Abstract
This work presents an environmentally friendly approach to synthesizing silver-zinc oxide (Ag-Zn) bimetallic nanoparticles using domestic waste of kiwi (Actinidia chinensis var. deliciosa) peel extract that acts as a reducing and stabilizing agent. The fabricated nanoparticles were characterized using various techniques such as UV–Vis spectroscopy, Fourier transform infrared spectroscopy (FTIR), and field emission scanning electron microscopy (FESEM). The antimicrobial and catalytic activity of the fabricated Ag–Zn were investigated. The antimicrobial activity against gram-positive Staphylococcus aureus (SA), Bacillus subtilis (BS), and gram-negative Klebsiella pneumonia (KP) strains were evaluated and it was found that KP showed higher zone of inhibition i.e.12 mm, then followed by 11 mm, and 9 mm for SA and BS respectively. For the catalytic activity, more than 85% of methyl red, 93% of phenol red, and 78% of eosin yellow dyes were degraded in 1 h. The green synthesis method presented in this study provides a sustainable and non-toxic synthesis route while simultaneously remediating the environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Small particles of the size 1–100 nm have gained significant research interest due to their tremendous specific surface area which is unique and applicable in a wide range of applications [1]. Among the different types of nano-sized materials, transitional metal oxide-based nanomaterials like Ag and Zn are among the most efficient and effective nanomaterials exhibiting excellent catalytic, antioxidant, electrochemical, and antimicrobial activity [2]. This has made nanoparticles effective in detecting and quantifying many types of toxic molecules and contaminants found in different items such as liquid or solid food, drugs, water bodies, and others [3]. This is due to their cost effectiveness, low toxicity, good stability, biocompatibility, and excellent electron transfer capabilities.
Due to the low toxicity and small particulate size of nanoparticles, they have been used as antimicrobial agents to protect food, crops, and other products from different pathogenic microorganisms. For example, a pH-triggered reassembling Ag NPs in a bacteria-infected micro-environment has been synthesized by [3] and the NPs greatly improved the antibacterial activity with a minimum inhibitory concentration of 4 & 32 µm mL−1 against methicillin-resistant Staphylococcus aureus and 8 & 32 µm mL−1 respectively. Another Ag nanoparticles synthesized using turmeric extract was found to possess antibacterial and catalytic activity [4]. Also, green synthesized ZnO was found to exhibit strong antibacterial activity against B. subtilis, K. pneumonia, P. aeruginosa, and Proteus mirabilis [1]. Similarly, an Ag film nanocomposite was developed by [5] to inactivate food pathogenic and improve the shell life of strawberry fruits. Moreover, sunlight-activated ZnO has been synthesized and used as a bio-fungicide against strawberry plant pathogen Botrytis cinerea [6] and the results obtained indicated that ZnO inhibited the growth of Botrytis cinerea by 12% and 80% when used in the dark and sunlight respectively. ZnO has also been applied in the lining of canned food items like corn, meat, and fish [6]. In terms of electrochemical, optical and electron transfer capabilities, Ag and ZnO-modified carbon paste electrode with an ionic surfactant was developed and used for the determination of paraquat (II), nonlinear absorption with superior optical limitation, and the detection of trimethoprim (TMP) respectively [7,8,9]. The developed electrode shows the highest peak current compared to nascent CPE.
Even though metallic nanoparticles have these advantages, they have some limitations like reusability and poor recovery, stability, high surface energy, and susceptibility to oxidation that limit their applications in some fields [10]. To overcome those problems, scientists have developed some strategies over the years to overcome them such as entrenching the nanoparticles on a polymer matrix providing support using a solid material [11], or blending two monometallic nanoparticles to create a new material called bimetallic nanoparticles.
Bimetallic nanoparticles are nanoparticles that are made up of two different types of metal. This combination results in the creation of new properties and characteristics that curtail the synergy between the two metals. As such, bimetallic nanoparticles have found significant research interest and application in many fields including electrochemical sensors, catalysis, and biosensors. The properties of the bimetals can be adjusted by adjusting the percentage of composition while the morphology such as size and structure depends on the synthesis parameters like the concentration of the metal salts/reducing and stabilizing agent and the method of synthesis [12]. For instance, zinc oxide and silver-zinc oxide bimetallic nanoparticles were green synthesized by [13] and the resulting bimetallic nanoparticles were smaller in size and had better antibacterial activity as compared to the zinc oxide monometallic nanoparticles. Similarly, Au–Ag were synthesized using the leaf extract of Ocimum tenuiflorum and the bimetallic nanoparticles good antibacterial activity against gram –ve E.coli, gram + ve Bacillus subtilis and showed good degradation activity against Coomassie brilliant blue R250 dye [14].
