Abstract
Most ants live in closed societies from which non-members are excluded through fighting or ritualized displays to protect colony resources. Nestmate recognition is the process by which ants discriminate nestmate from non-nestmate ants. Ants use cues coded in mixtures of long-chain hydrocarbon compounds on the cuticle as nestmate recognition cues. Pavement ants (Tetramorium caespitum) form conspicuous wars between neighboring colonies that are organized after workers meet and make the decision to fight after assessing nestmate recognition cues. These wars involve thousands of individuals. Fighting is ritualized and few ants die in the process. We identified 24 cuticular hydrocarbon compounds, above 1% in relative abundance, in the profile of pavement ants with chain lengths ranging from 15 to 31 carbon atoms. Cuticular lipids contained, in order of abundance: mono-methyl alkanes (45–56%), n-alkanes (range: 16–40% relative abundance), and alkenes (10–20%), with small or trace amounts of di-methyl, tri-methyl alkanes and fatty acids. Results from behavioral tests show that pavement ants assess information in cuticular hydrocarbon profiles to recognize both conspecific and heterospecfic (Pogonomyrmex occidentalis and Camponotus modoc) non-nestmate ants and that the relative abundance of methyl-branched alkanes and alkenes codes for nestmate status, at least for conspecific interactions. Our data add to a growing body of knowledge about how ants use cuticular hydrocarbon based nestmate recognition cues to prevent the intrusion of non-nestmates in to colony space.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most ants live in closed societies, called colonies, from which workers prevent the intrusion of non-nestmates into colony space to avoid competition or exploitation of colony resources. Nestmate recognition is the process used by social insects to recognize and discriminate nestmates from non-nestmates (Espanoda and Gordon 2015; Sturgis and Gordon 2012; vander Meer 1998; van Zweden and d'Etorre 2010). Responses to non-nestmate ants often, but not always, lead to agonistic behavior directed at a non-nestmate intruder, including biting, stinging, and spraying of formic acid, in order to prevent the non-nestmate ant from accessing colony resources (Roulston et al. 2003; Bjoerkman-Chiswell et al. 2008). Nestmate recognition also acts to maintain colony cohesiveness and cooperation (Crozier and Pamilo 1996; Sturgis and Gordon 2012).
Ants recognize both conspecific and heterospecfiic non-nestmates through the detection of hydrocarbon cues (Akino et al. 2004; Astruc et al. 2001; Buchowski et al. 2005; Ichinose 1991; Ichinose and Lenoir 2010; Lahav et al. 1999; Liang and Silverman 2000; Liang et al. 2001; Lucas et al. 2005; Greene and Gordon 2007; Martin et al. 2008; Menzel et al. 2009; Ozaki et al. 2005; Roux et al. 2009; Torres et al. 2007; van Wilgenburg 2010; Wagner et al. 2000). Cuticular hydrocarbons serve a primary function of preventing trans-cuticular water loss and abrasion to the cuticle (Gibbs 1998; Gibbs and Rajpurohit 2010). Over 1000 different ant hydrocarbon compounds from over 78 ant species have been described (Martin and Drijfhout 2009b). The major structural classes of ant hydrocarbons, in order of abundance, include n-alkanes, mono- and di-methyl-alkanes, and alkenes (Martin and Dirjfhou 2009b). The majority of ant cuticular hydrocarbon compounds range from 19 to 33 carbons in chain-length with odd-numbered n-alkanes of 25 to 33 carbons in chain-length being especially prominent (Martin and Drijfhout 2009b). Ants of the same species share most hydrocarbon compounds in their profiles although the relative proportions of the compounds can differ among colonies. Ants of different species also exhibit qualitative differences in their profiles along with quantitative differences in the relative abundance of shared compounds (Martin and Drijfhout 2009b). Experimental approaches have typically assessed the ability of focal ants to discriminate between cuticular hydrocarbon profiles from nestmates and non-nestmates (Roulston et al. 2003). Hydrocarbon profiles, or, the mixture of hydrocarbons on the cuticle, are often presented on non-living ant mimics or are used to supplement the profiles of living ants (e.g., Greene and Gordon 2003; Greene and Gordon 2007; Roulston et al. 2003; Roux et al. 2009; Brandsteater et al. 2011; van Wilgenburg 2010).
Cuticular hydrocarbon based nestmate recognition cues have been shown to be detected by specialized sensilla on the antennae (Ozaki et al. 2005; Sharma et al. 2015). Information in cues detected by sensilla are compared to a template; mis-matches between the cue and template trigger nestmate recognition behavior (Bos and D’Ettore 2012). Ants can detect hydrocarbons at low amounts; workers of the ant Aphaenogaster senilis can discriminate non-nestmate hydrocarbon profiles at very low concentrations equivalent to 10−4 workers-worth of hydrocarbons (Ichinose and Lenoir 2010).
Although most ant species fight territorial intruders after nestmate recognition responses in effort to kill, some species use ritualized fighting and displays during conflicts with neighbors in order to minimize the costs of fighting, especially death of workers, yet communicate colony size. Honeypot ant (Myrmecocystus mimicus) colonies participate in tournaments in which colonies recruit workers to an area at which workers form two colonies perform displays to each other (Hölldobler 1976). Tournaments end when one colony has recruited more displaying workers. Meat ants (Iridomyrmex purpureus) line up at territorial boundaries and display to ants of neighboring colonies in order to prevent intrusion (Ettershank and Ettershank 1982; van Wilgenbrug et al. 2005). Tournaments can involve hundreds of ants and last for days. Displays begin after two ants antennate and last for about 15 s. Less commonly, meat ants will engage in fighting with neighbors; territory holders were more often initiate fighting while territorial intruders were more likely to initiate displays (Ettershank and Ettershank 1982; van Wilgenbrug et al. 2005). Forelius pruinosis display to conspecific intruders by shaking and aligning their bodies but do not do not engage in fighting behavior (Ettershank and Ettershank 1982; Hölldobler 1982).
