Abstract
In present work three EOSs (The modified Lenard Jones EOS, Birch–Murnaghan (3rd) EOS, Vinet–Rydburg EOS) are used to study thermo elastic properties of carbon nanotubes at high pressure. The results obtained in the calculation of pressure at the different value of V/V0 are in agreement with the experimental data this indicates that these EOSs can also be used for the calculation of nanomaterials. The present work also is in agreement to the work of Stacey that pressure increases with decreases in volume while Bulk modulus increases with increase in pressure, First order pressure derivative of isothermal Bulk modulus decreases with increase in pressure. Gruneisen parameter calculated from different EOSs also satisfies the fact that for a good approximation the ratio of Gruneisen parameter to V/V0 is constant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Research on Nanomaterials is very hot topic for the researcher because of their demand in different sectors (Industrial area, Biological area, application in electrical area etc.). Nanomaterials not just nano sized materials but this is a entirely different area because at this level a lot of properties of materials are governed by quantum mechanics. In this paper our main concern is on carbon nanotubes (CNTs) because in the field of nanotechnology CNTs are unique and are material of future. In the present work we have theoretically predicted the thermo elastic properties of carbon nanotubes. We have used three different Equation of states (The modified Lenard Jones EOS, Birch–Murnaghan (3rd) EOS, Vinet–Rydburg EOS) for calculation. These equations of states (EOSs) are sufficient to describe almost all the thermo elastic properties of CNTs.
2 Method of analysis
To investigate thermo elastic properties of carbon nanotubes (CNTs) under high pressure we have used three different EOSs. They are given below:
-
(a)
The modified Lenard Jones EOS [1]: the modified Lenard Jones EOS is given below,
$$ P = \left( {\frac{{K_{0} }}{n}} \right)\left( y \right)^{ - n} \left[ {y^{ - n} - 1} \right] $$(1)where \(n = \frac{{K_{0}{\prime} }}{3}\) and \(y = \left( {\frac{V}{{V_{0} }}} \right)\).
Isothermal Bulk modulus can be found by Eq. (1) using the formula \(K{}_{T} = - V\left( {\frac{\partial P}{{\partial V}}} \right){}_{T}\)
$$ K_{T} = K_{0} y^{ - n} [2y^{ - n} - 1] $$(2)First order pressure derivative of Bulk Modulus (\(K_{T}^{\prime}\)) can be obtained by equation \(K_{T}^{\prime} = \left( {\frac{{\partial K_{T} }}{\partial P}} \right)_{T}\)
$$ K_{T}^{\prime} = n\left[ {\frac{{ - 4y^{ - n} + 1}}{{ - 2y^{ - n} + 1}}} \right] $$(3) -
(b)
Birch–Murnaghan (3rd) EOS [2]: the Birch–Murnaghan (3rd) EOS have been derived using finite strain theory is given below:
$$ P = \frac{3}{2}K_{0} [x^{ - 7} - x^{ - 5} ][1 + \frac{3}{4}(K_{0}^{\prime} - 4)(x^{ - 2} - 4)] $$(4)where \(x = \left( {\frac{V}{{V{}_{0}}}} \right)^{\frac{1}{3}}\).
Isothermal Bulk modulus can be found by Eq. (4) using the formula \(K{}_{T} = - V\left( {\frac{\partial P}{{\partial V}}} \right){}_{T}\)
$$ K_{T} = \frac{{K_{0} }}{2}[7x^{ - 7} - 5x^{ - 5} ] + \frac{3}{8}K_{0} (K_{0}^{\prime} - 4)(9x^{ - 9} - 14x^{ - 7} + 5x^{ - 5} ) $$(5)First order pressure derivative of Bulk Modulus (\(K_{T}^{\prime}\)) can be obtained by equation \(K_{T}^{\prime} = \left( {\frac{{\partial K_{T} }}{\partial P}} \right)_{T}\)
$$ K_{T}^{\prime} = \frac{{K_{0} }}{{8K_{T} }}[(K_{0}{\prime} - 4)(81x^{ - 9} - 98x^{ - 7} + 25x^{ - 5} ) + \frac{4}{3}(49x^{ - 7} - 25x^{ - 5} )] $$(6) -
(c)
(c) Vinet–Rydburg EOS [3, 4]: The Vinet–Rydburg EOS is based on universal relationship between binding energy and interatomic separation for solids, the Vinet–Rydburg EOS is given below:
$$ P = 3K{}_{0}x^{ - 2} (1 - x)\exp [\eta (1 - x)]\quad \quad $$(7)where \(x = \left( {\frac{V}{{V{}_{0}}}} \right)^{\frac{1}{3}}\) and \(\eta = \frac{3}{2}(K^{\prime}{}_{0} - 1)\).