Although, bimetallic nanoparticles can be synthesized via physical, chemical, or biological methods. The physical method has the advantage of maintaining a constant chemical composition but has the disadvantage of variation in particle size. Chemical synthesis on the other hand, while being effective in producing the desired nanoparticles, the process usually involves the use of chemicals during the production process that when discarded into the environment, can become harmful to animals and human health. However, in contrast to the chemical method, the biological method of nanoparticle synthesis utilizes reducing and stabilizing agents of biological origin like plant extract or microbes. This serves as an alternative for producing bimetallic nanoparticles that are more stable and biocompatible. For instance, silver and silver-oxide (Ag & AgO), zinc-oxide (ZnO), silver-zinc (Ag–Zn), and silver-zinc oxide (Ag–ZnO) bimetallic nanoparticles have been synthesized using Solanum elaeagnifolium [15], Ocimum lamifolium Leaf [16], Annona muricata [17], and Urginea epigea bulb extract [13] respectively. It is believed that the presence of phytochemicals and biomolecules in the plant extract made the reduction and stabilization of bimetallic nanoparticles to be possible. However, bio-waste like fruit or vegetable peels is another rich source of phytochemicals that can be utilized for bimetallic nanoparticle synthesis [18]. These bio-wastes have zero market value and are rich in phytochemicals. Thus, by utilizing and exploring their reduction potential, the cost of nanoparticle production can be further reduced while simultaneously remediating the environment. They can also serve as an alternative for nanoparticle synthesis using medicinal or endangered plant species. For instance, orange peels, pomegranate peels, avocado fruit peels, eggshells, and silkworm cocoons have been used to synthesize Au–Pd [19] and Ag–CuO [20], Ni–Fe [21], Ag–Au [22], NiO–ZnO [23] and Fe–Cu [24] respectively.
Actinidia chinensis var. deliciosa, commonly known as ‘Kiwifruit’, is a delicious and highly nutritious fruit that belongs to the Actinidiaceae family. It is widely recognized for its vibrant green flesh and unique flavor which has gained popularity due to its exceptional nutritional value, health benefits, and numerous applications. The fruit is considered a nutritional powerhouse, packed with essential vitamins (like vitamin C, K, E, and B-complex including folate), minerals (like copper, potassium, magnesium, calcium, phosphorus, and iron), carbohydrate, protein, water, sugar, dietary fiber, amino acids, flavonoids, and antioxidants with a single fruit containing more than the recommended daily nutritional intake [25]. These nutrients are essential for a wide range of pharmacological applications including anti-cancer, antioxidant, anti-tumor, anti-inflammatory, anti-diabetic, hypolipemic, maintaining proper heart function, maintaining a healthy digestive system, boosting the immune system, preventing constipation, regulating blood sugar levels and blood pressure, and protecting against oxidative stress [26]. In addition to its nutritional value, kiwifruit has gained attention for its potential applications in nanoparticle synthesis. The bioactive compounds present in kiwifruit, such as phenolic compounds and enzymes, can act as reducing agents and stabilizers for nanoparticle synthesis. The fruit is mostly eaten as a whole or processed into jam or juice after peeling the skin which leads to the accumulation and generation of a substantial amount of bio-waste (peels). The kiwifruit peel, which is often discarded, has been investigated for its potential use in nanoparticle synthesis. The peel contains high concentrations of bioactive compounds like dietary fiber, antioxidants, folate, vitamin E, flavonoid, and phenolic compounds making it a valuable resource for sustainable nanoparticle production [25]. Researchers can reduce environmental impact and promote a circular economy approach by utilizing kiwifruit waste. For instance, silver (Ag) [27], zinc oxide (ZnO) [28], titanium dioxide (TiO2) [29], tin oxide (SnO2) [30], and sulfur (S) [31] nanoparticles have all been synthesized using kiwifruit peel extract as the reducing and as well as the stabilizing agent. This shows that kiwi peel extract has a lot of potential for nanoparticle synthesis. In this study, potential of kiwi peel extract to synthesize silver-zinc bimetallic nanoparticles were evaluated. To our knowledge, there is no report of bimetallic nanoparticle synthesis of Ag–Zn bimetallic nanoparticles from kiwi peel extract. Thus, this study aims to explore the reduction potential of kiwifruit (Actinidia chinensis var. deliciosa) peel extract for the synthesis and application of Ag–Zn bimetallic nanoparticles.
2 Materials and Methods
2.1 Materials
Kiwifruit (Actinidia chinensis var. deliciosa) peel. Silver nitrate (AgNO3) purity 99.8% and zinc sulphate pentahydrate (ZnSO4.7H2O) purity 99.6% were used as the metal salt precursors, and methyl red (MR), and phenol red (PR), dyes were purchased from Himedia. All of the chemicals were of analytical grade and didn’t undergo any further purification. Double distilled water was used throughout the entire experiment.
2.2 Extract Preparation
Kiwifruit peel extract was collected domestically in a zip-lock bag. The peels were washed and rinsed with double distilled water before drying in a hot air oven at 70–80 °C. The dried peels were then pulverized using a mortar and pestle. The extract was obtained by adding 1 g of the kiwi peel powder to 25 ml of double distilled water and then boiling for 3–5 min on a heating mantle. The mixture was allowed to cool down and then filtered using Whatman’s Grade 1 filter paper to obtain the final extract. The extract was then stored at 4 °C for further use.
2.3 Synthesis of Ag–Zn Bimetallic Nanoparticles
The Ag–Zn was prepared using a single-pot simultaneous co-reduction method. A stock solution of 3 mM of AgNO3 and ZnSO4.7H2O was made separately at room temperature. The solutions were subsequently mixed in a beaker at room temperature. To that mixture, an aqueous solution of the kiwifruit peel extract was added on a ratio of 9:1 while subjected to heating at 70–80 °C. A visible color change was observed in the aqueous solution turning from pale yellow to a-dark brown color within 30 min. The color stayed the same even after heating for 1 h. The solution was then purified by centrifuging at 10,000 RPM for 5 min. The supernatant was discarded and then rinsed with distilled water before centrifuging again. The process was repeated 3 times before drying the particles in a hot air oven at 80 °C. The final particles were stored in an air-tight container until further use.
