiphoton microscopy, to probe the complex world of the tumor micro-architecture and tumor-stromal cell interactions. Current advances using expression profiling of subpopulations of cells in tumors are focusing on the understanding of the different determinants behind tumor cell and stromal cell interactions in the primary tumor by finding a gene expression signature of metastatic tumor cells, and relating these findings to current clinical observations.
A novel technique for studying the invasion of cancer cells inside tumors is the in vivo invasion assay which utilizes a microneedle filled with ECM, and chemoattractants, such as EGF or CSF-1 [26]. This method is very useful in mimicking the chemotaxis and signaling that takes place between cancer cells and blood vessels during intravasation [12, 26]. When studied using intravital imaging, the in vivo invasion assay showed that macrophages and tumor cells migrate together toward the microneedles, which further supported the importance of the paracrine loop between these two cell types for invasion in vivo [14]. Results show that macrophages and cancer cells are the only cells that migrate into the microneedles in mammary tumors, and that this co-migration is independent of how the mammary tumor was formed (i.e., using either transgenic oncogene expression or orthtopically injected tumor cell lines) [14]. Microarray-based gene expression profiling of tumor cells collected from the microneedles led to the discovery of the invasion signature, a gene expression pattern of invasive tumor cells that predicts metastatic outcome in animal models [32, 57] and confirmed that these cells had up-regulated genes for chemotaxis and repression of apoptosis, and simultaneously down regulated pro-apoptotic genes. The invasive cells were also characterized as being non-proliferative and resistant to chemotherapy [58]. These observations led to the ‘tumor microenvironment invasion model’ [59], which attributes the progression of tumors to the development of different microenvironments within the tumor that can elicit the transient expression of genes that lead to invasion [53]. The challenge for the future will be to apply the in vivo invasion assay to human tumors to derive an invasion signature that may be used in the development of diagnostics and therapeutics for use in humans.
Abbreviations
- ECM:
-
extracellular matrix
- MPIVI:
-
multiphoton-based intravital imaging
- SHG:
-
second harmonic generation
- MMPs:
-
matrix metallo-proteases
- GFP:
-
green fluorescent protein
- CFP:
-
cyan fluorescent protein
References
Weigelt B, Peterse JL, van ’t Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer 2005;5(8):591–602.
Callahan R, Smith GH. MMTV-induced mammary tumorigenesis: gene discovery, progression to malignancy and cellular pathways. Oncogene 2000;19(8):992–1001.
Varghese HJ, Mackenzie LT, Groom AC, Ellis CG, Ryan A, MacDonald IC, et al. In vivo videomicroscopy reveals differential effects of the vascular-targeting agent ZD6126 and the anti-angiogenic agent ZD6474 on vascular function in a liver metastasis model. Angiogenesis 2004;7(2):157–64.
Graham KC, Wirtzfeld LA, MacKenzie LT, Postenka CO, Groom AC, MacDonald IC, et al. Three-dimensional high-frequency ultrasound imaging for longitudinal evaluation of liver metastases in preclinical models. Cancer Res 2005;65(12):5231–7.
Al-Mehdi AB, Tozawa K, Fisher AB, Shientag L, Lee A, Muschel RJ. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nat Med 2000;6(1):100–102.
Im JH, Fu W, Wang H, Bhatia SK, Hammer DA, Kowalska MA, et al. Coagulation facilitates tumor cell spreading in the pulmonary vasculature during early metastatic colony formation. Cancer Res 2004;64(23):8613–9.
Hoffman RM. The multiple uses of fluorescent proteins to visualize cancer in vivo. Nat Rev Cancer 2005;5(10):796–806.
Brown EB, Boucher Y, Nasser S, Jain RK. Measurement of macromolecular diffusion coefficients in human tumors. Microvasc Res 2004;67(3):231–6.
Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 2005;307(5706):58–62.
Tyrrell JA, Mahadevan V, Tong RT, Brown EB, Jain RK, Roysam B. A 2-D/3-D model-based method to quantify the complexity of microvasculature imaged by in vivo multiphoton microscopy. Microvasc Res 2005;70(3):165–78.
Farina KL, Wyckoff JB, Rivera J, Lee H, Segall JE, Condeelis JS, et al. Cell motility of tumor cells visualized in living intact primary tumors using green fluorescent protein. Cancer Res 1998;58(12):2528–32.
Wang W, Wyckoff JB, Frohlich VC, Oleynikov Y, Huttelmaier S, Zavadil J, et al. Single cell behavior in metastatic primary mammary tumors correlated with gene expression patterns revealed by molecular profiling. Cancer Res 2002;62(21):6278–88.