Isothermal Bulk modulus can be found by Eq. (7) using the formula \(K{}_{T} = - V\left( {\frac{\partial P}{{\partial V}}} \right){}_{T}\)
$$ K_{T} = K_{0} x^{ - 2} [1 + (\eta x + 1)(1 - x)]\exp \{ \eta (1 - x)\} \quad $$(8)First order pressure derivative of Bulk Modulus (\(K_{T}^{\prime}\)) can be obtained by equation \(K_{T}^{\prime} = \left( {\frac{{\partial K_{T} }}{\partial P}} \right)_{T}\)
$$ K_{T}^{\prime} = \frac{1}{3}\left[ {\frac{{x(1 - \eta ) + 2\eta x^{2} }}{1 + (1 + \eta x)(1 - x)} + \eta x + 2} \right] $$(9)For doing calculation we can also use another EOS like Brennan–Stacey [5, 6] EOS but in the present work we are interested to find some thermo elastic properties of CNT so three EOS will be sufficient for calculations.
2.1 Relative isothermal expansion coefficient (\(\alpha_{r}\))
\(\alpha_{r} = \left( {\frac{\alpha }{{\alpha_{0} }}} \right)\), coefficient of thermal expansion is the ratio of relative change of volume and change in temperature [7]:
The value of \(\alpha_{r}\) can be calculated as follows:
2.2 The Gruneisen parameter
The value of Gruneisen parameter \((\gamma )\) can be calculated by using the formula given by Borton and Stacey [8]:
where \(f\)=2.35.
3 Result and discussion
Generally we use EOSs for Bulk materials but in this paper we have used EOSs for nanomaterials (CNTs). The input parameter used in this paper for performing calculations are given in the Table 1.
Here we have calculated the effect of pressure or compression on thermoelastic properties of carbon nano tubes. Figure 1 represents variation of pressure with respect to compression (V/V0) by using different equation of states and we notice that all the three EOSs used in these calculations gives exactly identical results and the theoretically obtained values from EOSs gives complete agreement with experimental data [10].
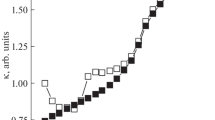
Figure 2 represents the variations of isothermal bulk modulus with respect to temperature and we notice bulk modulus is increasing with the increase in pressure and this completely agree with the work of Stacey that bulk modulus increases with the increase in pressure [11].
Figure 3 represents the variations of \(K_{T}^{\prime}\) with respect to pressure, we can notice that \(K_{T}^{\prime}\) is decreasing with increase in pressure with all the three EOSs used in the calculation and this also completely agree with the work of Stacey that \(K_{T}^{\prime}\) is decreases with increase in pressure [11]. Here we notice that Vinet–Rydburg EOS give very small deviation from other two.
Figure 4 represent the variations of Relative isothermal expansion coefficient \(\alpha_{r}\) with respect to pressure for CNT and we notice that it also decreasing with increase in pressure from theoretical point of view in all the three EOSs used in this calculations.
Figure 5 represents the variation of Gruneisen parameter with respect to different compression V/V0. Here we notice that the graph between \(\gamma\) and V/V0 is straight line in all the three EOSs used in this calculation. So such type of variation of \(\gamma\) with V/V0 completely agree with the fact that for a good approximation the ratio of Gruneisen parameter to V/V0 is constant [12]. Here we see that Vinet–Rydburg EOS is showing disagreement from the other two EOSs this is due to this EOS was showing was also showing deviation in value of \(K_{T}^{\prime}\).
