Abstract
Species in the Polysphincta genus group, as far as is known, are exclusively koinobiont ectoparasitoids of spiders. These wasps attack their hosts, inflicting a temporary paralysis, and then lay one egg on the host’s abdomen or prosoma. Parasitoid attack behavior is highly variable among species, including occasions where the wasp darts directly and holds the spider, as well as instances involving complex behavioral sequences. In the present study, we describe the attack behavior of Polysphincta sp. nr. purcelli and P. janzeni on Cyclosa fililineata and C. morretes, respectively. All attacks occurred at night. Initially, the female wasp landed on the web hub at the position occupied by the spider, with the spider always escaping from this initial attack. Subsequently, the wasp waited for up to 14 h at the web hub for the spider’s return. The wasp then inserted its ovipositor into the mouth of the spider, after which the spider became paralyzed and remained motionless for at least 30 min. The wasp laid one egg on the surface of the host’s abdomen and remained on the web for at least 1 h thereafter. The lie-in-wait and attack only after the return of the host to the web hub, as well as the permanence of the wasp on the web after the attack are not frequent behaviors described for polysphinctines. Behavioral idiosyncrasies, such as those observed here, are common among polysphinctines, suggesting a high level of specific adaptive matching of polysphinctine parasitoid behavior to their hosts’ biological characteristics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ichneumonid wasps of the subfamily Pimplinae include a subgroup of 22 genera, the monophyletic Polysphincta genus group (sensu Gauld and Dubois 2006, hereafter polysphinctines), which are distributed worldwide (Gauld and Dubois 2006; Palacio et al. 2007). Polysphinctines are exclusively koinobiont ectoparasitoids of spiders (Gauld et al. 2002; Gauld and Dubois 2006) and are known to change their hosts’ web building behaviors, inducing the construction of modified structures that increase cocoon protection, reduce the probability of web collapse, and reduce risks associated with prey interception (Eberhard 2000a, 2001, 2010; Gonzaga and Sobczak 2007, 2011; Matsumoto and Konishi 2007; Matsumoto 2009; Gonzaga et al. 2010; Korenko et al. 2014; Kloss et al. 2016).
Oviposition of polysphinctines requires contact between the parasitoid wasp and its potentially harmful predatory host. To minimize the risks during this process, the wasps inject paralyzing venom into their host’s body. Gonzaga and Sobczak (2007) demonstrated that Hymenoepimecis veranii Loffredo & Penteado-Dias, 2009 females insert the tip of their ovipositor into the host spider’s mouth, probably reaching the subesophageal ganglion, and then inspect the abdomen of the spider in search of a spot to lay an egg and for the presence of other wasp eggs. Hymenoepimecis veranii venom has an immediate effect, leaving the attacked spider motionless for at least 18 min. The critical stage of this process is between the initial approach and delivery of the paralyzing venom.
The small number of descriptions of polysphinctine attack behavior in the literature indicate that polysphinctines exhibit a high degree of variation and plasticity in their attack strategies (Eberhard 2000b; Gonzaga and Sobczak 2007; Weng and Barrantes 2007; Matsumoto 2009; Takasuka et al. 2009; Takasuka and Matsumoto 2011; Sobczak 2013). For example, the wasps H. bicolor (Brulle, 1846) and H. argyraphaga Gauld, 2000 that attack Nephila clavipes (Linnaeus, 1767) (Nephilidae) and Leucauge argyra (Walckenaer, 1842) (Tetragnathidae), respectively, tend to hover around the target spider before executing a direct attack, darting rapidly at the spider and grasping it with their legs (Eberhard 2000b; Sobczak 2013). Alternatively, H. argyraphaga has been observed hanging immobile from web radii in the free zone near the hub until the host spider approached (Eberhard 2000b). This behavior is probably one alternative to the grasp and parasite on the spider technique, after an unsuccessful initial attack. Similarly, H. veranii will stand immobile on barrier threads and wait until the target spider leaves its retreat to capture prey, at which point the wasp will dart for the spider (Gonzaga and Sobczak 2007). Similarly, Brachyzapus nikkoensis (Uchida, 1928) lands on the funnel web of its host spider Agelena limbata (Thorell, 1897) (Agelenidae) and waits until the spider approaches to attack it (Matsumoto 2009).