The pavement ant, Tetramorium caespitum (subfamily Myrmicinae) is a tramp ant species and a pest to human structures and agriculture (McGlynn 1999; Scharf et al. 2004). It is commonly found in urban and suburban habitats in the Northern Hemisphere. Colonies are uni-modal, can be polygynous and commonly nest under foundations, sidewalks, and pathways (Steiner et al. 2003). Colonies of this species are well-known for their ant wars which involve thousands of ants fighting for the purpose of establishing colony boundaries (Hoover et al. 2016; Plowes 2008). In these wars, ants almost always fight in pairs, grasping mandibles, and fighting for hours. Fighting in pavement ant wars is ritualized and few ants die in the process (Plowes 2008). Some ants do not fight but recruit workers from colonies to the war using pheromone trails and wars establish quickly, on a timeframe of about 30 min (Hoover et al. 2016; Plowes 2008). Pavement ant wars are likely ritualized tournaments to display colony size at territorial boundaries, like has been reported for some other ants (Hölldobler 1976; Ettershank and Ettershank 1982; van Wilgenbrug et al. 2005).
Methods
Research Sites
Pavement ants were collected by aspiration along foraging trails in urban areas of Denver, Colorado, including on Auraria Campus, home to University of Colorado Denver. Colonies were most often found on sidewalks or near cement foundations adjacent to irrigated grass. Ants were collected during their typical seasonal foraging activity period, most often at or after dusk, by using food bait and aspirating the ants. Pavement ants recruit in high numbers to foods with high levels of sugar and the workers are attracted to fats (Colignon and Detrain 2010). Pavement ant colonies are monodomous; we collected workers from colonies that were separated by at least 10 m, a distance that ensured we collected from distinct colonies.
Western harvester ant (Pogonomyrmex occidentalis) and carpenter ant (Camponotus modoc) foragers were collected by hand using clean forceps at colonies found in central Denver. In the Denver, Colorado area, T. caespitum interacts with both species as it can be found nesting near P. occidentalis nest mounds and tends to be active with C. modoc in evenings along curbs and sidewalks.
Ants collected for surface lipid extraction were frozen after collection at −20 °C until used for solvent extraction. Behavioral bioassays using live ants were always initiated up to 24 h after capture.
Extraction of Surface Lipids and Isolation of Cuticular Hydrocarbons
To extract and isolate the cuticular hydrocarbon component of the surface lipids, thawed ants were immersed in 1.0 ml of 100% pentane for 10 min with occasional mild agitation (Howard and Blomquist 2005; Nelson and Blomquist 1995; Greene and Gordon 2003, 2007). Cuticular hydrocarbons were isolated from more polar lipids by eluting surface lipid extracts through a 2-cm column of silica gel (Sigma-Aldrich, Grade 60, 70–230 mesh, 60 Å) packed in a 5 ¾ inch Pasteur pipette plugged with filter paper. Before adding cuticular hydrocarbon extracts to columns, the silica gel was activated with about 1 ml of 100% pentane. Three ml of 100% pentane was used to elute the cuticular hydrocarbon fractions into clean tubes. This processes yielded approximately 1.5 micrograms of cuticular hydrocarbon per ant. Cuticular hydrocarbon extracts were stored at −20 °C until used in an experiment or another chemical procedure.
For structural analysis, hydrocarbons were collected from 200 workers each from five colonies and another pooled sample of ants containing 1000 workers from three colonies. We used the pooled samples to create an injectable sample that would have clear peaks relative to background noise. The pooled samples also allowed us to better capture all CHC compounds in the population.
Separation of Cuticular Hydrocarbon Structural Classes
Separation of n-alkanes, methyl branched-alkanes, and alkenes was conducted according to methods detailed by Nelson and Blomquist (1995) and Greene and Gordon (2007). To separate saturated cuticular hydrocarbons from the unsaturated n-alkane fraction, cuticular hydrocarbon extracts were eluted through a 2 cm silica gel column (Sigma-Aldrich, Grade 60, 70–230 mesh, 60A) impregnated with a silver nitrate solution (10:1, v:v; Sigma-Aldrich). The column was covered with aluminum foil to avoid exposure of silver nitrate to light. The column was activated with 1 ml of 100% pentane. The cuticular hydrocarbon extracts were added and saturated hydrocarbons (n-alkanes and methyl-branched alkanes) were eluted with 3.0 ml of 100% pentane. Unsaturated hydrocarbons (n-alkenes) were eluted with 3.0 ml of 10% ethyl acetate/90% pentane.
n-alkanes were separated from methyl-branched alkanes using 5 Å molecular sieves (Sigma, M-0258; 1/16th inch pellets, nominal pore diameter 5 Å; Nelson and Blomquist (1995) and Greene and Gordon (2007)). Prior to separation, the molecular sieves were desiccated by baking them at 170 °C for 48 h. The sieves were washed with isooctane twice after desiccation and stored in an Erlenmeyer flask covered in isooctane. The saturated hydrocarbon fractions were dried and re-solvated with about 1 ml of 100% isooctane. Fractions in isooctane were placed in a capped tube with the 5 Å sieves for 48 h on a heater block set to 75 °C. The sieves were then washed with isooctane, separated from the solvent, and discarded. The isooctane in the methyl-alkane fraction was allowed to evaporate and the hydrocarbons were re-solvated with 100% pentane. The methyl-alkane fraction in pentane was eluted through a 2 cm silica gel column, as above, to remove contaminants from the molecular sieves.