In the present study we have used EOSs for calculations of thermo elastic properties of carbon nanotubes which is nanomaterials so this leads the conclusion that the EOSs which are commonly used for calculation of Bulk materials can also used for calculation of properties of nanomaterials [13]. So EOSs can be used to study the properties of nanomaterials at high pressure or compression [14, 15].
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author.
References
J. Sun, A modified Lennard–Jone type equation of state for solids strisfying the spinodal condition. J. Phys. Condensed Matter 17(12), L103–L111 (2005)
F. Birch, Finite elastic strain of cubic crystals. Phys. Rev. 71, 809–824 (1947)
P. Vinet, J. Ferrante, J.R. Smith, J.H. Rose, J. Phys. C 92, L467 (1986)
P. Vinet, J. Ferrante, J.H. Rose, J.R. Smith, J. Phys. Res. 19, 9319 (1987)
F.D. Stacey, B.J. Brennan, R.D. Irvine, Geophys. Surv. 4, 189 (1981)
H.K. Rai, S.P. Shukla, A.K. Mishra, A.K. Pandey, Electron. J. Chem. 5, 385–394 (2008)
G. Borelius, Solid State Physics (Academic Press, New York, 1958), pp.6–65
M.A. Barton, F.D. Stacey, Phys. Earth Planet. Inter. 39, 167 (1985)
J. Tang, L.C. Qin, T. Sasaki, M. Yudasaka, A. Matsushita, S. Iijima, Compressibility and polygonization of single-walled carbon nanotubes under hydrostatic pressure. Am. Phys. Soc. 85(9), 1887 (2000)
S.M. Sharma et al., Pressure-induced phase transformation and structural resilience of single-wall carbon nanotube bundles. Phys. Rev. Condensed Matter Mater. Phys. (2001). https://doi.org/10.1103/PhysRevB.63.205417
F.D. Stacey, Phys. Earth Planet. Inter. 89, 219 (1995)
R. Boehler, Phys. Rev. B 11, 6754 (1983)
A.I. Ghazal, A.M. Al-Sheikh, J. Phys. Conf. Ser. (2021). https://doi.org/10.1088/1742-6596/1999/1/012075
M.S. Ghiorso, Am. J. Sci. 1999, 304–752 (2004)
R. Gupta, M. Gupta, Indian Acad. Sci. 44, 218 (2021)
Funding
The authors declared that they have not receive any funding for the paper entitled Theoretical prediction for Thermoelastic properties of carbon nanotubes (CNTs) at different pressure or compression using Equation of States.
Author information
Authors and Affiliations
Contributions
All persons who meet authorship criteria are listed as authors, and all authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript. Furthermore, each author certifies that this material or similar material has not been and will not be submitted to or published in any other publication before its appearance in the “Journal of Mathematical Chemistry”. Category 1 Conception and design of study: AKP, acquisition of data: SS, analysis and/or interpretation of data: CKD, Category 2 Drafting the manuscript: SS. Approval of the version of the manuscript to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Hereby, Prof. Chandra Kumar Dixit, Dr. Anjani Kumar Pandey and Shivam Srivastava consciously assure that for the manuscript Theoretical prediction for thermoelastic properties of carbon nanotubes (CNTs) at different pressure or compression using Equation of States the following is fulfilled: (1) This material is the author’s own original work, which has not been previously published elsewhere. (2) The paper is not currently being considered for publication elsewhere. (3) The paper reflects the author's own research and analysis in a truthful and complete manner. (4) The paper properly credits the meaningful contributions of co-authors and co-researchers. (5) The results are appropriately placed in the context of prior and existing research. (6) All sources used are properly disclosed (correct citation). Literally copying of text must be indicated as such by using quotation marks and giving proper reference. (7) All authors have been personally and actively involved in substantial work leading to the paper, and will take public responsibility for its content. I agree with the above statements and declare that this submission follows the policies of Solid State Ionics as outlined in the Guide for Authors and in the Ethical Statement.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Srivastava, S., Pandey, A.K. & Dixit, C.K. Theoretical prediction for thermoelastic properties of carbon nanotubes (CNTs) at different pressure or compression using equation of states. J Math Chem 61, 2098–2104 (2023). https://doi.org/10.1007/s10910-023-01506-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10910-023-01506-3