Abstract
A new Fe3O4/Mo-MOF (metal–organic framework) magnetic nano polymer was synthesized in one step and Ultrasound assisted. Fe3O4/Mo-MOF magnetic nano polymer was characterized by XRD, SEM, EDX mapping BET, VSM, and FT-IR. The results of the structure confirmation revealed that the ultrasonic method had created unique properties in the Fe3O4/Mo-MOF magnetic nano polymer, and its biological and catalytic applications were investigated. A new pyrano[2,3-d]pyrimidines derivative was synthesized using the catalytic activity of Fe3O4/Mo-MOF magnetic nano polymer in a three-component reaction involving malononitrile, aldehyde derivatives, and barbituric acid derivatives with Fe3O4/Mo-MOF magnetic nano polymer as a magnetic catalyst. In the antimicrobial activity of Fe3O4/Mo-MOF magnetic nano polymer: Antimicrobial properties on gram-positive, gram-negative, and fungal strains based on MIC, MBC, and MFC value. The results of catalytic and biological studies proved that nanoparticles have unique properties attributed to their structure and synthesis method.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
An essential group of compounds in organic chemistry are heterocyclic. More than half of organic chemists' research is in heterocyclic chemistry. Heterocyclic structure is observed in many chemical, pharmaceutical, veterinary and agricultural products [1,2,3,4,5].
Research shows that the structure of many natural drugs, such as codeine, morphine, quinine, atropine, papaverine, reserpine, emetine, theophylline, and procaine, are heterocyclic compounds. All synthetic chemical compounds used as drugs, like metronidazole, diazepam, azidothymidine, captopril, antipyrine, barbiturates, isoniazid, methotrexate, and chlorpromazine, belong to the heterocyclic branch. A heterocyclic structure is also present in many herbicides, pesticides, luminophores, and dyes [6, 7].
Meanwhile, a group of heterocyclic compounds proven in medicinal chemistry is called pyrimidine. Pyrimidine is a six-membered aromatic ring molecule with two nitrogen heteroatoms. This heterocyclic compound is similar to pyridine and benzene and has common biological properties with pyridine [8,9,10].
There are methods to obtain pyrimidine. Among these methods is the organic synthesis of these compounds in the laboratory. One of the classical methods in synthesizing this valuable heterocyclic compound was Pietro Biginelli’s 1891 Biginelli reaction, which produced a pyrimidine (3,4 dihydropyrimidine-2(1H)-ones) derivative. This product is used in the pharmaceutical industry. It has a wide range of applications. It can be said that heterocyclic compounds containing a nitrogen hetero atom, phenyl ring, and carbonyl functional group in their structure undoubtedly show high biological activity [11].
One of the essential polycyclic heterocyclic compounds is called pyranopyrimidine. The pyranopyrimidine is produced from the connection of the pyran ring to a pyrimidine. These compounds have extraordinary biological properties, including antitumor activity [12], antioxidant activity [13], anti-HIV activity [14], anticancer activity [15] and antimicrobial activities [16].
Several methods and catalysts have been reported for synthesizing pyranopyrimidine derivatives. One of the most essential reported catalysts for synthesizing these compounds is nanocatalysts [17,18,19,20]. Recently, many applications of nanoparticles have been reported due to their fantastic structure [21,22,23,24,25].
Metal–organic frameworks are a new class of porous nanomaterials with one, two, or three-dimensional structures that include metal ions or clusters coordinated with organic ligands. Investigations show that an organic–metallic framework is a coordination network with organic ligands containing potential pores [26, 27]. Sometimes these pores are stable during the reaction and the elimination of guest molecules and can be filled again with other compounds. This property of MOF has made it widely used in storing and separating gases and as solid materials in supercapacitors for storing gases such as hydrogen and carbon dioxide. Metal–Organic Frameworks Applications from Catalysis to Gas Storage [28].
For efficient MOFs in the gas storage process, a helpful patterning method uses metal-binding solvents such as water and N,N-diethylformamide. The mechanism of action is that when the solvent is discharged, the metal sites are exposed and allowed. They allow hydrogen to connect in these places [29].
Other applications of MOF are electrocatalysis [30], imaging and biological measurement [31], nuclear waste materials [32], drug delivery systems [33], semiconductors [34] and biomimetic mineralization [35].
Core–shell nanocomplexes, which include mineral cores surrounded by metal–organic frameworks, are considered emerging multifunctional materials. Investigations show that the new NP@MOF core–shell nanostructures, which are a controlled combination of metal–organic frameworks MOFs and inorganic nanoparticles NPs, have a vast range of efficiency in different fields, including in medicine, environment, devices, and energy [36, 37].