Because n-alkanes could not be recovered efficiently from the molecular sieves, an n-alkane fraction was re-constructed by dissolving of each of the following in 40 ml of 100% pentane: 5.0 mg pentadecane (Fluka), 3.4 mg of tricosane (Aldrich), 4.3 mg of tetracosane (Fluka), 2.7 mg of pentacosane (Fluka), 5 mg of hexacosane (Supelco), 3.1 mg of heptacosane (Aldrich), and 3.1 mg of henitriacontane (Aldrich). This sample was aliquoted into 10 test tubes and the solvent was allowed to evaporate.
Previous use of these fractionation techniques confirmed that the fractionation techniques lead to pure structural class fractions (Greene and Gordon 2007). Here, we confirmed separation of the hydrocarbon structural classes by comparing retention times of peaks from gas chromatograms. For this study, the n-alkane fraction was created using synthetic hydrocarbons and was composed of 100% n-alkane molecules. No methyl-alkane or n-alkene peaks were found in the n-alkane fraction. Furthermore, the methyl-alkane fraction was found to contain 98% methyl-alkanes, with only n-pentacosane found as a contaminant (see supplementary data). The n-alkene fraction was composed of 85% n-alkenes with n-alkanes accounting for the majority of contaminants (supplemental data).
Creation of Ant Mimics
Interactions with hydrocarbon-coated glass beads can mimic chemical interactions with live ants (e.g., Greene and Gordon 2003, 2007, 2013; Roulston et al. 2003). Ant mimics were prepared by placing 5 mm diameter glass beads (Fisher Scientific) in the vials containing isolated cuticular hydrocarbons in 100% pentane as in Greene et al. (2007, 2013). The glass beads were left in the solvent overnight at room temperature to allow pentane to evaporate. Thus the cuticular hydrocarbons from the ants were isolated and then transferred to the surface of glass beads. The beads were stored at −20 °C until the day of experiment, performed one to 2 days after extraction.
For the nestmate recognition bioassays, cuticular hydrocarbons from 10 pavement workers from each colony were extracted and added to a single ant mimic. A total of 30 colonies were sampled for all experiments. For the species recognition bioassay, 5 workers of P. occidentalis (from one colony) worth of hydrocarbon were applied to each glass bead and 5 Camponotus workers worth of (from one colony) hydrocarbon were added to each glass bead. The number of ant-equivalents used per mimic was designed to provide similar amounts of hydrocarbons on all beads as T. caespitum are much smaller ants than C. modoc or P. occidentalis workers and to account for hydrocarbons coating the tube walls rather than the surface of the bead. As a blank control, beads were treated with 100% pentane which was allowed to completely evaporate.
Chemical Analyses
In order to measure relative abundances of known peaks, analysis by gas chromatography was conducted using a Varian 3900 gas chromatograph with a DB-5 fused silica capillary column (30 m, 0.25 um ID, 0.25 um film thickness; J&W Scientific). Oven temperature was held at 170 °C for 5 min during split-less injection, raised to 220 °C at 25 °C per min, and then to 310 °C at a rate of 3 °C·min per min with a 5 min hold. The injection temperature was 280 °C. The carrier gas was helium, set to a flow of 1.0 ml/min, and flame ionization detector was used. The relative abundances of the major hydrocarbon peaks were measured on gas chromatograms by dividing the area of each peak by the sum of all peak areas.
Identification of cuticular hydrocarbons was performed using gas chromatography mass spectrometry (GCMS; Agilent 6890 N/5973 MSD). Two microliter samples were injected by splitless injection onto a capillary column (OV-5 fused silica capillary column, 30 m, 0.32 mm ID, 0.5 μm; Ohio Valley). Samples were purged after 1 min. The carrier gas was helium with the flow rate set to 1.1 ml/min. The injection temperature was 280 °C. For electron ionization, the initial oven temperature was 170 °C for 1 min, and then the temperature was increased from 170 °C to 300 °C at 100 °C/min, then more slowly from 300 °C to 330 °C at 15 °C/min with a 10 min hold. To ensure of the consistency of retention time between runs, a mixture of n-alkane standard (C10, C20, C22, C24, C26, C28, C30, C32, C34, C36, C38, and C40, Sigma-Aldrich) were injected before sample runs. For chemical ionization GC, the initial temperature was 75 °C, after 2 min the temperature was increased from 75 °C to 330 °C at 15 °C /min and held at 330 °C for 10 min.
N-Alkanes were identified by [M-15]+/base peak in CI spectra and EI mass spectra library search with ECL and Kováts Indices as references. Mono-methyl alkanes were first identified by [M-15]+/base peak in CI spectra and EI mass spectra. Di-methyl alkanes were characterized by [M-15]+/base peak in CI mass spectra and EI mass spectra. Cuticular hydrocarbons were quantitatively analyzed by measuring the peak areas of the resulting chromatograms using Agilent MSD ChemStation Software. RTE Integrator was selected for integration. Only the peak area > 1% of the largest peak area were selected for integration for quantitation. We manually checked and corrected all peak identifications used for the integration methods to provide the most accurate estimate of relative abundance for each peak. Both Kovats indices and equivalent chain length analyses were conducted to confirm the identity of compounds. When available, mass spectra of unknowns were compared to standards.