Condeelis J, Segall JE. Intravital imaging of cell movement in tumours. Nat Rev Cancer 2003;3(12):921–30.
Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res 2004;64(19):7022–9.
Emptage NJ. Fluorescent imaging in living systems. Curr Opin Pharmacol 2001;1(5):521–5.
Centonze VE, White JG. Multiphoton excitation provides optical sections from deeper within scattering specimens than confocal imaging. Biophys J 1998;75(4):2015–24.
Williams RM, Zipfel WR, Webb WW. Interpreting second-harmonic generation images of collagen I fibrils. Biophys J 2005;88(2):1377–86.
Agarwal AR, Mih J, George SC. Expression of matrix proteins in an in vitro model of airway remodeling in asthma. Allergy Asthma Proc 2003;24(1):35–42.
Sahai E, Wyckoff J, Philippar U, Segall JE, Gertler F, Condeelis J. Simultaneous imaging of GFP, CFP and collagen in tumors in vivo using multiphoton microscopy. BMC Biotechnol 2005;5:14.
Brown E, McKee T, diTomaso E, Pluen A, Seed B, Boucher Y, et al. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nat Med 2003;9(6):796–800.
Campagnola PJ, Millard AC, Terasaki M, Hoppe PE, Malone CJ, Mohler WA. Three-dimensional high-resolution second-harmonic generation imaging of endogenous structural proteins in biological tissues. Biophys J 2002;82(1 Pt 1):493–508.
Agarwal A, Coleno ML, Wallace VP, Wu WY, Sun CH, Tromberg BJ, et al. Two-photon laser scanning microscopy of epithelial cell-modulated collagen density in engineered human lung tissue. Tissue Eng 2001;7(2):191–202.
Cahalan MD, Parker I, Wei SH, Miller MJ. Two-photon tissue imaging: seeing the immune system in a fresh light. Nat Rev Immunol 2002;2(11):872–80.
Jain RK, Brown EB, Munn LL, Fukumura D. Intravital Microscopy of Normal and Diseased Tissue in the Mouse. In: Live Cell Imaging: a Laboratory Manual. Cold Spring Harbor, New York: CSHL; 2004.
Lin EY, Jones JG, Li P, Zhu L, Whitney KD, Muller WJ, et al. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol 2003;163(5):2113–26.
Wyckoff JB, Segall JE, Condeelis JS. The collection of the motile population of cells from a living tumor. Cancer Res 2000;60(19):5401–4.
Motoike T, Loughna S, Perens E, Roman BL, Liao W, Chau TC, et al. Universal GFP reporter for the study of vascular development. Genesis 2000;28(2):75–81.
Faust N, Varas F, Kelly LM, Heck S, Graf T. Insertion of enhanced green fluorescent protein into the lysozyme gene creates mice with green fluorescent granulocytes and macrophages. Blood 2000;96(2):719–26.
Sasmono RT, Oceandy D, Pollard JW, Tong W, Pavli P, Wainwright BJ, et al. A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood 2003;101(3):1155–63.
Stalder AK, Ermini F, Bondolfi L, Krenger W, Burbach GJ, Deller T, et al. Invasion of hematopoietic cells into the brain of amyloid precursor protein transgenic mice. J Neurosci 2005;25(48):11125–32.
Wyckoff JB, Jones JG, Condeelis JS, Segall JE. A critical step in metastasis: in vivo analysis of intravasation at the primary tumor. Cancer Res 2000;60(9):2504–11.
Wang W, Goswami S, Lapidus K, Wells AL, Wyckoff JB, Sahai E, et al. Identification and testing of a gene expression signature of invasive carcinoma cells within primary mammary tumors. Cancer Res 2004;64(23):8585–94.
Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 2006;124(2):263–6.
Quaranta V. Motility cues in the tumor microenvironment. Differentiation 2002;70(9–10):590–8.
Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer 2003;3(5):362–74.
Ingman W, Wyckoff J, Xue C, Lin EY, Wang W, Goswami S, et al. Imaging invasion and metastasis in vivo. In: Wells A, editor. Cell motility in cancer invasion and metastasis. Massachusetts: Kluwer; 2005 (chapter 3). Kluwer; 2005.
Bailly M, Condeelis J. Cell motility: insights from the backstage. Nat Cell Biol 2002;4(12):E292–4.
Buccione R, Orth JD, McNiven MA. Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nat Rev Mol Cell Biol 2004;5(8):647–57.