The highest variation in attack behavior has been reported within the genus Zatypota. Zatypota albicoxa Walker, 1874, for example, has at least four strategies for attacking its host Parasteatoda tepidariorum (Koch, 1841) (Theridiidae). In one strategy, known as ambush-style, the wasp hangs onto the web, pulling the thread from where it is hanging with its foreleg until the spider lifts up the thread containing the hanging wasp. In its alternative ambush-style strategy, the wasp hangs motionless at the mid-height of the web until the spider approaches, while in its climbing-style strategy, the wasp climbs the web until it reaches the spider. When employing its reclining-style attack, the wasp wanders around under the web and lays its dorsum on the ground, pulling at a gumfooted thread with its fore- and midlegs, or leaning against the thread motionlessly with both pairs of legs directly touching a gumfooted thread, until the spider climbs down along the vertical thread, at which point it is attacked by the wasp (Takasuka et al. 2009; Takasuka and Matsumoto 2011). Z. petronae Gauld, 1991 invades the retreat of the host spider Theridion evexum Keyserling, 1884 (Theridiidae) and attacks the spider inside its retreat; the details concerning Z. petronae’s immobilization and egg laying processes were not reported (Weng and Barrantes 2007).
Aside from the above-mentioned wasp genera, there is no information about the attack behaviors of most of the remaining polysphinctine genera (Gauld et al. 2002; Gauld and Dubois 2006), including the species-rich Polysphincta genus. The Polysphincta genus includes 27 species (Yu et al. 2012), but it comprises a set of polysphinctine species that lack the defining features of the other wasp genera (Gauld and Dubois 2006). This fact resulted in the inclusion of several pimplines associated with spiders within this genus. Attaining a reliable definition for Polysphincta requires an expansion of our knowledge of tropical wasp fauna, which may result in a series of monophyletic genera (Gauld and Dubois 2006).
There is information on host identities for several Polysphincta species, for example: P. boops Tschek, 1868, which attacks Araniella cucurbitina (Clerck, 1757) (Araneidae) and A. opisthographa (Kulczyński, 1905) (Fritzen and Shaw 2014; Korenko et al. 2014) in Europe; P. gutfreundi Gauld,1991, which attacks Allocyclosa bifurca (McCook, 1887) (Araneidae) and Cyclosa monteverde Levi, 1999 (Araneidae) in Costa Rica (Barrantes et al. 2008; Eberhard 2010; Gonzaga et al. 2010); P. janzeni Gauld, 1991, which has been observed parasitizing C. fililineata Hingston, 1932 (Araneidae) and C. morretes Levi, 1999 (Araneidae) in Brazil (Gonzaga 2004); P. koebelei Howard 1892, which attacks Araneus gemmoides Chamberlin & Ivie, 1935 (Araneidae) (Bovee and Leech 2014) in Canada and Larinioides cornutus (Clerck, 1757) in the United States (Howard 1892); P. longa Kasparyan, 1976, which attacks Araneus angulatus Clerck, 1757 (Araneidae) (Fritzen and Shaw 2014) in Poland; P. rufipes Gravenhorst, 1829, which attacks L. cornutus (Clerck, 1757) (Araneidae) and Zygiella x-notata (Clerck, 1757) (Araneidae) in Germany (Schmitt et al. 2012); P. sp. nr. purcelli, which attacks Cryptachaea sp. (Theridiidae) in the Brazilian Atlantic rainforest (T. G. Kloss, unpub. data); and P. tuberosa Gravenhorst, 1829, which has been observed parasitizing Araneus quadratus Clerck, 1757 (Araneidae) (Nielsen 1923), Araniella ophistographa Kulczyński, 1905, Araneus diadematus Clerck, 1757 (Araneidae) and A. cucurbitina Clerck, 1757 (Korenko et al. 2014) in Europe. Some of these records include details of host behavioral changes (e.g., Gonzaga 2004; Eberhard 2010; Bovee and Leech 2014; Korenko et al. 2014), but female wasp behavior during attacks has been reported only for P. rufipes (Schmitt et al. 2012).