In addition to the mentioned cases, nanoparticles as catalysts help in the synthesis of organic and heterocyclic compounds using multi-component reactions. These compounds have recently attracted the attention of many scientists due to their greenness and reusability. There have been several reports of nanoparticles in the synthesis of nitrogen-containing heterocyclic compounds such as quinolone [38,39,40], pyrazolopyranopyrimidines derivatives [41, 42] and chromene derivatives organic derivatives [43,44,45], etc.
Considering the importance of the synthesis of heterocyclic compounds such as pyranopyrimidine derivatives and providing new catalysts in their synthesis, in this research, a new Fe3O4/Mo-MOF magnetic nano polymer was synthesized and reported as a unique catalyst with high efficiency and recyclability in the synthesis of pyranopyrimidine derivatives. Antimicrobial activities such as the antibacterial and antifungal activity of Fe3O4/Mo-MOF magnetic nano polymer were evaluated and reported as bioactive agents. The synthesis of Fe3O4/Mo-MOF magnetic nano polymer nanoparticles with magnetic properties, antimicrobial activity, recyclable catalytic properties, easy separation after the reaction, and new pyranopyrimidine derivatives are critical innovations of this research.
2 Experimental
2.1 General
All materials, such as raw materials and solvents with high purity, were prepared from Merck. Fe3O4 nanostructures (purity of 99.99) were prepared from Sigma- Aldrich. Philips XPERT PRO Cu-Ka radiation was used for the XRD pattern. Scanning electron microscopy of the product was analyzed using FEI Nova NanoSEM230. We examined the adsorption/desorption isotherms of N2 using Micromeritics. VSM curves by using MDKFD were measured. Thermo Scientific Nicolet-6700 for the FT-IR spectrum was used. 1H and 13C-NMR spectra were recorded by Bruker FT-NMR Ultrashield-400 spectrometer. KSP1N Krus’s melting point meter was used for the melting points of derivatives.
2.2 Synthesis of Fe3O4/Mo-MOF Magnetic Nano Polymer
In ultrasonic-assisted for one-pot synthesis of Fe3O4/Mo-MOF magnetic nano polymer first, in 30 mL acetic acid/double-distilled water (1:1), added 1 mmol (0.2315 g) Fe3O4 nanoparticle, 2 mmol (0.135 g) MoCl5 and 4 mmol (0.6685 g) pyridine-2,6 dicarboxylic acid and stirred at 80 °C for 15 min. Afterward, a mixture was placed in an ultrasonic reactor at a power of 370 W at room temperature for 20 min. Using an external magnet, the synthesized Fe3O4/Mo-MOF magnetic nano polymer was separated, washed with distilled water and acetic acid, and dried at ambient temperature.
2.3 General Method of Synthesis of Pyrano[2,3-d]pyrimidines Derivatives Using Fe3O4/Mo-MOF Magnetic Nano Polymer
For the synthesis of pyrano[2,3-d]pyrimidines derivatives under optimal conditions; aldehyde derivatives (1 mmol), 0.066 mg malononitrile (1 mmol), 2 mg Fe3O4/Mo-MOF magnetic nano polymer, and 0.1561 gr 1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione (1 mmol) in 5 ml H2O/EtOH (1:1) at 40 °C were stirred. The performance of the reaction was checked by TLC (thin-layer chromatography). When the reaction was complete, by using an external magnet, the Fe3O4/Mo-MOF magnetic nano polymer was separated. The sediments were recrystallized in a mixture of water and ethanol for purification.
2.3.1 7-Amino-5-(2,4-dimethoxyphenyl)-1,3-dimethyl-2,4-dioxo-1,3,4,5-tetrahydro-2h-pyrano[2,3-D]pyrimidine-6-carbonitrile
1H NMR (400 MHz, DMSO-d6) δ = 3.14 (s, 3H, CH3), 3.29 (s, 3H, CH3), 3.57 (s, 3H, OCH3), 3.71 (s, 3H, OCH3), 4.66 (s, 1H, CH), 6.59 (d, J = 8.5 Hz, 1H, ArH), 6.67 (d, J = 8.5, 1H, ArH), 6.81 (s, 1H, ArH), 7.24 (s, 2H, NH2);
13C NMR (100 MHz, DMSO-d6): δ 26.95, 28.13, 30.17, 55.46, 56.09, 56.87, 88.37, 111.24, 112.01, 116.14, 118.74, 131.37, 150.42, 150.67, 151.84, 153.29, 157.66, 161.03.