2.4 Characterization of Ag–Zn Bimetallic Nanoparticles
The synthesized Ag–Zn was characterized with different characterization techniques, namely; Uv–visible spectrophotometer, FTIR, FESEM, EDX, and DLS. The initial UV–vis analysis was carried out in the range of 200–800 nm wavelength using a Shimadzu UV-1800 spectrophotometer, Japan. The resulting absorbance graph on the y-axis was plotted against the wavelength on the x-axis. The FESEM analysis of the synthesized Ag–Zn was carried out using field emission scanning electron microscopy in conjunction with EDX (Carl Zeiss Model Supra 55, Germany). The Ag–Zn FTIR analysis was carried out using a Cary 630 Agilent FTIR spectrometer ranging from 4000 to 700 cm-1 wavelengths. The particle size analyzer (Litesizer 500) was used to analyze the particle size distribution of the synthesized Ag–Zn bimetallic nanoparticles.
2.5 Catalytic Activity
The catalytic efficiency of the Ag–Zn bimetallic nanoparticles was evaluated in an aqueous solution by the reduction and degradation of methyl red and phenol red dyes. In a typical experiment, a stock solution of 30 mM of NaBH4, 1 mg/ml of Ag–Zn bimetallic nanoparticles, and 1 mM of MR, PR, and EY dyes were prepared. In separate test tubes, 1 ml of NaBH4 was added to 1 ml of MR, PR, and EY stock solution. The mixture was brought to 9 ml by adding 7 ml of distilled water. Then 1 ml of Ag–Zn was added and the entire solution was brought to 10 ml. UV–vis spectrophotometer readings (200–800 nm) of the mixture were taken at regular time intervals after keeping it in the dark. The mixture without the bimetallic nanoparticles was used as a control. The decrease in absorbance peak indicates the nano-catalytic efficiency of the bimetallic nanoparticles in the presence of NaBH4.
2.6 Antibacterial Studies
The in vitro antibacterial studies of the synthesized Ag–Zn bimetallic nanoparticles were evaluated against S. aureus, K. pneumonia, and B. subtilis using the disk diffusion method. The microbial cultures were grown overnight before spreading on agar plates. Ag–Zn bimetallic nanoparticles (1 mg/ml) stock solution was freshly made with doubled distilled water and different concentration of 25, 50, 100, and 200 µg/ml was used as treatment before incubating the agar plates for 24 h at 37 °C. Ampicillin (10 mg c) was used as the positive control and distilled water as the negative control. By measuring the zone of inhibition (ZOI), the antibacterial activity of the Ag–Zn bimetallic nanoparticles was quantified. The zone of inhibition is directly proportional to the bactericidal potential of the Ag–Zn bimetallic nanoparticles.
3 Results and Discussion
3.1 UV–Visible Spectrometric Analysis
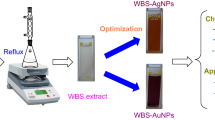
Nanoparticle synthesis generally requires a reducing agent that can reduce metal ions into their respective nanoparticles. The reducing agent can also serve as the stabilizing agent, preventing the nanoparticles from forming agglomeration. But at times, a separated stabilizing agent is required to stabilize the nanoparticles depending on the method of synthesis used. Kiwifruit peel extract has been demonstrated to aid the synthesis of bimetallic nanoparticles. As reported in previous literature, kiwifruit peel extract contains different kinds of plant phytochemicals like flavonoid, tannin, and phenolic compounds [25] which are more than capable of reducing metal ions effectively while simultaneously stabilizing them. Before the synthesis began, the appearance of the aqueous solution of the metal precursors and extract was pale yellow. After it was subjected to the reaction process, it became opaque, and a dark brown precipitate was observed after 30 min. The precipitate remains even after the reaction time elapsed for more than 1.5 h. The precipitate that physically appeared in the solution, however, suggests the formation of the bimetallic Ag–Zn nanoparticles (Fig. 1). UV and visible light are known to be energetic while having the ability to increase the electron energy level. The absorbance of the synthesized bimetallic nanoparticles was measured in the range of 200–800 nm. Similar results have been reported by [32] (Fig. 2.)
During the UV reading analysis, two distinctive peaks were observed (Fig. 2). The increasing peak that was observed at 278 nm confirmed the presence of zinc (Zn) nanoparticles. This is in line with the absorbance range of 263–339 nm for ZnO as reported by [16] depending on the concentration of Zn. Other studies, however, suggested absorbance peaks of 267 nm [33], 270 nm [34], 376 nm [1], 378 nm [35], and 385 nm [36] for ZnO respectively. Another distinct peak at 418 nm was characterized as the formation of silver nanoparticles. It has been reported that the absorption band of Ag nanoparticles is between 415 and 425 nm [37] depending on the molar concentration of Ag. However, a report suggested the absorption band of Ag at 390 nm, 430 nm, 431 nm, and 460 nm respectively [38]. Another study also reported the absorption of Ag at 430 nm [39]. However, when Ag is doped with Zn, the absorption band broadens due to strong interfacial electronic coupling between Ag & Zn. This leads to stronger light scattering showing a typical band gap in the visible region of 400–525 nm for Ag@Zn [36]. All these findings are consistent with the current findings in our study as well.