Experiment 1: Do Pavement Ants Use Cues in Cuticular Hydrocarbons to Discriminate Nestmates from Conspecific and Heterospecfic Non-Nestmates?
The following stimuli were tested at 14 focal colonies of T. caespitum: 1) nestmate hydrocarbon coated mimics, 2) two randomly assigned non-nestmate conspecific hydrocarbon coated mimics, 3) heterospecific hydrocarbon coated mimics from P. occidentalis, 4) heterospecific hydrocarbon coated mimics from C. modoc, and 5) a blank control mimic.
The agonistic behavior exhibited by focal ants was measured towards treated ant mimics. The mimics were placed about 10 cm from the nest entrances of focal T. caespitum colonies to ensure a high interaction rate with workers entering and exiting the colonies and also because fights with non-nestmate ants often occur in the vicinity of a nest entrance. All the behavioral tests were performed in the evening, between 7:00 PM and 12:00 AM when colonies were active and foraging. All behavioral tests were conducted as blind tests; the observer did not know which type of stimuli was being measured while recording data. At each colony, the stimuli were presented in a random sequential order all on the same evening, with about 3 min breaks between tests, to control for the effect of treatment order.
After placement of mimics, aggressive behavioral responses of all ants that made antennal contact with the mimics were measured during a 10 min observation period. Every 30 s, behavioral measurements were made by observing a focal ant for 10 s. During each 10 s duration, aggression was quantified by assigning a score = 0 for ants that antennated the mimic without flaring mandibles or biting the mimic; a score = 1 for ants that antennated the mimic and flared mandibles; and, score = 2 for ants that antennated the mimic and bit it. We chose these behaviors to measure as they are easily observed, even with a small ant like T. caespitum, and they are commonly observed in ant recognition responses including, as we had previously observed, by T. caespitum in fights in the field, also in Plowes (2008). For each replicate, and following Hefetz et al. (1996), an aggression score was calculated by summing the aggression scores measured every 30 s and dividing by the total number of observations of antennal contact made by the focal ant with the ant mimic.
Median scores of aggressive ants were compared using a Kruskal-Wallis test and post-hoc analyses were conducted using Wilcoxon Paired-Sample tests with a revised alpha = 0.0033 (Bonferroni correction). A non-parametric test was used because the data distributions did not mimic a normal distribution (raw data displayed in Fig. 2 as filled dots).
Experiment 2: How are Nestmate Recognition Cues Coded in Pavement Ant Cuticular Hydrocarbon Profiles?
Supplementation of Cuticular Hydrocarbon Profiles of Live Ants
The cuticular hydrocarbon profiles of pavement ant nestmates were supplemented with hydrocarbon structural classes – n-alkanes, n-alkenes, methyl-branched alkanes – in order to determine which structural class of hydrocarbon coded for nestmate recognition cues in T. caespitum. Two hundred workers were used from each of 8 colonies. Six sets of 20 ants from each colony were created and housed in Petri dishes for hydrocarbon supplementation. From five of the sets for each colony, ants were vortexed for 1 min using a Scientific Industries VWR Scientific Vortex Genie 2 model # G-560 on its lowest setting of 1 in a test tube containing: 1) evaporated pentane (blank control), 2) 400 ant-equivalents of pooled non-nestmate hydrocarbons, 3) 400 ant-equivalents of synthetic n-alkanes, 4) 400 ant-equivalents of non-nestmate methyl-alkanes, or 5) 400 ant-equivalents of non-nestmate n-alkenes (Torres et al. (2007)). Following vortexing, groups were placed back in their respective petri dish. The same vortexing process was performed on two subsequent mornings. This process allowed supplementation of cuticular hydrocarbons on workers with what appeared to be minimal or no obvious damage to the ants. Blank control ants were neither vortexed or treated with hydrocarbons. The 6th set was saved to be used as un-treated focal ants in the following behavioral bioassay; these ants were contained in a Petri dish for 3 days. In each Petri dish, a damp cotton ball and approximately 0.5 g of cream cheese for food were added.
Bioassay to Test for Aggression
Similar to the bioassay used by Chen and Nonacs (2000), an arena was created by placing a bottomless Fluon-coated polystyrene Petri dish (Fisherbrand, 60 mm × 15 mm) on a sidewalk placed about 10 cm from nest entrances of focal colonies. Putty was placed around the outside of the arena in order to keep ants from escaping. Ten ants from the focal colony were placed inside the arena and allowed to acclimate for 1 min after which 10 treated ants were placed in the arena as opponents. Each of the following were tested as opponents in the using 8 colony replicates: 1) 10 nestmates that had been isolated from the mother colony for 3 days; 2) 10 nestmates vortexed in a clean test tube previously treated with pentane that was allowed to evaporate; 3) 10 nestmate supplemented with non-nestmate cuticular hydrocarbons; 4) 10 nestmate supplemented with n-alkanes; 5) 10 nestmate supplemented with methyl-alkanes; and, 6) 10 nestmate supplemented with n-alkenes. Here we used treated live ants instead of ant mimics because, unlike for the ant mimics used in experiment 1, live ants fight back, allowing for easier identification of aggression.