The aim of this study was to provide information on the attack behavior of P. sp. nr. purcelli and P. janzeni on C. fililineata and C. morretes, respectively. We also investigated the frequency of parasitized spiders in the population over two seasons, encompassing the development of the parasitoids from eggs to adult life stages. The general characteristics of these two polysphinctines’ attack behaviors are discussed.
Materials and Methods
Field observations were conducted at the Biological Station of Santa Lucia (Estação Biológica de Santa Lúcia – EBSL) (19°57’56” S, 40°32’24” W) and the Biological Reserve Augusto Ruschi (Reserva Biológica Augusto Ruschi – REBIO) (19°54’26” S, 40°33’11” W). Both sites are found in the Atlantic rainforest reserves in the Santa Teresa municipality, Espírito Santo State, Brazil.
We performed both diurnal and nocturnal visual searching of C. fililineata and C. morretes webs along all of the trails at EBSL and REBIO. When an adult polysphinctine was encountered on a web, we maintained uninterrupted observation from the initial attack (wasp landing on the web hub) until 1 h after the lie-in-wait attack (when the wasp grasped the spider with its legs after remaining motionless at the web hub until the spider returned) success, which was determined by wasp oviposition. When there was no successful oviposition after the lie-in-wait attack, we maintained our observation until the wasp departed and we returned to the web locality the next day to verify if the spider had left the web.
During these observations, we recorded the behaviors of wasps and spiders with a Nikon D5100 camera with lens AF-S VR Micro-NIKKOR 105 mm f/2.8G IF-ED, which allowed observation of details of the attack behaviors. To evaluate the duration of the polysphinctine’s life cycle and investigate the morphological changes in the parasitoid larvae during development, we monitored all host spiders subjected to oviposition (n = 4) and spiders that were already parasitized by larvae in different stages (C. fililineata: n = 33; C. morretes: n = 42), until the polysphinctine larvae built their cocoons. We classified larval development into three stages: (1) first-stage larvae exhibited the absence of body divisions; (2) second-stage larvae showed segmented bodies; and (3) third-stage larvae developed characteristic dorsal tubercles, which were absent in the previous stages. However, it is possible that these stages do not correspond exactly to larval instars. To study pupae development time, we marked 13 parasitized spiders of each species, including the two individuals of C. fililineata and two individuals of C. morretes where we observed oviposition. We observed host individuals daily, to evaluate the period between the molt of last stage larvae to pupae and parasitoid wasp emergence.
The frequency of parasitized spiders was estimated by inspecting all C. fililineata and C. morretes encountered along the trails during a 30-day period in the wet season of 2013 (November) and a 30-day period in the dry season of 2014 (July). These inspections were performed by nocturnal visual searching along all trails at EBSL and REBIO. Each spider was inspected only once within each month. Voucher P. sp. nr. purcelli and P. janzeni specimens were deposited in the collection at Universidade Federal de São Carlos (curator: A. M. P. Dias), and specimens of C. morretes and C. fililineata were deposited in the arachnid collection of Centro de Coleções Taxonômicas da Universidade Federal de Minas Gerais (curator: A. J. Santos) in Minas Gerais, Brazil.
Results
We observed six parasitism attempts by P. sp. nr. purcelli on C. fililineata and eight by P. janzeni on C. morretes, with two successful ovipositions for each species. Only one individual from each spider species had a parasitoid larva previously attached to its abdomen, but oviposition attempts on these two parasitized individuals were unsuccessful.