3.2 DLS Analysis
The dynamic light scattering analysis was employed to study the size distribution of the synthesized Ag–Zn bimetallic nanoparticles. The synthesized Ag–Zn bimetallic nanoparticles were found to possess a larger size distribution with an average hydrodynamic diameter of 272 nm which is larger than expected as shown in the Fig. 3. The larger size distribution of the bimetallic nanoparticles is due to the agglomeration of the nanomaterials. As a result, the size of the bimetallic nanoparticles will appear to be much larger than the expected size. Similar hydrodynamic diameter of 188 ± 18 nm for Ag–Zn has been reported by [40].
3.3 FTIR Analysis
Figure 4 shows the FTIR spectra of both the kiwifruit peel extract and the synthesized Ag-Zn bimetallic nanoparticles. The FTIR spectra were recorded in the range of 400–4000 cm−1. A broad spectrum within the range of 3000 to 3500 cm−1 was observed with a distinctive peak at 3352 cm-1 on both the extract and synthesized bimetallic nanoparticles respectively thus, indicating the presence of phenols and as well as the bending mode of the OH group [41]. This result is similar and was observed during the synthesis of Ag–ZnO using Urginea epigea extract where the FTIR spectrum shows a broad band in the 3294 cm−1 region indicating the presence of OH group [13]. The intense peak at 1637 cm−1 is attributed to C=C stretching vibration [13]. This is in line with the report of Zn synthesis using Cayratia pedata leaf extract [42]. The FTIR spectrum of Ag-Zn bimetallic nanoparticles shows identical peaks to kiwifruit peel extract with a slight shift in the position in which they appear. This could be possible as a result of the interaction between the Ag–Zn bimetallic nanoparticles and the functional groups.
3.4 XRD Analysis
In order to evaluate the crystalline nature of the synthesized bimetallic nanoparticles, the XRD analysis was employed. The XRD spectrum in Fig. 5 shows a weak peak at 38.44° and did not exhibit any other diffraction peak. The peak at 38.44 might be attributed to the (111) lattice plane of Zn [43]. We are not sure of the reason why the XRD spectrum didn’t show much peaks but a similar XRD spectrum of Ag–Zn was also observed by [32].
3.5 FESEM and EDX
The characteristics of the surface morphology of the synthesized Ag–Zn bimetallic nanoparticles were analyzed using FE-SEM. As shown in Fig. 5, the Ag–Zn bimetallic nanoparticles were dispersed evenly. The bimetallic nanoparticles were photographed at 200 nm, WD-9 mm, 10 kV, and Mag-100 and 75 K X, which reveals spherical and irregular morphology. It also suggests uniform particle formation with a size variation in their sizes. The morphology also revealed a smooth surface of the bimetallic nanoparticles. EDX analysis further estimated the presence of the bimetals in a designated region of the FE-SEM image. After further examination of the EDX data (Fig. 6), the Ag–Zn bimetallic nanoparticles produced are made up of 75.24 wt% of Ag and 24.76 wt% of Zn which deviated from the stoichiometric standard that was used during synthesis (1:1). This may have happened as a result of the crystallographic orientation or surface energies of the metal atoms. However, the overall result suggests a crystalline nature of the Ag–Zn nanoparticles of which the Ag has higher peak intensity as compared to Zn peaks (Fig. 7).
4 Dye Degradation
4.1 Methyl Red
Methyl red is an organic dye that is also known as azo dye. Its chemical name is 2-(N, N-dimethyl-4-aminophenyl) azobenzenecarboxylic acid, serving as a standard pH indicator. Although it is not classified as a highly hazardous chemical, it is widely used in many industries like the textile industry to impact color on fabrics, ink, and printing industry and used in research and development in the fields of chemistry and biology. However, methyl red is a significant cause of water contamination when industrial or textile wastewater pollutants are discharged into the environment which may subsequently find their way to underground or open water bodies causing harm to the ecosystem. As a result, treatment of methyl red contaminated water is of great importance. The reaction of Ag–Zn bimetallic nanoparticles as nano-catalyst to degrade methyl red was achieved and within 1 h, more than 85% was degraded in the presence of NaBH4. The UV absorption spectra are shown in the Fig. 8 below. When methyl red was treated with NaBH4, there was an insignificant reduction in the absorption peak. However, when the methyl red dye was treated with Ag–Zn bimetallic nanoparticles in the presence of NaBH4, a reduction was observed which tends to decrease with time. The experiment was carried out at room temperature using doubled distilled water and 1 mM concentration of the dye solutions.
Several previous studies have reported the degradation of dyes. For instance, a research reported the synthesis of gold nanospheres and Fe–Cu bimetallic nanoparticles using Cyclea peltata extract and resulting nanoparticle was found to degrade up 86.02% and 82% of methyl green dye in 105 min respectively [44, 45]. Similarly, green synthesized Ag nanoparticles were investigated for their catalytic activity against methylene blue dye and were observed to have positive reduction potential [4].