Fighting was defined to have occurred when an ant clasped another’s body with its mandibles; this usually occurs immediately after antennating a non-nestmate ant (Bubak et al. 2016; Hoover et al. 2016; Plowes 2008). Fighting was measured from two digital pictures taken 30 s and 1 min into the assay, time scales similar to other ant nestmate recognition assays (e.g., Morel et al. 1988; Roulston et al. 2003; Whitehouse and Jaffe 1995). The number of ants fighting, out of 20, in each picture was counted. We analyzed the average number of ants fighting from the two pictures. We predicted that increasing, or changing, the relative abundance of a structural class would cause treated nestmate ants to fight at a high rate compared to control-treated nestmate ants.
Results
Identification of T. caespitum Cuticular Hydrocarbon Profile
We measured 24 common hydrocarbon peaks on the cuticle of pavement ants, all with a relative abundance greater that 1% (Fig. 1). The chain length in pavement ant cuticular hydrocarbon ranges from 21 to 31 carbon atoms. Cuticular lipids contain n-alkanes (range: 16–40% relative abundance), mono-methyl alkanes (45–56%) and alkenes (10–20%), with small or trace amounts of di-methyl, tri-methyl alkanes and fatty acids. The most abundant compound was 11-methylpentacosane (11-meC25) in mono-methyl alkanes; n-pentacosane in n-alkanes, and heptacosene in alkenes.
Experiment 1: Do Pavement Ants Use Cues in Cuticular Hydrocarbons to Discriminate Nestmates from Conspecific and Heterospecfic Nestmates?
.
Workers displayed high levels of aggression to both conspecific non-nestmate hydrocarbon samples and hydrocarbon samples from heterospecific ants in comparison to blank controls and nestmate hydrocarbon samples when presented on mimics (Kruskal-Wallis, X2 = 60.396, df = 5, p < 0.0001; Fig. 2). All alpha levels were adjusted to 0.0033 using a Bonferroni correction. Mimics coated with conspecific non-nestmate hydrocarbons stimulated more workers to fight compared to the glass beads coated with nestmate hydrocarbon (Wilcoxon test, p < 0.00012 for both tests) and the blank control (Wilcoxon test, p < 0.00012). Mimics coated with heterospecific non-nestmate hydrocarbons also stimulated a higher proportion of aggressive ants score compared to the glass beads coated with nestmate hydrocarbon (Wilcoxon test, p < 0.00012 for both tests) and the blank control (Wilcoxon test, p < 0.00012). There was no difference in the median responses to the conspecific non-nestmate ant stimuli and heterospecific ant stimuli (Wilcoxon test, all p-values >0.0033). There was not a significant difference in responses to the nestmate cuticular hydrocarbon stimulus and the blank control (Wilcoxon test, p > 0.0033).
Pavement ants displayed higher levels of aggression to ant mimics treated with conspecific non-nestmate cuticular hydrocarbons (CHC) (TcNonCHC1 and TcNonCHC2) and CHCs from Camponotus modoc (CmCHC) and Pogonomyrmex barbatus (PoCHC) compared to the blank control and nestmate CHC (TcNMCHC) treated mimics. Bars display the median aggression score for each treatment. Boxes display the 25% and 75% inter-quartile ranges. Outliers are plotted as open circles. Raw data are plotted as filled dots
Experiment 2: How are Nestmate Recognition Cues Coded in Pavement Ant Cuticular Hydrocarbon Profiles?
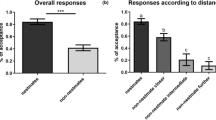
The median number of aggressive ants was affected by the hydrocarbon supplementation of nestmate cuticular hydrocarbon profiles (Kruskal-Wallis, X2 = 52.85, df = 5, p < 0.0001; Fig. 3). Supplementation with methyl-branched alkanes elicited aggression from a mean of 5.5 ants (range: 3.715 to 7.285), supplementation with n-alkenes elicited aggression from a mean of 2.4 ants (range: 0.822 to 3.978), and n-alkanes elicited aggression from a mean of 0 ants. The blank control treatment elicited aggression from a mean of 0 ants, the vortex control treatment elicited aggression from a mean of 0.2 ants (range: 0 to 2), and the pooled cuticular hydrocarbon treatment elicited aggression from a mean of 7.35 ants (range: 0.822 to 3.978). There was no difference in the levels of aggression among the n-alkane fraction treatment, the vortex control, and the blank control (Wilcoxon rank-sum test; p > 0.05; adjusted alpha = 0.003 (Bonferroni)). Supplementation with pooled hydrocarbons, the methyl-alkane fraction and the n-alkene fraction stimulated mean levels of aggression higher than for the non-vortex blank control, vortex control, and the n-alkane fraction (Wilcoxon rank-sum test; p < 0.0001; adjusted alpha = 0.003 (Bonferroni)). There was a difference in aggression levels with supplementation with methyl-alkanes and n-alkenes (Wilcoxon rank-sum test; p < 0.0007; adjusted alpha = 0.003 (Bonferroni)). Supplementation with an intact non-nestmate pooled hydrocarbon fraction elicited higher levels of aggression than all other treatments except for supplementation with the methyl-alkane fraction (Wilcoxon rank-sum test; p < 0.0003; adjusted alpha = 0.003 (Bonferroni)).