Yamaguchi H, Lorenz M, Kempiak S, Sarmiento C, Coniglio S, Symons M, et al. Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. J Cell Biol 2005;168(3):441–52.
Yamaguchi H, Wyckoff J, Condeelis J. Cell migration in tumors. Curr Opin Cell Biol 2005;17(5):559–64.
Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer 2005;5(5):341–54.
Barnes CJ, Kumar R. Epidermal growth factor receptor family tyrosine kinases as signal integrators and therapeutic targets. Cancer Metastasis Rev 2003;22(4):301–7.
Klijn JG, Look MP, Portengen H, Alexieva-Figusch J, van Putten WL, Foekens JA. The prognostic value of epidermal growth factor receptor (EGF-R) in primary breast cancer: results of a 10 year follow-up study. Breast Cancer Res Treat 1994;29(1):73–83.
Siegel PM, Hardy WR, Muller WJ. Mammary gland neoplasia: insights from transgenic mouse models. Bioessays 2000;22(6):554–63.
Xue C, Wyckoff J, Liang F, Sidani M, Violini S, Tsai KL, et al. Epidermal growth factor receptor overexpression results in increased tumor cell motility in vivo coordinately with enhanced intravasation and metastasis. Cancer Res 2006;66(1):192–7.
Pal SK, Pegram M. Epidermal growth factor receptor and signal transduction: potential targets for anti-cancer therapy. Anticancer Drugs 2005;16(5):483–94.
Bailly M, Wyckoff J, Bouzahzah B, Hammerman R, Sylvestre V, Cammer M, et al. Epidermal growth factor receptor distribution during chemotactic responses. Mol Biol Cell 2000;11(11):3873–83.
Lichtner RB, Kaufmann AM, Kittmann A, Rohde-Schulz B, Walter J, Williams L, et al. Ligand mediated activation of ectopic EGF receptor promotes matrix protein adhesion and lung colonization of rat mammary adenocarcinoma cells. Oncogene 1995;10(9):1823–32.
Wyckoff JB, Insel L, Khazaie K, Lichtner RB, Condeelis JS, Segall JE. Suppression of ruffling by the EGF receptor in chemotactic cells. Exp Cell Res 1998;242(1):100–9.
Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res 2006;66(2):605–12.
Leek RD, Harris AL. Tumor-associated macrophages in breast cancer. J Mammary Gland Biol Neoplasia 2002;7(2):177–89.
Henderson IC, Patek AJ. The relationship between prognostic and predictive factors in the management of breast cancer. Breast Cancer Res Treat 1998;52(1-3):261–88.
Goswami S, Sahai E, Wyckoff JB, Cammer M, Cox D, Pixley FJ, et al. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res 2005;65(12):5278–83.
Shestakova EA, Wyckoff J, Jones J, Singer RH, Condeelis J. Correlation of beta-actin messenger RNA localization with metastatic potential in rat adenocarcinoma cell lines. Cancer Res 1999;59(6):1202–5.
Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol 2004;22(12):1567–72.
Levene MJ, Dombeck DA, Kasischke KA, Molloy RP, Webb WW. In vivo multiphoton microscopy of deep brain tissue. J Neurophysiol 2004;91(4):1908–12.
Wang W, Mouneimne G, Sidani M, Wyckoff J, Chen X, Makris A, et al. The activity status of cofilin is directly related to invasion, intravasation, and metastasis of mammary tumors. J Cell Biol 2006;173(3):395–404.
Goswami S, Wang W, Wyckoff JB, Condeelis JS. Breast cancer cells isolated by chemotaxis from primary tumors show increased survival and resistance to chemotherapy. Cancer Res 2004;64(21):7664–7.
Wang W, Goswami S, Sahai E, Wyckoff JB, Segall JE, Condeelis JS. Tumor cells caught in the act of invading: their strategy for enhanced cell motility. Trends Cell Biol 2005;15(3):138–45.
Acknowledgements
The authors would like to thank current and former laboratory members for their contributions to the work discussed in this review. The research summarized here was supported by CA100324.
Author information
Authors and Affiliations
Corresponding author
Additional information
Figures are available in color format in the online version of the article.
Rights and permissions
About this article
Cite this article
Sidani, M., Wyckoff, J., Xue, C. et al. Probing the Microenvironment of Mammary Tumors Using Multiphoton Microscopy. J Mammary Gland Biol Neoplasia 11, 151–163 (2006). https://doi.org/10.1007/s10911-006-9021-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10911-006-9021-5