4.2 Phenol Red
Phenol red is a pH indicator that appears as a dark red powder. It is usually used to track and monitor the pH of growth media and physiological fluids in biological and cell culture applications. It transitions from yellow to red when the pH increases. Typically, red is around 8.0–8.2 and becomes yellow below 6.8. Phenol red is a stable organic compound that is widely used in many industries and when the wastewater is released into the environment, it is difficult to degrade by natural process. Therefore, efforts have been made to break down its chemical structure. The Ag–Zn bimetallic nano-catalyst was found to be effective and degraded more than 93% of phenol red in 10 min as shown in the UV absorption spectra Fig. 9.
4.3 Eosin Yellow
Eosin yellow is a yellow powder or crystal that can serve as a pH indicator and is also used in histology and microbiology to stain certain biological specimens and to indicate the pH of a certain media. It is a synthetic ionic dye that is usually yellow in an acidic solution and turns pink in the alkaline solution used in textile, food, and cosmetic industries. It is a potential pollutant due to its toxicity and persistence in water bodies. As such, Ag–Zn bimetallic nano-catalyst was employed for the degradation of eosin yellow dye. As shown in the UV spectra in Fig. 10 below, eosin yellow had more than 78% degradation in 1 h.
It is important to note that the Ag–Zn bimetallic nanoparticle act as a catalyst for methyl red, phenol red and eosin yellow dye degradation. This is true because, nanoparticles are known for their high surface area which facilitates the breaking down of NaBH4 (electron donor) and transfer of electrons to the dyes (electron acceptor) thus, causing a reduction reaction. Although, it has been reported that NaBH4 on its own can’t reduce dyes due to redox potential differences while a catalyst, whose redox potential is in-between the electron donor and acceptor is required to have a smooth electron transfer [44, 45]. This makes bimetallic nanoparticles a very good nano-catalyst for degradation of dyes. However, in another research, NaBH4 was replaced with solar radiation while the Ag metal nanoparticles play the role of a photocatalyst. The visible light illumination propagated the SPR (Surface Plasmon Resonance) of the Ag metal nanoparticles which in return, increased the adsorption of the Ag metal nanoparticles on to the dye solution and subsequently, decolourization of the dye. It is believed that Ag nanoparticles are also stable, and good photocatalysts solar radiation for degradation of organic dyes [46, 47] (Fig. 11).
5 Antibacterial Studies
The biogenic Ag–Zn bimetallic nanoparticles’ antimicrobial effect was tested against gram-positive bacterial strains (S. aureus, B. subtilis) and gram-negative (K. pneumonia) bacteria using the disk diffusion method which demonstrates the microorganism susceptibility. A diffused ring was created by the aqueous solution of the Ag-Zn on all the bacterial strains ranging from a minimum of 0 mm to a maximum of 18 mm against all the bacterial strains. The Ag–Zn bimetallic nanoparticles show greater inhibitory on K. pneumonia (12 mm) to compared B. subtilis (9 mm), and S. aureus (11 mm). Ampicillin effect was also observed (2-11 mm) against the bacterial pathogen (Table 1) (Fig. 12).
In literature various studies reported the antimicrobial properties of nanoparticles. For example, a study reported Ag nanoparticles synthesie using Jatropha curcas seed cake extract and the resulting nanoparticles were found to be effective against P. aeruginosa, E. coli and B. subtilis bacterial strains [48]. Similarly, green synthesized Au–Ag bimetallic nanoparticles were found to possess substantial bactericidal effect against all bacterial strains used for the study [14]. Nanoparticles are able to exhibit antibacterial activity via a variety of mechanism. They can be able to release ROS, depletion of ATP or interact with the cell membrane of the bacteria, thus, causing physical damage and compromising the integrity of the bacteria that can result to bacteria death [49, 50]. The nanomaterials can interact with the bacterial cell membrane, causing physical damage and compromising the integrity of the bacteria1. These mechanisms make metallic nanomaterials effective against bacteria, and they are being researched for their potential in combating antibiotic resistance.
6 Conclusion
This study summarized the a safe, simple, and environmentally friendly route for the synthesis of Ag–Zn bimetallic nanoparticles using kiwifruit peel extract as the reducing and stabilizing agent. The bimetallic nanoparticles were successfully synthesized and characterized using UV spectroscopy, DLS, FESEM, and FTIR. The antimicrobial activity and catalytic activity of the bimetals were studied. The bimetallic nanoparticles showed effective catalytic activity against methyl red, phenol red, and eosin yellow. More than 85% of methyl red, 93% of phenol red, and 78% of eosin yellow dyes were degraded in 1 h. Furthermore, Ag-Zn shows a promising inhibition against gram-positive bacterial strains (S. aureus, B. subtilis) and gram-negative (K. pneumonia) bacterial strains with the zone of inhibition on K. pneumonia (12 mm), compared to B. subtilis (9 mm), and S. aureus (11 mm) respectively. Ampicillin was used as a control and the effect was observed (2–11 mm). This study demonstrated the green synthesis approach of Ag–Zn bimetallic nanoparticles and its potential application in many fields.