A higher number of pavement ants were aggressive towards live nestmates that had their cuticular hydrocarbon (CHC) profiles supplemented with non-nestmate CHCs (Pooled CHC), methyl-branched CHCs (Methyl-Branched), and n-alkenes (n-Alkenes) compared to the blank control, vortex control, and supplementation with n-alkanes (n-Alkane). Bars display the median aggression score for each treatment. Boxes display the 25% and 75% inter-quartile ranges. Outliers are plotted as open circles. Raw data are plotted as filled dots
Discussion
Our data provide evidence that cues in cuticular hydrocarbons are sufficient to code for nestmate and non-nestmate identity in pavement ants. Pavement ant workers recognize cues present in cuticular hydrocarbons during interactions with both conspecific non-nestmates and heterospecific non-nestmate ants to discriminate nestmates from non-nestmates. More specifically, our data provide evidence that nestmate recognition cues for T. caespitum are specifically coded in the relative abundance of methyl-branched alkanes and n-alkenes in conspecific nestmate recognition cues. Our results, using live ants modified with hydrocarbon structural classes, could have resulted from either the response of the receiver ant or the treated ant, or perhaps both, to the hydrocarbon supplementation treatments, although each explanation provides evidence of the role of methyl-branched and n-alkenes as recognition cues. Pavement ants also use cues in their cuticular hydrocarbon profile to recognize nestmate ants, an important aspect to the collective-organization of ant wars between neighboring colonies (Hoover et al. 2016; Bubak et al. 2016).
Mono-methyl alkanes, di-methylalkanes and tri-methylalkanes, referred to as methyl-branched alkanes, and their isomers exist in all insect cuticular lipids (Nelson and Blomquist 1995; Martin and Drijfhout 2009b). Methyl-alkanes are the most abundant hydrocarbon component found on the surface lipids of most ants (Martin and Drijfhout 2009b). These data add to a growing body of research showing the importance of cues coded in ant cuticular hydrocarbon profiles (reviewed in Sturgis and Gordon 2012; van Zweden and d’Ettorre 2010).
It has been found that many species encode these group identification signals in methyl-branched alkanes and n-alkenes. In a closely related species, Tetramorium bicarinatum, colonies differ only in methyl-branched alkanes, not in n-alkenes or n-alkanes, implying that only methyl-branched alkanes can serve as recognition cues (Astruc et al. 2001). Guerrieri et al. (2009) demonstrated that supplementing the ant Camponotus herculeanus with 3,11-di-methylheptacosane was enough to disrupt colony and species signatures. Martin et al. (2008) found that proportions of di-methylalkanes, with chain lengths ranging from 27 to 35, are putative species signals for Formica uralensis, Formica lugubris, Formica aquilonia, Formica pratensis, Formica polyctena, Formica rufa, and Formica truncorum. Mono-methylalkanes also serve as recognition cues in Formica sanguinea (Martin et al. 2008).
n-alkenes are also used as nestmate recognition cues in ants. Formica exsecta and Formica lemani use only the (Z)-9-alkene component of the cuticular hydrocarbon profile as species and nestmate cues (Martin and Drijfhout 2009a). (Z)-9-alkenes are also used to designate nest-membership in Formica japonica although (Z)-9-alkenes alone are not sufficient to elicit aggression in this species (Akino et al. 2004). Formica japonica relies on colony-specific ratios of five n-alkanes as well as five (Z)-9-alkenes (Akino et al. 2004). An analogous mechanism could be used by T. caespitum.
Supplementing T. caespitum hydrocarbon profiles with n-alkanes did not induce aggression with untreated nestmates. This suggests that n-alkanes are not involved in the nestmate recognition response. n-alkanes are ubiquitous and readily fluctuate in response to environmental stimuli (Martin & Drijfhout 2009a). These qualities make them excellent candidates for conveying task membership as has been demonstrated in the harvester ant Pogonomyrmex barbatus (Greene and Gordon 2003). These same qualities make n–alkanes a poor choice to use in species and colony recognition (Sturgis and Gordon 2012). This may explain why Tetramorium caespitum do not respond with aggression to nestmates supplemented with n-alkanes. This is consistent with behavior seen in honeybees that attack nestmates when their n-alkene profile is changed but ignore nestmates when their n-alkane profile is altered (Dani et al. 2005). In the ant Linepihithema humile supplementation with pharmacological amounts of n-alkanes can result in aggressive nestmate recognition responses, however, the response is stimulated by increasing the relative abundance of n-alkanes beyond the range seen in nature (Greene and Gordon 2007).
Tetramorium caespitum workers respond with similar recognition responses to cues from conspecific and heterospecfic non-nestmate ants. Ant species seem to differ in their comparative response to conspecific and heterospecific cues. Lucas et al. (2005) showed differences in reactions of three species in the genus Polychondicyla to intra-specific and inter-specific hydrocarbon profiles; species that were more closely related in a cluster analysis of hydrocarbon profiles were less aggressive towards each other. Another tramp ant species, Linepithema humile, also showed similarly high levels of aggression to heterospecific non-nestmate hydrocarbons and nestmate hydrocarbon, but only with very high, non-physiological levels of n-alkanes standards (Greene and Gordon 2007). A congener of T. caespitum, Tetramorium bicarinatum, exhibits similar hydrocarbon profiles at different colonies in an invaded area and does not show aggression towards conspecific non-nestmates, but the ants are aggressive towards ants of different species (Astruc et al. 2001). Our data suggest that T. caespitum workers simply exclude all non-nestmate ant regardless of their species membership. A similar response occurs even though there are both qualitative and quantitative differences in cuticular hydrocarbon profiles between T. caespitum workers and workers of P. occidentalis, at least.