El Anal. Calcd for C18H18N4O5: C, 58.37; H, 4.90; N, 15.13; O, 21.60. Found: C, 58.39; H, 4.91; N, 15.12; O, 21.58.
2.3.2 7-Amino-5-(2,6-dimethoxyphenyl)-1,3-dimethyl-2,4-dioxo-1,3,4,5-tetrahydro-2h-pyrano[2,3-D]pyrimidine-6-carbonitrile
1H NMR (400 MHz, DMSO-d6) δ = 3.10 (s, 3H, CH3), 3.19 (s, 3H, CH3), 3.59 (s, 3H, OCH3), 3.66 (s, 3H, OCH3), 4.75 (s, 1H, CH), 7.52 (t, J = 8.4 Hz, 1H, ArH), 7.71 (d, J = 8.4, 21H, ArH), 7.24 (s, 2H, NH2);
13C NMR (100 MHz, DMSO-d6): δ 25.94, 27.03, 31.47, 55.92, 56.51, 57.09, 88.73, 111.51, 112.88, 115.99, 119.27, 132.26, 151.07, 151.64, 152.00, 153.47, 159.21, 160.78.
El Anal. Calcd for C18H18N4O5: C, 58.37; H, 4.90; N, 15.13; O, 21.60. Found: C, 58.38; H, 4.88; N, 15.15; O, 21.59.
2.4 Hot Filtration Test
A hot filtration test was done based on previous reports, and no enhancement in conversion was noticed in the filtrate [46].
2.5 Antimicrobial Properties of Fe3O4/Mo-MOF Magnetic Nano Polymer
Antimicrobial properties of Fe3O4/Mo-MOF magnetic nano polymer against gram-positive, gram-negative, and fungal strains. In antimicrobial properties, Minimum Inhibitory Concentration (MIC), Minimum Bactericidal Concentration (MBC), and Minimum Fungicidal Concentration (MFC) were studied. Guidelines M07-A9, M26-A, M02-A11, M44-A, and M27-A2 [47,48,49] for antimicrobial research were used.
3 Results and Discussion
3.1 Results of Synthesis Fe3O4/Mo-MOF Magnetic Nano Polymer
Fe3O4/Mo-MOF magnetic nano polymer nanoparticles were synthesized by the ultrasonic-assisted and one-pot reaction. Techniques such as XRD pattern, SEM image, EDX mapping, BET curve, VSM curve, and FT-IR spectrum were used to identify and confirm the structure of the Fe3O4/Mo-MOF magnetic nano polymer.
The XRD pattern of the Fe3O4/Mo-MOF magnetic nano polymer is shown in Fig. 1, and based on that, the peaks related to the Fe3O4 and Mo nanoparticles [50, 51] can be seen in it. The crystalline and stable structure observed in nanoparticles can result from the ultrasonic-assisted synthesis method.
Using the Debby Scherer equation and XRD pattern, the size of nanoparticles can be calculated [52, 53], and here the size of Fe3O4/Mo-MOF magnetic nano polymer nanoparticles were 86 nm.
Based on the SEM image of the Fe3O4/Mo-MOF magnetic nano polymer (Fig. 2), the synthesized Fe3O4/Mo-MOF magnetic nano polymer have the same morphology and nano size, which can be attributed to its synthesis method using ultrasonic-assisted. The morphology of the catalyst sample can greatly affect their performance of the final products. In fact, in case of synthesizing samples with uniform morphology, it is expected that their effective surface will increase. Therefore, the morpholigical features can significantly affect the catalytic applications of the sample. Carbon, oxygen, and nitrogen elements were mixed in EDX mapping of the Fe3O4/Mo-MOF magnetic nano polymer, as shown in Fig. 3.
Specific surface area is one of the essential parameters in nanoparticles, and one of the factors that cause unique properties in nanoparticles and their diverse applications is the high specific surface area. One of the practical factors in the amount of Specific surface area is the synthesis method of nanoparticles. The high specific surface area in the catalytic applications of nanoparticles increases the surface area of all reactants. As a result, the reaction time decreases, and the efficiency increases. In biological applications, the high specific surface area increases the contact of nanoparticles with pathogenic agents and, as a result, the high effectiveness of nanoparticles [54, 55]. The N2 adsorption/desorption isotherms related to the Fe3O4/Mo-MOF magnetic nano polymer are shown in Fig. 4. BET characterization of Fe3O4/Mo-MOF magnetic nano polymer BET characterization showed a value of 1500 m2/g, which was relatively high. The high specific surface area of the Fe3O4/Mo-MOF magnetic nano polymer result from its centering by the ultrasonic-assisted method.