Polysphincta sp. nr. purcelli and P. janzeni presented similar initial and lie-in-wait attack behaviors. All of the initial and lie-in-wait attacks occurred at night on immature females of C. morretes and mature females of C. fililineata, with no attacks observed on male host spiders. We observed the beginning of initial attacks on five occasions (two in C. fililineata and three in C. morretes). For the other nine attack observations (four in C. fililineata and five in C. morretes), the wasp was already in the lie-in-wait attack mode, which was determined by the wasp being at the web hub when we encountered it. The initial attack started with the female wasp landing on the web hub, near the position occupied by the spider, but never directly on the spider. In all observations of initial attacks, the spider jumped off the web immediately or moved quickly towards the web edge (Fig. 1a). After the initial attack, the wasp remained motionless at the web hub until the spider returned and touched the body of the wasp. We observed wasps waiting for the hosts to return for periods ranging from 30 min up to 14 h. Only one female, however, was observed waiting for up to 14 h. One spider (C. morretes) did not return to the web hub after the wasp landed on the web, which occurred in the lie-in-wait attack attempt of 14 h. In this instance, the wasp departed off the web at sunrise, after remaining for 14 h on the web.
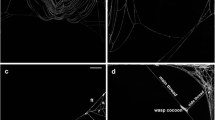
Life cycle of the parasitoids Polysphincta sp. nr. purcelli and P. janzeni: a Adult P. sp. nr. purcelli landing at the center of the web and the host spider escaping (arrow) to the edge of the web; b Immobilization of an adult female Cyclosa fililineata host by P. sp. nr. purcelli; c P. janzeni individual at the center of a C. morretes web post-attack; d Second-stage larva of P. janzeni on its C. morretes host; e Third-stage larva of P. sp. nr. purcelli on its adult female C. fililineata host; f Parasitoid larva of P. sp. nr. purcelli attached to the center of the cocoon web by small hooks on its dorsal pseudopods (arrow) after killing the host spider; g P. janzeni cocoon (arrow) in the center of the stabilimentum of a C. morretes cocoon web; h Adult male P. sp. nr. purcelli emerging from a cocoon (arrow) located on a C. fililineata cocoon web. Scale bars: 0.5 cm
We observed that the lie-in-wait attack started immediately after the spider touched the body of the wasp, when the wasp grasped the spider with its legs and the spider fought to escape, resulting in both animals moving on the web (away from the web hub), rolling one over the other. In ten observations of lie-in-wait attacks (four in C. fililineata and six in C. morretes), the wasps failed to grasp the spider, which resulted in the spider jumping from the web to the ground/vegetation and the wasp then departing. All spiders that escaped the lie-in-wait attacks returned to the web hub the next morning. After a struggle lasting a few seconds, the wasp remained positioned with its head facing towards the posterior part of the spider’s abdomen and inserted its ovipositor into the spider’s mouth (Fig. 1b), leading to immediate paralysis of the host. The wasp then inserted and withdrew its ovipositor into the spider’s mouth repeatedly for about 5 min. Subsequently, the wasp apparently started to inspect the spider’s body, repeatedly rubbing and jabbing the base of its ovipositor all over the host’s abdomen (near the location where the egg was later attached) for approximately 4 min. After this behavior, the wasp again proceeded to insert and withdraw its ovipositor in and out of the spider’s mouth repeatedly for another 3 min. Finally, the wasp deposited a single egg on the anterodorsal surface of the host’s abdomen, and returned to the hub of the web (Fig. 1c), where it remained for at least 1 h.
The spider recovered its mobility about 30 min after the oviposition, and immediately rubbed its posterior legs on its abdomen. Upon recovery, the spider went back to the web hub, where it touched the parasitoid wasp and then moved away quickly from the center. After a successful oviposition, the wasp remained static in the center of the web when touched by the spider, in contrast to its active response during the attack. The spider’s movements towards and away from the center of the web were repeated several times, over at least 1 h, when the observations were finalized.
Two days after oviposition, first-stage larvae emerged, without body divisions discernible to the naked eye. After a further few days, we observed marked body divisions, which we considered indicative of the second stage (see Fig. 1d). During the first and second parasitoid larval stages, the host spiders of both species often moved their hind legs towards the larva. We never observed the spiders’ legs touching a parasitizing larva, much less removing it. However, on two occasions, second-stage larvae that were parasitizing C. morretes hosts disappeared from 1 day to the next.