References
P. Ramesh, K. Saravanan, P. Manogar, J. Johnson, E. Vinoth, M. Mayakannan, Green synthesis and characterization of biocompatible zinc oxide nanoparticles and evaluation of its antibacterial potential. Sens. Bio-Sens. Res. 31, 100399 (2021). https://doi.org/10.1016/j.sbsr.2021.100399
M. Hosny, M. Fawzy, A.S. Eltaweil, Green synthesis of bimetallic Ag/ZnO@Biohar nanocomposite for photocatalytic degradation of tetracycline, antibacterial and antioxidant activities. Sci. Rep. (2022). https://doi.org/10.1038/s41598-022-11014-0
X. Xie et al., Ag nanoparticles cluster with pH-Triggered reassembly in targeting antimicrobial applications. Adv. Func. Mater. 30(17), 2000511 (2020). https://doi.org/10.1002/adfm.202000511
S. Nayak, L.C. Goveas, C. Vaman Rao, Biosynthesis of Silver nanoparticles using turmeric extract and evaluation of its anti-bacterial activity and catalytic reduction of methylene blue, in Materials, Energy and Environment Engineering. ed. by R.B. Mohan, G. Srinikethan, B.C. Meikap (Springer, Singapore, 2017), pp.257–265. https://doi.org/10.1007/978-981-10-2675-1_31
V. Kanikireddy, K. Varaprasad, M.S. Rani, P. Venkataswamy, B.J. Mohan Reddy, M. Vithal, Biosynthesis of CMC-Guar gum-Ag0 nanocomposites for inactivation of food pathogenic microbes and its effect on the shelf life of strawberries. Carbohydr. Polym. 236, 116053 (2020). https://doi.org/10.1016/j.carbpol.2020.116053
Z. Luksiene, N. Rasiukeviciute, B. Zudyte, N. Uselis, Innovative approach to sunlight activated biofungicides for strawberry crop protection: ZnO nanoparticles. J. Photochem. Photobiol., B 203, 111656 (2020). https://doi.org/10.1016/j.jphotobiol.2019.111656
A. Farahi, M. Achak, L. El Gaini, M.A. El Mhammedi, M. Bakasse, Silver particles-modified carbon paste electrodes for differential pulse voltammetric determination of paraquat in ambient water samples. J. Assoc. Arab Univ. Basic Appl. Sci. 19(1), 37–43 (2016). https://doi.org/10.1016/j.jaubas.2014.06.011
S. Nayak, M.K.B. Dr, L. Goveas, C.V. Rao, S. Sajankila, Investigation of nonlinear optical properties of AgNPs synthesized using Cyclea peltata leaf extract post OVAT optimization. BioNanoScience (2021). https://doi.org/10.1007/s12668-021-00875-w
V.B. Patil, D. Ilager, S.M. Tuwar, K. Mondal, N.P. Shetti, Nanostructured ZnO-based electrochemical sensor with anionic surfactant for the electroanalysis of trimethoprim. Bioengineering (2022). https://doi.org/10.3390/bioengineering9100521
H. Kumar, N. Venkatesh, H. Bhowmik, A. Kuila, Metallic nanoparticle: a review. Biomed. J. Sci. Tech. Res 4(2), 3765–3775 (2018)
D.S. Idris et al., Polymer-based nanocarriers for biomedical and environmental applications. e-Polymers (2023). https://doi.org/10.1515/epoly-2023-0049
T. Mazhar, V. Shrivastava, R.S. Tomar, Green synthesis of bimetallic nanoparticles and its applications: a review. J. Pharm. Sci. Res. 9(2), 102 (2017)
M.C. Jobe, D.M. Mthiyane, M. Mwanza, D.C. Onwudiwe, Biosynthesis of zinc oxide and silver/zinc oxide nanoparticles from Urginea epigea for antibacterial and antioxidant applications. Heliyon 8(12), e12243 (2022)
S. Nayak, C.V. Rao, S. Mutalik, Exploring bimetallic Au–Ag core shell nanoparticles reduced using leaf extract of Ocimum tenuiflorum as a potential antibacterial and nanocatalytic agent. Chem. Pap. 76(10), 6487–6497 (2022). https://doi.org/10.1007/s11696-022-02299-6
M. Barwant et al., Eco-friendly synthesis and characterizations of Ag/AgO/Ag2O nanoparticles using leaf extracts of Solanum elaeagnifolium for antioxidant, anticancer, and DNA cleavage activities. Chem. Pap. 76(7), 4309–4321 (2022). https://doi.org/10.1007/s11696-022-02178-0
E. Tilahun, Y. Adimasu, Y. Dessie, Biosynthesis and optimization of ZnO nanoparticles using Ocimum lamifolium leaf extract for electrochemical sensor and antibacterial activity. ACS Omega 8(30), 27344–27354 (2023). https://doi.org/10.1021/acsomega.3c02709
A. Velidandi, N.P.P. Pabbathi, S. Dahariya, R.R. Baadhe, Green synthesis of novel Ag–Cu and Ag–Znbimetallic nanoparticles and their in vitro biological, eco-toxicity and catalytic studies. Nano-Struct. Nano-Objects 26, 100687 (2021)
D.S. Idris, A. Roy, Synthesis of Bimetallic nanoparticles and applications—an updated review. Crystals (2023). https://doi.org/10.3390/cryst13040637