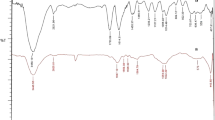
The FT-IR spectrum of nanoparticles (Fig. 5) showed the peaks related to the O–H groups of water in the region of 3300–3400 cm−1. Peaks related to C-H groups were observed in the 2800–3000 cm−1. Carbonyl groups showed absorption in the region of 1630 cm−1. In the region of 1500 cm−1, absorption is related to C=N. The observed peaks near 1000 cm−1 are related to Mo–O [51, 56]. The observed peak in the region of 500–600 cm−1 was related to Fe–O [57, 58].
FT-IR spectrum data proved that all the peaks related to raw materials were present in the final product.
Magnetic saturation for Fe3O4 MNPs, near 0.060 emu/g, was obtained. For Fe3O4/Mo-MOF magnetic nano polymer, magnetic saturation near 0.030 emu/g was obtained (Fig. 6).
The extreme difference in magnetic saturation of Fe3O4 nanoparticles and Fe3O4/Mo-MOF magnetic nano polymer proves that groups covered Fe3O4 nanoparticles. Therefore, it can be concluded that Fe3O4 nanoparticles were placed as cores around Mo-MOF as shells.
Based on the spectral data of the XRD pattern (observation of Fe3O4 and molybdenum nanoparticles patterns), EDX mapping images (Observing the elements of raw materials in the final product), FT-IR spectrum (observation of desired functional groups and bonds), VSM curves (reduction of magnetism), a possible structure for Fe3O4/Mo-MOF magnetic nano polymer were proposed in Fig. 7.
3.2 Results of Synthesis of Pyrano[2,3-D]pyrimidines Derivatives by Fe3o4/Mo-Mof Magnetic Nano Polymer
We synthesized the novel pyrano[2,3-d]pyrimidines derivatives by the three-component reaction of aldehyde derivatives, malononitrile, 1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione and using of Fe3O4/Mo-MOF magnetic nano polymer as catalyst (according to the Scheme 1).
For the synthesis of pyrano[2,3-d]pyrimidines derivatives, at first, the reaction conditions, such as solvent, temperature, and amount of catalyst, were optimized. Reaction optimization for 4f was done according to Table 1.
The optimal conditions were as described in H2O: EtOH (1:1) as a solvent, the temperature of 40 o C, and 2 mg Fe3O4/Mo-MOF magnetic nano polymer as the catalyst and other derivatives in Table 2 were synthesized under optimal conditions (two derivatives were novel).
The Inductively coupled plasma mass results showed that 0.002 g of Fe3O4/Mo-MOF magnetic nano polymer contains 3.6 × 10–4 g of molybdenum (3.8 × 10–6 mol), so the TON and TOF values for the products were calculated and listed in the table.
For the synthesis of pyrano[2,3-d]pyrimidines derivatives using Fe3O4/Mo-MOF magnetic nano polymer, the mechanism of Scheme 2 was suggested.
Intermediate (I) was formed by condensation of aldehyde with malononitrile. In next step, from Michael reaction of (I) and pyrimidine, intermediate (II) was formed which is in equilibrium with intermediate (III). Intermediate (III) performs cyclization reaction and creates intermediate (IV). Finally, by the tautomerization of (IV), the desired product was synthesized. In this mechanism, Fe3O4/Mo-MOF magnetic nano polymer play the role of Lewis acid and has significant role in the synthesis of intermediates (I), (II) and (III).
The catalyst used in this research, due to its magnetic property, after the reaction was completed, quickly separated using an external magnet, washed with water and ethanol, and reused up to 6 times after drying. The results for 4f are shown in Fig. 8.
The results of product efficiency after reuse prove that the catalyst can be used up to 6 times without a significant change in efficiency.
Also, previous reports used different conditions and catalysts to synthesize pyrano[2,3-d]pyrimidines derivatives. These catalysts include urea-based ionic liquid stabilized on silica-coated Fe3O4 magnetic nanoparticles [64], liquid glass/milled in a stainless steel grinding vial [65], polyethylene glycol 400 [60], heptakis (6-amino-6-deoxy)-β-cyclodextrin [59], ChCl/PE DES [66], L-proline nitrate [62], zinc ferrite [67], Orthoboric acid [68], nano-basic silica [69] and Mn-ZIF-8@ZnTiO3 [70] Table 3 compares some of the conditions that have been reported recently compared to this study.
In addition to the synthesis of new derivatives, we can mention the high efficiency and less time method as an advantage of this research compared to recently reported methods.