The third-stage larvae (Fig. 1e and f) originated 2 days before the wasp larvae killed the spider host. We observed that dorsal tubercles were used by the larvae to hold onto the web, after killing the host spider (see arrow in Fig. 1f). On the last day of this larval stage, the host spiders built a modified web (Kloss et al. 2016). After construction of the modified web, the parasitoid larvae attached themselves to the web hub and proceeded to consume the host spider (Fig. 1f). Then, the parasitoid larvae initiated spinning of a cocoon at the center of the web or into the stabilimentum (Fig. 1g). The period of larval development, from the egg stage to cocoon building, varied from 23 to 26 days (n = 4). The adult polysphinctine emerged 11 to 14 days (12.3 ± 0.91, mean ± SE) after its cocoon had been built (Fig. 1h, P. janzeni: n = 13; Polysphincta sp. nr. purcelli: n = 13).
The frequency of parasitism differed between the evaluated seasons. In the wet season, we registered 111 C. fililineata webs and 124 C. morretes webs, of which 11 C. fililineata (9.91 %) and 5 C. morretes (4.03 %) were parasitized. In the dry season, we registered 102 C. fililineata individuals and 124 C. morretes individuals, of which 15 (14.71 %) C. fililineata and 29 (23.39 %) C. morretes were parasitized.
Discussion
In the present study, we observed three main aspects of P. sp. nr. purcelli and P. janzeni oviposition behavior: (i) wasp flies and lands on the web hub, at the position occupied by the spider, and waits an extended time for the spider to return; (ii) wasp attacks the host after it has returned to the hub and touched the wasp; and (iii) wasp remains on the web for an extended period of time after attacking the spider.
The wasp’s behavior of waiting for the host spider’s return, instead of immediately attacking it, is probably linked to the high value of the web for these host spider species. Cyclosa fililineata and C. morretes build characteristic detritus stabilimenta on their webs. Stabilimenta are thought to reduce the conspicuity of the web to visually oriented predators (Gonzaga and Vasconcellos-Neto 2005) and prey insects (Tan and Li 2009). The columns of detritus are built gradually, with a progressive accumulation of food debris and other particles that fall onto the web (Gonzaga and Vasconcellos-Neto 2005, 2012). Spiders may remain better concealed in long structures that have approximately the same width as their bodies. Thus, abandoning one web to move to another site would require the spiders to rebuild these stabilimenta, during which time they would be exposed to predators. The parasitoid wasps may favor waiting on webs with completed stabilimenta to exploit this risk avoidance behavior in their hosts. The presence of a detritus stabilimentum at the hub of the web, concealing the spider’s position, may also be important to influence the indirect attack behavior by P. janzeni and P. sp. nr. purcelli. Direct attacks by polysphinctines were previously observed only against spider hosts that remained exposed and clearly visible at the hub of their orbs, such as N. clavipes (Sobczak 2013) and L. argyra (Eberhard 2000b). In circumstances where host spiders are protected by a complex structure of silk threads (e.g., Manogea porracea – Sobczak et al. 2009) or inside shelters (e.g., A. omnicolor – Gonzaga and Sobczak 2007) attacks involve landing on the web or retreating to chase the host or waiting until the host departs from its protected position. Polysphincta sp. nr. purcelli and P. janzeni may be unable to accurately detect the position occupied by the spider within the detritus column to conduct a direct attack, adopting instead an alternative strategy of waiting for the spider at the hub after causing an initial disturbance. In fact, studies calculating color contrasts between spiders and their stabilimenta conducted using other Cyclosa species as models have showed that spiders are invisible to hymenopterans at close distances (Chou et al. 2005; Tan and Li 2009).
Attacking the host spider only after the spider had returned to the hub and touched the wasp, as opposed to pursuing it aggressively immediately, may reduce the probability that the host spider will escape by jumping away. In the field, we observed frequent jumping behavior in both C. fililineata and C. morretes in response to the approach of a researcher, moths hovering over the web, and Mimetidae predator spiders climbing on the web.