W.P. Wicaksono et al., A green synthesis of gold–palladium core–shell nanoparticles using orange peel extract through two-step reduction method and its formaldehyde colorimetric sensing performance. Nano-Struct. Nano-Objects 24, 100535 (2020). https://doi.org/10.1016/j.nanoso.2020.100535
D.S. Idris, A. Roy, Biogenic synthesis of Ag–CuO nanoparticles and its antibacterial, antioxidant, and catalytic activity. J. Inorg. Organomet. Polym. (2023). https://doi.org/10.1007/s10904-023-02873-9
K.V.G. Ravikumar, S.V. Sudakaran, K. Ravichandran, M. Pulimi, C. Natarajan, A. Mukherjee, Green synthesis of NiFe nano particles using Punica granatum peel extract for tetracycline removal. J. Clean. Prod. 210, 767–776 (2019). https://doi.org/10.1016/j.jclepro.2018.11.108
A.E. Adebayo et al., Biosynthesis of silver, gold and silver–gold alloy nanoparticles using Persea americana fruit peel aqueous extract for their biomedical properties. Nanotechnol. Environ. Eng. 4(1), 13 (2019). https://doi.org/10.1007/s41204-019-0060-8
S. PrashannaSuvaitha, P. Sridhar, T. Divya, P. Palani, K. Venkatachalam, Bio-waste eggshell membrane assisted synthesis of NiO/ZnO nanocomposite and its characterization: evaluation of antibacterial and antifungal activity”. Inorganica Chim. Acta 536, 120892 (2022). https://doi.org/10.1016/j.ica.2022.120892
S.R. Dhruval et al., Rapid synthesis of antimicrobial Fe/Cu alloy nanoparticles using Waste Silkworm Cocoon extract for cement mortar applications. Adv. Nat. Sci: Nanosci. Nanotechnol. 11(2), 025006 (2020). https://doi.org/10.1088/2043-6254/ab8790
J. Zhang, J. Tian, N. Gao, E.S. Gong, G. Xin, C. Liu, B. Li, Assessment of the phytochemical profile and antioxidant activities of eight kiwi berry (Actinidia arguta (Siebold & Zuccarini) Miquel) varieties in China. Food. Sci. Nutr. 9(10), 5616–5625 (2021)
X. He et al., “Actinidia chinensis Planch.: A Review of Chemistry and Pharmacology,” Frontiers in Pharmacology, vol. 10, 2019, [Online]. Available: https://www.frontiersin.org/articles/https://doi.org/10.3389/fphar.2019.01236. Accessed 04 Jul 2023
X. Sun et al., Sodium alginate-based nanocomposite films with strong antioxidant and antibacterial properties enhanced by polyphenol-rich kiwi peel extracts bio-reduced silver nanoparticles. Food Packag. Shelf Life 29, 100741 (2021)
J. Li, Y. Li, H. Wu, S. Naraginti, Y. Wu, Facile synthesis of ZnO nanoparticles by Actinidia deliciosa fruit peel extract: bactericidal, anticancer and detoxification properties. Environ. Res. 200, 111433 (2021)
N. Ajmal, K. Saraswat, M.A. Bakht, Y. Riadi, M.J. Ahsan, M. Noushad, Cost-effective and eco-friendly synthesis of titanium dioxide (TiO2) nanoparticles using fruit’s peel agro-waste extracts: characterization, in vitro antibacterial, antioxidant activities. Green Chem. Lett. Rev. 12(3), 244–254 (2019)
E. Gomathi, M. Jayapriya, M. Arulmozhi, Environmental benign synthesis of tin oxide (SnO2) nanoparticles using Actinidia deliciosa (Kiwi) peel extract with enhanced catalytic properties. Inorg. Chem. Commun. 130, 108670 (2021)
R. Priyadarshi, S. Roy, J.-W. Rhim, Enhanced functionality of green synthesized sulfur nanoparticles using kiwifruit (Actinidia deliciosa) peel polyphenols as capping agents. J. Nanostruct. Chem. (2021). https://doi.org/10.1007/s40097-021-00422-
Tanuja, A. Lagashetty, Synthesis and characterization of silver doped zinc oxide nanoparticle from beetle leaf extract- evaluation of antimicrobial activity against human pathogenic bacteria and fungi. IJRASET 10(5), 2703–2716 (2022). https://doi.org/10.22214/ijraset.2022.42914
Green synthesis of Ag, Zn and Cu nanoparticles from aqueous extract of Spondias mombin leaves and evaluation of their antibacterial activity, African J. Clin. Exp. 21(2), 106–113 (2020). https://doi.org/10.4314/AJCEM.V21I2.4
S. Kunwar et al., Bio-fabrication of Cu/Ag/Zn Nanoparticles and their antioxidant and dye degradation activities. Catalysts (2023). https://doi.org/10.3390/catal13050891
M. Pudukudy, Z. Yaakob, Facile synthesis of quasi spherical ZnO nanoparticles with excellent photocatalytic activity. J. Clust. Sci. 26(4), 1187–1201 (2015). https://doi.org/10.1007/s10876-014-0806-1
S.S. Patil et al., Nanostructured microspheres of silver @ zinc oxide: an excellent impeder of bacterial growth and biofilm. J. Nanopart. Res. 16(11), 2717 (2014). https://doi.org/10.1007/s11051-014-2717-3