3.3 Results of Antimicrobial Properties of Fe3O4/Mo-MOF Magnetic Nano Polymer
Table 4 shows the results of the antimicrobial properties of Fe3O4/Mo-MOF magnetic nano polymer. We tested the antimicrobial study, antibacterial properties, and fungal properties against Staphylococcus epidermidis and Bacillus cereus (as Gram-positive bacteria); Shigella dysenteriae and Klebsiella pneumonia (as Gram-negative bacteria); Candida albicans; F: Fusarium oxysporum (as fungi strain).
Minimum Inhibitory Concentration (MIC), Minimum Bactericidal Concentration (MBC), and Minimum Fungicidal Concentration (MFC) value study of Fe3O4/Mo-MOF magnetic nano polymer (Table 4) showed that it had high antimicrobial properties against all Gram-positive bacteria, Gram-negative bacteria and Fungi strain that were study on it. The observed values for MIC of Fe3O4/Mo-MOF magnetic nano polymer nanoparticles were 16–256 μg/mL. The remarkable thing about the antibacterial and antifungal activity of Fe3O4/Mo-MOF magnetic nano polymer nanoparticles was that they were more effective than Cefazolin and Terbinafine, known as common antimicrobial drugs. One of the influential factors in the significant antimicrobial properties of Fe3O4/Mo-MOF magnetic nano polymer nanoparticles can be their high specific surface [54, 55].
4 Conclusion
A new Fe3O4/Mo-MOF magnetic nano polymer was synthesized using ultrasonic-assisted and one-pot reaction methods. The synthesized Fe3O4/Mo-MOF magnetic nano polymer using XRD, SEM, EDX mapping BET, VSM, and FT-IR were identified, and their structure was confirmed. We observed the same morphology and high specific surface area from the synthesized Fe3O4/Mo-MOF magnetic nano polymer. The same morphology and its high specific surface can be attributed to the synthesis method, which was ultrasonic assisted, and it can be concluded that the use of this method gives unique properties to nanoparticles. Fe3O4/Mo-MOF magnetic nano polymer was used as a catalyst in the synthesis of pyrano[2,3-d]pyrimidine derivatives. Some results of using Fe3O4/Mo-MOF magnetic nano polymer as the catalyst compared to previously reported methods are the synthesis of new pyrano[2,3-d]pyrimidine derivatives, easy separation after reaction using the magnet, reusability, and less time for synthesis of derivatives with higher efficiency. The Fe3O4/Mo-MOF magnetic nano polymer nanoparticles also showed antimicrobial properties against the studied bacteria and fungi, which were more effective than some commercial drugs.
References
G. Green, J. Evans, A. Vong, A. Katritzky, C. Rees, E. Scriven, Comprehensive heterocyclic chemistry II (Pergamon Press, Oxford, 1995), p.469
S.Ç. Yavuz, S. Akkoç, B. Tüzün, O. Şahin, E. Saripinar, Efficient synthesis and molecular docking studies of new pyrimidine-chromeno hybrid derivatives as potential antiproliferative agents. Synth. Commun. 51, 2135–2159 (2021)
A.R. Morsy, S.K. Ramadan, M.M. Elsafty, Synthesis and antiviral activity of some pyrrolonyl substituted heterocycles as additives to enhance inactivated Newcastle disease vaccine. Med. Chem. Res. 29, 979–988 (2020)
H. Dong, Y. Xian, H. Li, Y. Wu, W. Bai, X. Zeng, Analysis of heterocyclic aromatic amine profiles in Chinese traditional bacon and sausage based on ultrahigh-performance liquid chromatography–quadrupole-Orbitrap high-resolution mass spectrometry (UHPLC–Q-Orbitrap-HRMS). Food Chem. 310, 125937 (2020)
M.M. Heravi, V. Zadsirjan, Prescribed drugs containing nitrogen heterocycles: an overview. RSC Adv. 10, 44247–44311 (2020)