The post-oviposition behavior of returning to the web hub and remaining there for at least 1 h, instead of leaving the web immediately, has never been registered before for polysphinctines. The function of this behavior is not known, however, given that we observed the spiders attempting to remove the deposited eggs upon recovering from paralysis, by moving their hind legs towards their abdomen (that can also explain the larvae disappearance that was observed in two C. morretes specimens – see Results), the perpetuity of the wasp may be a strategy to assure a successful oviposition. However, the existence of a visual or chemical mechanism used by the wasps to detect egg removal is not known. In addition, other polysphinctine species are known to practice this behavior of the spider’s efforts to remove the egg (Eberhard 2000b; Gonzaga and Sobczak 2007; Takasuka and Matsumoto 2011). Conversely, it could be argued that the time spent by the mother wasp on the web after oviposition is likely too short to significantly decrease the probability of egg mortality. Other parasitoids have been documented removing not only recently laid eggs, but also larvae (Takasuka and Matsumoto 2011), with the risk of eviction being dispersed along the whole period of larval development.
Although some characteristics of P. sp. nr. purcelli and P. janzeni oviposition behavior appear to be unique among polysphinctines, they exhibited some behavioral sequences that may be more widespread. The insertion of the ovipositor into the mouth of the host, and consequent paralysis of the spider, are one of several ways known in H. argyraphaga (Eberhard 2000b) and H. veranii (Gonzaga and Sobczak 2007). The behavior of groping the host spider’s abdomen after paralysis has also been described for other species. This behavior, which has been interpreted as being related to searching for pre-existing larvae or eggs (Takasuka and Matsumoto 2011), was observed for Hymenoepimecis (Eberhard 2000b; Gonzaga and Sobczak 2007; Fincke et al. 1990), Reclinervellus (Matsumoto and Konishi 2007), and Zatypota (Takasuka et al. 2009) genera. Although not proven, nocturnal attack behaviors similar to those described here, have also been suggested for species of Hymenoepimecis and Acrotaphus (Gauld and Dubois 2006) genera.
In conclusion, the present observations suggest that the oviposition behavior of P. sp. nr. purcelli and P. janzeni may be related to the characteristic reactions of their hosts. The oviposition behavior sequences of other polysphinctine species also appear to be well suited to specific behaviors or web architectures of their hosts. Hymenoepimecis veranii, for example, would almost certainly fail to subdue its host within the host’s curled leaf shelter. Thus, instead of invading the host’s shelter, H. veranii waits outside the host’s retreat until the spider departs to capture spontaneously an intercepted insect. These fine-tuned interactions most likely occur in other polysphinctine species and may restrict the range of host species used by each parasitoid.
References
Barrantes G, Eberhard WG, Weng JL (2008) Seasonal patterns of parasitism of the tropical spiders Theridion evexum (Araneae, Theridiidae) and Allocyclosa bifurca (Araneae, Araneidae) by the wasps Zatypota petronae and Polysphincta gutfreundi (Hymenoptera, Ichneumonidae). Rev Biol Trop 56:749–754
Bovee J, Leech R (2014) Araneus gemmoides (Araneae: Araneidae) death by a parasitoid (Hymenoptera: Ichneumonidae). Bull Entomol Soc Can 46:86–91