Ritualized fighting by pavement ants is initiated if: 1. antennal contact is made with an opponent – during which the ants assess colony membership using cues coded in the relative abundance of methyl-alkanes and n-alkenes – and; 2. after they have had a recent history of interactions with nestmate ants (Bubak et al. 2016; Hoover et al. 2016). Interactions with nestmates increase brain levels of 5-HT and octopamine while dopamine increases in brains correlate to fighting behavior (Bubak et al. 2016; Hoover et al., 2016). The decision of a pavement ant to fight depends upon its brain state; elevated brain levels of the monoamines serotonin (5-HT) and octopamine above a threshold make it likely an ant will fight a non-nestmate when they meet and assess colony membership using the cuticular hydrocarbon coded cues (Hoover et al. 2016).
References
Akino T, Yamamura K, Wakamura S, Yamaoka R (2004) Direct behavioral evidence for hydrocarbons as nestmate recognition cues in Formica japonica (Hymenoptera : Formicidae). Appl Entomol Zool 39:381–387
Astruc C, Malosse C, Errard C (2001) Lack of intraspecific aggression in the ant Tetramorium bicarinatum: A chemical hypothesis. J Chem Ecol 27:1229–1248
Bjorkman-Chiswell BT, Van Wilgenburg E, Thomas ML, Swearer SE, Elgar MA (2008) Absence of aggression but not nestmate recognition in an Australian population of the Argentine ant Linepithema humile. Insect Soc 55:207–212
Bos N, d’Ettorre P (2012) Recognition of social identity in ants. Front Psychol 3:83–93
Brandstaetter AS, Rössler W, Kleineidam CJ (2011) Friends and foes from an ant brain's point of view–neuronal correlates of colony odors in a social insect. PLoS One 6:e21383
Buback AN, Yaeger JDW, Renner KJ, Swallow JG, Greene MJ (2016) Neuromodulation of nestmate recognition decisions by pavement ants. PLoS One 11:e0166417
Buczkowski G, Kumar R, Suib SL, Silverman J (2005) Diet-related modification of cuticular hydrocarbon profiles of the Argentine ant, Linepithema humile, diminishes intercolony aggression. J Chem Ecol 31:829–843
Chen JSC, Nonacs P (2000) Nestmate recognition and intraspecific aggression based on environmental cues in Argentine ants (Hymenoptera : Formicidae). Ann Entomol Soc Am 93:1333–1337
Collignon B, Detrain C (2010) Distributed leadership and adaptive decision-making in the ant Tetramorium caespitum. Proc Roy Soc B 277:1267–1273
Crozier RH, Pamilo P. (1996) Evolution of social insects. Oxford University Press
Dani FR, Jones GR, Corsi S, Beard R, Pradella D, Turillazzi S (2005) Nestmate Recognition Cues in the Honey Bee: Differential Importance of Cuticular Alkanes and Alkenes. Chem Senses 30:477–489
Esponda F, Gordon DM (2015) Distributed nestmate recognition in ants. Proc Royal Soc B. https://doi.org/10.1098/rspb.2014.2838
Ettershank G, Ettershank JA (1982) Ritualised fighting in the meat ant Iridomyrmex purpureus (Smith) (Hymenoptera: Formicidae). Aust J Entomol 21:97–102
Gibbs AG (1998) Water-proofing properties of cuticular lipids. Am Zool 38:471–482
Gibbs AG, Rajpurhoit G (2010) Cuticular lipids and water balance. Insect Hydrocarbons: Biology, biochemistry, and Chemical Ecology. Cambridge Press, Cambridge, pp 100–120
Greene MJ, Gordon DM (2003) Cuticular hydrocarbons inform task decisions. Nature 423:32
Greene MJ, Gordon DM (2007) Structural complexity of chemical recognition cues affects the perception of group membership in the ants Linepithema humile and Aphaenogaster cockerelli. J Exp Biol 210:897–905
Greene MJ, Pinter-Wollman N, Gordon DM (2013) Interactions with combined chemical cues inform harvester ant foragers' decisions to leave the nest in search of food. PLoS One 8(1):e52219. https://doi.org/10.1371/journal.pone.0052219
Guerrieri FJ, Nehring V, Jorgensen CG, Nielsen J, Galizia CG, d'Ettorre P (2009) Ants recognize foes and not friends. Proc R Soc Lond B Biol Sci 276:2461–2468
Hefetz A, Errard C, Chambris A, Le Negrate A (1996) Postpharyngeal gland secretion as a modifier of aggressive behavior in the myrmicinc ant Manica rubida. J Insect Behav 9:709–717
Hölldobler B (1976) Tournaments and slavery in a desert ant. Science 192:912–914
Hölldobler B (1982) Interference Strategy of Iridomyrmex pruinosum (Hymenoptera: Formicidae) during Foraging. Oecologia 52:208–213
Hoover KM, Bubak AN, Law IJ, Yeaeger JDW, Renner KJ, Swallow JG, Greene MJ (2016) The organization of societal conflicts by pavement ants Tetramorium caespitum: an agent-based model of amine mediated decision making. Current Zool 62:277–284
Howard RW, Blomquist GJ (2005) Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu Rev Entomol 50:371–393
Ichinose K (1991) Seasonal variation in nestmate recognition in Paratrechina flavipes (Smith) worker ants (Hymenoptera: Formicidae). Anim Behav 41:1–6
Ichinose K, Lenoir A (2010) Hydrocarbons detection levels in ants. Insect Soc 57:453–455
Lahav S, Soroker V, Hefetz A (1999) Direct behavioral evidence for hydrocarbons as ant recognition discriminators. Naturwissenschaften 86:246–249