D.K. Adeyemi, A.O. Adeluola, M.J. Akinbile, O.O. Johnson, G.A. Ayoola, Green synthesis of Ag, Zn and Cu nanoparticles from aqueous extract of Spondias mombin leaves and evaluation of their antibacterial activity. Afr. J. Clin. Exp. Microbiol. (2020). https://doi.org/10.4314/ajcem.v21i2.4
H. Yousaf, A. Mehmood, K.S. Ahmad, M. Raffi, Green synthesis of silver nanoparticles and their applications as an alternative antibacterial and antioxidant agents. Mater. Sci. Eng., C 112, 110901 (2020). https://doi.org/10.1016/j.msec.2020.110901
N. Jomehzadeh, Z. Koolivand, E. Dahdouh, A. Akbari, A. Zahedi, N. Chamkouri, Investigating in-vitro antimicrobial activity, biosynthesis, and characterization of silver nanoparticles, zinc oxide nanoparticles, and silver-zinc oxide nanocomposites using Pistacia Atlantica Resin. Mater. Today Commun. 27, 102457 (2021). https://doi.org/10.1016/j.mtcomm.2021.102457
S.M. Mousavi-Kouhi, A. Beyk-Khormizi, M.S. Amiri, M. Mashreghi, M.E. TaghavizadehYazdi, Silver-zinc oxide nanocomposite: from synthesis to antimicrobial and anticancer properties. Ceram. Int. 47(15), 21490–21497 (2021). https://doi.org/10.1016/j.ceramint.2021.04.160
A. Kadam et al., Facile synthesis of Ag–ZnO core–shell nanostructures with enhanced photocatalytic activity. J. Ind. Eng. Chem. 61, 78–86 (2018). https://doi.org/10.1016/j.jiec.2017.12.003
A. Jayachandran, T.R. Aswathy, A.S. Nair, Green synthesis and characterization of zinc oxide nanoparticles using Cayratia pedata leaf extract. Biochemistry and Biophysics Reports 26, 100995 (2021). https://doi.org/10.1016/j.bbrep.2021.100995
A. Daphedar, T.C. Taranath, Green synthesis of zinc nanoparticles using leaf extract of Albizia saman (Jacq.) Merr. and their effect on root meristems of Drimia indica (Roxb.) Jessop. Caryologia 71(2), 93–102 (2018). https://doi.org/10.1080/00087114.2018.1437980
A. Velidandi, M. Sarvepalli, N.P.P. Pabbathi, R.R. Baadhe, Biogenic synthesis of novel platinum-palladium bimetallic nanoparticles from aqueous Annona muricata leaf extract for catalytic activity. 3 Biotech 11(8), 385 (2021). https://doi.org/10.1007/s13205-021-02935-0
R. Vijayan, S. Joseph, B. Mathew, Eco-friendly synthesis of silver and gold nanoparticles with enhanced antimicrobial, antioxidant, and catalytic activities. IET Nanobiotechnol. 12(6), 850–856 (2018). https://doi.org/10.1049/iet-nbt.2017.0311
P. Kumar, M. Govindaraju, S. Senthamilselvi, K. Premkumar, Photocatalytic degradation of methyl orange dye using silver (Ag) nanoparticles synthesized from Ulva lactuca. Colloids Surf. B 103, 658–661 (2013). https://doi.org/10.1016/j.colsurfb.2012.11.022
S. Nayak, L.C. Goveas, R. Selvaraj, S. Mutalik, S.P. Sajankila, Use of Cyclea peltata mediated gold nanospheres for adsorptive degradation of methyl green dye. Bioresour. Technol. Rep. 20, 101261 (2022). https://doi.org/10.1016/j.biteb.2022.101261
A.R. Suvarna, A. Shetty, S. Anchan, N. Kabeer, S. Nayak, Cyclea peltata leaf mediated green synthesized bimetallic nanoparticles exhibits methyl green dye degradation capability. BioNanoScience 10(3), 606–617 (2020). https://doi.org/10.1007/s12668-020-00739-9
S. Nayak, S.P. Sajankila, C.V. Rao, A.R. Hegde, S. Mutalik, Biogenic synthesis of silver nanoparticles using Jatropha curcas seed cake extract and characterization: evaluation of its antibacterial activity. Energy Sour. Part A: Recovery, Util. Environ. Eff. 43(24), 3415–3423 (2021). https://doi.org/10.1080/15567036.2019.1632394
S. Zhang, L. Lin, X. Huang, Y.-G. Lu, D.-L. Zheng, Y. Feng, Antimicrobial properties of metal nanoparticles and their oxide materials and their applications in oral biology. J. Nanomater. 2022, e2063265 (2022). https://doi.org/10.1155/2022/2063265
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through the Small Group Research Project under grant number (RGP1/71/44).
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
AR planned the overall content of the paper. DSI perform all the experimentation work. AR and DSI wrote the manuscript. AS, SA, KC and NFQ review the manuscript and provide suggestions to improve it. All the authors approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Idris, D.S., Roy, A., Subramanian, A. et al. Bio-fabrication of Silver–Zinc Bimetallic Nanoparticles and Its Antibacterial and Dye Degradation Activity. J Inorg Organomet Polym 34, 1908–1919 (2024). https://doi.org/10.1007/s10904-023-02936-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-023-02936-x