Chou IC, Wang PH, Shen PS, Tso IM (2005) A test of prey-attracting and predator defence functions of prey carcass decorations built by Cyclosa spiders. Anim Behav 69:1055–1061
Eberhard WG (2000a) Spider manipulation by a wasp larva. Nature 406:255–256
Eberhard WG (2000b) The natural history and behavior of Hymenoepimecis argyraphaga (Hymenoptera: Ichneumonidae) a parasitoid of Plesiometa argyra (Araneae, Tetragnathidae). J Hymenopt Res 9:220–240
Eberhard WG (2001) Under the influence: webs and building behavior of Plesiometa argyra (Araneae, Tetragnathidae) when parasitized by Hymenoepimecis argyraphaga (Hymenoptera, Ichneumonidae). J Arachnol 29:354–366
Eberhard WG (2010) Recovery of spiders from the effects of parasitic wasps: implications for fine-tuned mechanisms of manipulation. Anim Behav 79:375–383
Fincke OM, Higgins L, Rojas E (1990) Parasitism of Nephila clavipes (Araneae, Tetragnathidae) by an ichneumonid (Hymenoptera, Polysphinctini) in Panama. J Arachnol 18:321–329
Fritzen NR, Shaw MR (2014) On the spider parasitoids Polysphincta longa Kasparyan and P. boops Tschek (Hymenoptera, Ichneumonidae, Pimplinae), with the first host records of P. longa. J Hymenopt Res 39:71–82
Gauld ID, Dubois J (2006) Phylogeny of the Polysphincta group of genera (Hymenoptera: Ichneumonidae: Pimplinae): a taxonomic revision of spider ectoparasitoids. Syst Entomol 31:529–564
Gauld ID, Wahl DB, Broad GR (2002) The suprageneric groups of the Pimplinae (Hymenoptera: Ichneumonidae): a cladistic re-evaluation and evolutionary biological study. Zool J Linn Soc-Lond 136:421–485
Gonzaga, MO (2004) Funções e variabilidade estrutural dos estabilimentos construídos por Cyclosa fililineata Hingston 1932 e Cyclosa morretes Levi, 1999 (Araneae: Araneidae). [Ph.D. thesis], Universidade Estadual de Campinas, Brazil
Gonzaga MO, Sobczak JF (2007) Parasitoid-induced mortality of Araneus omnicolor (Araneae, Araneidae) by Hymenoepimecis sp. (Hymenoptera, Ichneumonidae) in southeastern Brazil. Naturwissenschaften 94:223–227
Gonzaga MO, Sobczak JF (2011) Behavioral manipulation of the orb-weaver spider Argiope argentata (Araneae: Araneidae) by Acrotaphus chedelae (Hymenoptera: Ichneumonidae). Entomol Sci 14:220–223
Gonzaga MO, Vasconcellos-Neto J (2005) Testing the functions of detritus stabilimenta in webs of Cyclosa fililineata and Cyclosa morretes (Araneae: Araneidae): do they attract prey or reduce the risk of predation? Ethology 111:479–491
Gonzaga MO, Vasconcellos-Neto J (2012) Variation in the stabilimenta of Cyclosa fililineata Hingston, 1932, and Cyclosa morretes Levi, 1999 (Araneae: Araneidae), in southeastern Brazil. Psyche. doi:10.1155/2012/396594
Gonzaga MO, Sobczak JF, Penteado-Dias AM, Eberhard WG (2010) Modification of Nephila clavipes (Araneae: Nephilidae) webs by the parasitoids Hymenoepimecis bicolor and H. robertsae (Hymenoptera: Ichneumonidae). Ethol Ecol Evol 22:151–165
Howard LO (1892) The Hymenopterous parasites of spiders. Proc Entomol Soc Wash 2:290–302
Kloss TG, Gonzaga MO, Roxinol JAM, Sperber CF (2016) Host behavioural manipulation of two orb-weaver spiders by parasitoid wasps. Anim Behav 111:289–296
Korenko S, Isaia M, Satrapová J, Pekár S (2014) Parasitoid genus-specific manipulation of orb-web host spiders (Araneae, Araneidae). Ecol Entomol 39:30–38
Matsumoto R (2009) “Veils” against predators: modified web structure of a host spider induced by an ichneumonid parasitoid, Brachyzapus nikkoensis (Uchida) (Hymenoptera). J Insect Behav 22:39–48
Matsumoto R, Konishi K (2007) Life histories of two ichneumonid parasitoids of Cyclosa octotuberculata (Araneae): Reclinervellus tuberculatus (Uchida) and its new sympatric congener (Hymenoptera: Ichneumonidae: Pimplinae). Entomol Sci 10:267–278