Liang D, Silverman J (2000) “You are what you eat”: Diet modifies cuticular hydrocarbons and nestmate recognition in the Argentine ant, Linepithema humile. Naturwissenschaften 87:412–416
Liang D, Blomquist GJ, Silverman J (2001) Hydrocarbon-released nestmate aggression in the Argentine ant, Linepithema humile, following encounters with insect prey. Comp Biochem Physiol B, Biochemistry & Molecular Biology 129:871–882
Lucas C, Pho DB, Jallon JM, Fresneau D (2005) Role of cuticular hydrocarbons in the chemical recognition between ant species in the Pachycondyla villosa species complex. J Insect Physiol 51:1148–1157
Martin SJ, Helanterae H, Drijfhout FP (2008) Evolution of species-specific cuticular hydrocarbon patterns in Formica ants. Biol J Linn Soc 95:131–140
Martin SJ, Drijfhout FP (2009a) Nestmate and Task Cues are Influenced and Encoded Differently within Ant Cuticular hydrocarbon Profiles. J Chem Ecol 35:368–374
Martin S, Drijfhout FP (2009b) A Review of Ant Cuticular Hydrocarbons. J Chem Ecol 35:1151–1161
McGlynn TP (1999) Other ant invaders. TREE 14:489–489
Menzel F, Schmitt T, Blüthgen N (2009) Intraspecific nestmate recognition in two parabiotic ant species: acquired recognition cues and low inter-colony discrimination. Insect Soc 56:251–260
Morel L, Vander Meer RK, Lavine BK (1988) Ontogeny of nestmate recognition cues in the red carpenter ant (Camponotus floridanus) – behavioral and chemical evidence for the role of age and social experience. Behav Ecol Sociobiol 22:175–183
Nelson DR, Blomquist GJ (1995) Insect Waxes. In: Hamilton RJ (ed) Waxes: Chemistry, Molecular Biology and Functions. The Oily Press, Christie, pp 1–90
Ozaki M, Wada-Katsumata A, Fujikawa K, Iwasaki M, Yokohari F, Satoji Y, Nisimura T, Yamaoka R (2005) Ant nestmate and non-nestmate discrimination by a chemosensory sensillum. Science 309:311–314
Plowes NJR (2008) Self organized conflicts in territorial ants. ProQuest, Ann Arbor
Roulston TH, Buczkowski G, Silverman J (2003) Nestmate discrimination in ants: effect of bioassay on aggressive behavior. Insect Soc 50:151–159
Roux O, Martin J-M, Ghomsi NT, Dejean A (2009) A non-lethal water-basd removal-reapplication technique for behavioral analysis of cuticular compounds of ants. J Chem Ecol 35:904–912
Scharf ME, Ratliff CR, Bennett GW (2004) Impacts of residual insecticide barriers on perimeter-invading ants, with particular reference to the odorous house ant, Tapinoma sessile. J Econ Entomol 97:601–605
Sharma KR, Enzmann BL, Schmidt Y, Moore D, Jones GR, Parker J, Berger SL, Reinberg D, Zwiebel LJ, Breit B, Liebig J, Ray A (2015) Cuticular Hydrocarbon Pheromones for Social Behavior and Their Coding in the Ant Antenna. Cell Rep 12:1261–1271
Steiner FM, Schlick-Steiner BC, Buschinger A (2003) First record of unicolonial polygyny in Tetramorium cf. caespitum (Hymenoptera, Formicidae). Insect Soc 50:98–99
Sturgis S, Gordon DM (2012) Nestmate recognition in ants (Hymenoptera, Formicidae): A review. Myrmecol News 16:101–110
Torres CW, Brandt M, Tsutsui ND (2007) The role of cuticular hydrocarbons as chemical cues for nestmate recognition in the invasive Argentine ant (Linepithema humile). Insect Soc 54:363–373
Vander Meer RK, Morel L (1998) Nest-mate recognition in ants. In: Vander Meer RK, Breed MD, Espelie KE, Winston ML (eds) Pheromone Communication in Social Insects: Ants. Bees and TermitesWestview Press, Boulder, Wasps, pp 79–103
van Wilgenburg E, van Lieshout E, Elgar M (2005) Conflict resolution strategies in meat ants (Iridomyrmex purpureus): ritualised displays versus lethal fighting. Behaviour 142:701–716
van Wilgenburg EV, Torres CW, Tsutsui ND (2010) The global expansion of a single ant supercolony. Evol Appl 3:136–143
van Zweden JS, d’Ettorre P (2010) Nestmate recognition in social insects and the role of hydrocarbons. Insect hydrocarbons: Biology, Biochemistry and Chemical Ecology 11. Cambridge Press, Cambridge, pp 222–243
Wagner D, Tissot M, Cuevas W, Gordon DM (2000) Harvester ants utilize cuticular hydrocarbons in nestmate recognition. J Chem Ecol 26:2245–2257
Whitehouse MEA, Jaffe K (1995) Nestmate recognition in the leaf-cutting ant Atta laevigata. Insect Soc 42:157–166
Acknowledgements
This work was supported, in part, by USDA/CSREES/NRI;UCD Project # 3533316. We thank Kevin Hoover and William Schuman for comments on the manuscript. The authors thank Claire Chen for her work identifying pavement ant cuticular hydrocarbon compounds.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(XLSX 11 kb)
Rights and permissions
About this article
Cite this article
Sano, K., Bannon, N. & Greene, M.J. Pavement Ant Workers (Tetramorium caespitum) Assess Cues Coded in Cuticular Hydrocarbons to Recognize Conspecific and Heterospecific Non-Nestmate Ants. J Insect Behav 31, 186–199 (2018). https://doi.org/10.1007/s10905-017-9659-4
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10905-017-9659-4