K. Goutham, N.R. Mangina, S. Suresh, P. Raghavaiah, G.V. Karunakar, Gold-catalysed cyclisation of N-propargylic β-enaminones to form 3-methylene-1-pyrroline derivatives. Org. Biomol. Chem. 12, 2869–2873 (2014)
A. Urbach, G.G. Muccioli, E. Stern, D.M. Lambert, J. Marchand-Brynaert, 3-Alkenyl-2-azetidinones as fatty acid amide hydrolase inhibitors. Bioorg. Med. Chem. Lett. 18, 4163–4167 (2008)
L.C. Ferreira Pimentel, A.C. Cunha, L.V. Boas Hoelz, H.F. Canzian, D.I. Leite Firmino Marinho, N. Boechat, M.M. Bastos, Phenylamino-pyrimidine (PAP) privileged structure: synthesis and medicinal applications. Curr. Top. Med. Chem. 20, 227–243 (2020)
S.G. El Gabaly, G.M. Youssif, A.M. Bayoumy, H. Ezzat, H. Elhaes, A. Refaat, M. Ibrahim, Modeling the effect of functional groups on the electronic properties of benzene. Pyridine Pyrimidine Egypt. J. Chem. 62, 1–11 (2019)
M. Albratty, H.A. Alhazmi, Novel pyridine and pyrimidine derivatives as promising anticancer agents: A review. Arab. J. Chem. (2022). https://doi.org/10.1016/j.arabjc.2022.103846
M. Bajda, S. Boryczka, J. Wietrzyk, B. Malawska, Investigation of lipophilicity of anticancer-active thioquinoline derivatives. Biomed. Chromatogr. 21, 123–131 (2007)
R.A. Haggam, M.G. Assy, E.K. Mohamed, A.S. Mohamed, Synthesis of Pyrano [2, 3-d] pyrimidine-2, 4-diones and Pyridino [2, 3-d] pyrimidine-2, 4, 6, 8-tetraones: Evaluation antitumor activity. J. Heterocycl. Chem. 57, 842–850 (2020)
A. Yousefi, R. Yousefi, F. Panahi, S. Sarikhani, A.R. Zolghadr, A. Bahaoddini, A. Khalafi-Nezhad, Novel curcumin-based pyrano [2, 3-d] pyrimidine anti-oxidant inhibitors for α-amylase and α-glucosidase: Implications for their pleiotropic effects against diabetes complications. Int. J. Biol. Macromol. 78, 46–55 (2015)
S. Maddila, K. Nagaraju, S.B. Jonnalagadda, Synthesis and antimicrobial evaluation of novel pyrano [2, 3-d]-pyrimidine bearing 1, 2, 3-triazoles. Chem. Data Collect. 28, 100486 (2020)
M.R. Bhosle, P. Andil, D. Wahul, G.M. Bondle, A. Sarkate, S.V. Tiwari, Straightforward multicomponent synthesis of pyrano [2, 3-d] pyrimidine-2, 4, 7-triones in β-cyclodextrin cavity and evaluation of their anticancer activity. J. Iran. Chem. Soc. 16, 1553–1561 (2019)
A.H. Bedair, H.A. Emam, N.A. El-Hady, K.A. Ahmed, A.M. El-Agrody, Synthesis and antimicrobial activities of novel naphtho [2, 1-b] pyran, pyrano [2, 3-d] pyrimidine and pyrano [3, 2-e][1, 2, 4] triazolo [2, 3-c]-pyrimidine derivatives. Il Farmaco 56, 965–973 (2001)
P. Nagaraju, P.N. Reddy, P. Padmaja, V.G. Ugale, Microwave-assisted synthesis of thiazole/benzothiazole fused pyranopyrimidine derivatives and evaluation of their biological activity. Lett. Org. Chem. 18, 49–57 (2021)
A.Y. El-Khateeb, S.E. Hamed, K.M. Elattar, Recent advancements in the multicomponent synthesis of heterocycles integrated with a pyrano [2, 3-d] pyrimidine core. RSC Adv. 12, 11808–11842 (2022)
R.R. Tehrani, E. Sheikhhosseini, D. Ghazanfari, M. Akhgar, Synthesis and Biological examination of novel tetra pyranopyrimidine heterocycles contained lipophilic spacers. Polycycl. Aromat. Compd. (2022). https://doi.org/10.1080/10406638.2022.2059530
H. Amini, S. Neamani, L. Moradi, Green synthesis of pyrazolo pyrano pyrimidine derivatives using ZnFe2O4/GA as a new effective catalyst in water media. Chem. Select 6, 9608–9615 (2021)
S. Naghdi, B. Jaleh, M. Eslamipanah, A. Moradi, M. Abdollahi, N. Einali, K.Y. Rhee, Graphene family, and their hybrid structures for electromagnetic interference shielding applications: Recent trends and prospects. J. Alloy. Compd. (2022). https://doi.org/10.1016/j.jallcom.2021.163176