Nielsen E (1923) Contributions to the life history of the Pimpline spider parasites (Polysphincta, Zaglyptus, Tromatobia) (Hym. Ichneum.). Entomol Medd 14:137–205
Palacio E, Sääksjärvi IE, Vahtera V (2007) Lamnatibia, a new genus of the Polysphincta group of genera from Colombia (Hymenoptera: Ichneumonidae; Pimplinae). Zootaxa 1431:55–63
Schmitt M, Richter D, Göbel D, Zwakhals K (2012) Beobachtungen zur Parasitierung von Radnetzspinnen (Araneidae) durch Polysphincta rufipes (Hymenoptera: Ichneumonidae). Arachnol Mitt 44:1–6
Sobczak, JF (2013) Estudos biológicos e ecológicos da interação entre Nephila clavipes (Araneae, Nephilidae) e o parasitoide Hymenoepimecis bicolor (Hymenoptera, Ichneumonidae, Pimplinae). [PhD thesis], Universidade Federal de São Carlos, São Carlos, Brazil
Sobczak JF, Loffredo APS, Penteado-Dias AM, Gonzaga MO (2009) Two new species of Hymenoepimecis (Hymenoptera: Ichneumonidae: Pimplinae) with notes on their spider hosts and behaviour manipulation. J Nat Hist 43:2691–2699
Takasuka K, Matsumoto R (2011) Lying on the dorsum, a unique host attacking behavior of Zatypota albicoxa (Hymenoptera, Ichneumonidae). J Ethol 29:203–207
Takasuka K, Matsumoto R, Ohbayashi N (2009) Oviposition behavior of Zatypota albicoxa (Hymenoptera, Ichneumonidae), an ectoparasitoid of Achaearanea tepidariorum (Araneae, Theridiidae). Entomol Sci 12:232–237
Tan EJ, Li D (2009) Detritus decorations of an orb-weaving spider, Cyclosa mulmeinensis (Thorell): for food or camouflage? J Exp Biol 212:1832–1839
Weng JL, Barrantes G (2007) Natural history and larval behavior of the parasitoid Zatypota petronae (Hymenoptera: Ichneumonidae). J Hymenopt Res 16:326–335
Yu DS, van Achterberb C, Horstmann K (2012) Taxapad 2012, World Ichneumonoidea 2011, Taxonomy, biology, morphology and distribution. http://www.taxapad.com Accessed 30 April 2015
Acknowledgments
We are grateful to the Estação Biológica de Santa Lúcia and Reserva Biológica Augusto Ruschi for access. This project was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (grant numbers 140508/2012-0, 306157/2014-4, 403733/2012-0, 445832/2014-2, 573802/2008-4, 309787/2012-2 and 300295/2016-2), Fundação de Amparo à Pesquisa e Inovação do Espírito Santo (grant number 1020/2015), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (research grant to José Augusto Martins Roxinol), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (grant numbers APQ-02104-14 and CRA-30058/12), and Instituto Nacional de Ciência e Tecnologia dos Hymenoptera Parasitoides da Região Sudeste (HYMPAR/Sudeste - CNPq/CAPES/Fapesp). We thank Thairine Mendes Pereira, José Luiz Molino, and Lourdes Corbellari Molino for assistance in fieldwork and Ana Paula S. Loffredo for the identification of Polysphincta sp. nr. purcelli and P. janzeni. We also thank Adalberto J. dos Santos, Flávia Maria S. Carmo, Karla S. C. Yotoko, José Henrique Schoereder and two anonymous referees for their comments on the manuscript. This study complies with the current laws of Brazil (Authorization ICMBIO N° 34711).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kloss, T.G., Gonzaga, M.O., Roxinol, J.A.M. et al. Attack Behavior of Two Wasp Species of the Polysphincta Genus Group (Hymenoptera, Ichneumonidae) on their Orb-Weaver Spider Hosts (Araneae, Araneidae). J Insect Behav 29, 315–324 (2016). https://doi.org/10.1007/s10905-016-9560-6
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10905-016-9560-6