N. Baig, I. Kammakakam, W. Falath, Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges, materials. Advances 2, 1821–1871 (2021)
L. Wang, M. Zhang, B. Yang, J. Tan, X. Ding, W. Li, Recent advances in multidimensional (1D, 2D, and 3D) composite sensors derived from MXene: Synthesis, structure, application, and perspective. Small Methods 5, 2100409 (2021)
W. Liu, F. Huang, Y. Liao, J. Zhang, G. Ren, Z. Zhuang, J. Zhen, Z. Lin, C. Wang, Treatment of CrVI-Containing Mg (OH) 2 Nanowaste. Angew. Chem. 120, 5701–5704 (2008)
W.-F. Lai, Non-conjugated polymers with intrinsic luminescence for drug delivery. J. Drug Deliv. Sci. Technol. 59, 101916 (2020)
S.R. Batten, N.R. Champness, X.-M. Chen, J. Garcia-Martinez, S. Kitagawa, L. Öhrström, M. O’Keeffe, M.P. Suh, J. Reedijk, Terminology of metal–organic frameworks and coordination polymers (IUPAC Recommendations 2013). Pure Appl. Chem. 85, 1715–1724 (2013)
Y. Yang, S.Q. Wang, H. Wen, T. Ye, J. Chen, C.P. Li, M. Du, Nanoporous gold embedded ZIF composite for enhanced electrochemical nitrogen fixation. Angew. Chem. Int. Ed. 58, 15362–15366 (2019)
D. Farrusseng, Metal-organic frameworks: Applications from catalysis to gas storage (Wiley, NY, 2011)
N.L. Rosi, J. Eckert, M. Eddaoudi, D.T. Vodak, J. Kim, M. O’Keeffe, O.M. Yaghi, Hydrogen storage in microporous metal-organic frameworks. Science 300, 1127–1129 (2003)
W. Cheng, X. Zhao, H. Su, F. Tang, W. Che, H. Zhang, Q. Liu, Lattice-strained metal–organic-framework arrays for bifunctional oxygen electrocatalysis, Nature. Energy 4, 115–122 (2019)
M.L. Cable, D.J. Levine, J.P. Kirby, H.B. Gray, A. Ponce, Luminescent lanthanide sensors Advances in Inorganic Chemistry (Elsevier, Amsterdam, 2011), pp.1–45
Y. Li, Z. Weng, Y. Wang, L. Chen, D. Sheng, J. Diwu, Z. Chai, T.E. Albrecht-Schmitt, S. Wang, Surprising coordination for low-valent actinides resembling uranyl (VI) in thorium (IV) organic hybrid layered and framework structures based on a graphene-like (6, 3) sheet topology. Dalton Trans. 45, 918–921 (2016)
M.X. Wu, Y.W. Yang, Metal–organic framework (MOF)-based drug/cargo delivery and cancer therapy. Adv. Mater. 29, 1606134 (2017)
A.A. Talin, A. Centrone, A.C. Ford, M.E. Foster, V. Stavila, P. Haney, R.A. Kinney, V. Szalai, F. El Gabaly, H.P. Yoon, Tunable electrical conductivity in metal-organic framework thin-film devices. Science 343, 66–69 (2014)
K. Liang, R. Ricco, C.M. Doherty, M.J. Styles, S. Bell, N. Kirby, S. Mudie, D. Haylock, A.J. Hill, C.J. Doonan, Biomimetic mineralization of metal-organic frameworks as protective coatings for biomacromolecules. Nat. Commun. 6, 1–8 (2015)
H. Zou, Z. Luo, X. Yang, Q. Xie, Y. Zhou, Toward emerging applications using core–shell nanostructured materials: A review. J. Mater. Sci. (2022). https://doi.org/10.1007/s10853-022-07328-z
Y. Zhao, Co-precipitated Ni/Mn shell coated nano Cu-rich core structure: A phase-field study. J. Market. Res. 21, 546–560 (2022)
A. Maleki, J. Rahimi, Synthesis of dihydroquinazolinone and octahydroquinazolinone and benzimidazoloquinazolinone derivatives catalyzed by an efficient magnetically recoverable GO-based nanocomposite. J. Porous Mater. 25, 1789–1796 (2018)
A. Maleki, T. Kari, M. Aghaei, Fe3O4@ SiO2@ TiO2-OSO3H: an efficient hierarchical nanocatalyst for the organic quinazolines syntheses. J. Porous Mater. 24, 1481–1496 (2017)
A. Maleki, M. Aghaei, T. Kari, Facile Synthesis of 7-Aryl-benzo [h] tetrazolo [5, 1-b] quinazoline-5, 6-dione fused polycyclic compounds by using a novel magnetic polyurethane catalyst. Polycyc. Aromat. Compd. 39(3), 266–278 (2017)
A. Maleki, V. Eskandarpour, Design and development of a new functionalized cellulose-based magnetic nanocomposite: Preparation, characterization, and catalytic application in the synthesis of diverse pyrano [2, 3-c] pyrazole derivatives. J. Iran. Chem. Soc. 16, 1459–1472 (2019)
A. Maleki, A.A. Jafari, S. Yousefi, Green cellulose-based nanocomposite catalyst: design and facile performance in aqueous synthesis of pyranopyrimidines and pyrazolopyranopyrimidines. Carbohyd. Polym. 175, 409–416 (2017)
A. Maleki, Z. Hajizadeh, K. Valadi, Green and eco-friendly mica/Fe3O4 as an efficient nanocatalyst for the multicomponent synthesis of 2-amino-4 H-chromene derivatives. Green Chem. Lett. Rev. 14, 62–72 (2021)
A. Maleki, H. Movahed, P. Ravaghi, Magnetic cellulose/Ag as a novel eco-friendly nanobiocomposite to catalyze synthesis of chromene-linked nicotinonitriles. Carbohyd. Polym. 156, 259–267 (2017)
A. Maleki, S. Azadegan, Amine-functionalized silica-supported magnetic nanoparticles: preparation, characterization and catalytic performance in the chromene synthesis. J. Inorg. Organomet. Polym Mater. 27, 714–719 (2017)
E. Babaei, B.B.F. Mirjalili, Fe3O4@ nano-dextrin/Ti (IV) as a bio-based magnetic nano-catalyst for facile synthesis of 2, 3-dihydroquinazolin-4 (1H)-ones, Iranian. J. Catal. 10, 219–226 (2020)
M. Moghaddam-Manesh, D. Ghazanfari, E. Sheikhhosseini, M. Akhgar, Synthesis of bioactive magnetic nanoparticles spiro [indoline-3, 4′-[1, 3] dithiine]@ Ni (NO3) 2 supported on Fe3O4@ SiO2@ CPS as reusable nanocatalyst for the synthesis of functionalized 3, 4-dihydro-2H-pyran. Appl. Organomet. Chem. 34, e5543 (2020)
M. Moghaddam-Manesh, S. Hosseinzadegan, Introducing new method for the synthesis of polycyclic compounds containing [1, 3] dithiine derivatives, with anticancer and antibacterial activities against common bacterial strains between aquatic and human. J. Heterocycl. Chem. 58, 2174–2180 (2021)
S. Hosseinzadegan, N. Hazeri, M.T. Maghsoodlou, Synthesis of novel thiazolo [3, 2-a] chromeno [4, 3-d] pyrimidine-6 (7H)-ones by bioactive Fe3O4@ gly@ thiophen@ Cu (NO3) 2 as reusable magnetic nanocatalyst. Appl. Organomet. Chem. 34, e5797 (2020)
B. Goswami, D. Mahanta, Fe3O4-Polyaniline Nanocomposite for non-enzymatic electrochemical detection of 2, 4-dichlorophenoxyacetic acid. ACS Omega 6, 17239–17246 (2021)
A.M. Kermani, A. Ahmadpour, T.R. Bastami, M. Ghahramaninezhad, Deep oxidative desulfurization of dibenzothiophene with Mo 132 nanoballs supported on activated carbon as an efficient catalyst at room temperature. New J. Chem. 42, 12188–12197 (2018)
R.O. Saleh, H. Achmad, B.T. Daminov, H.H. Kzar, A.B. Mahdi, A.T. Hammid, M.K. Abid, M.J.C. Opulencia, Y.F. Mustafa, H. Sharma, Synthesis of bioactive yttrium-metal–organic framework as efficient nanocatalyst in synthesis of novel pyrazolopyranopyrimidine derivatives and evaluation of anticancer activity. Front. Chem. (2022). https://doi.org/10.3389/fchem.2022.928047
W. Suksatan, P. Kazemzadeh, D. Afzali, M. Moghaddam-manesh, N.P.S. Chauhan, G. Sargazi, A controllable study on ultrasound assisted synthesis of a novel Ni/Zn based hybrid MOF nanostructures for dextranase immobilization. Inorg. Chem. Commun. 139, 109410 (2022)
H. Mirhosseini, T. Shamspur, A. Mostafavi, G. Sargazi, A novel ultrasonic reverse micelle-assisted electrospun efficient route for Eu-MOF and Eu-MOF/CA composite nanofibers: a high performance photocatalytic treatment for removal of BG pollutant. Environ. Sci. Pollut. Res. 28, 4317–4328 (2021)
O. Shekhah, J. Liu, R. Fischer, C. Wöll, MOF thin films: existing and future applications. Chem. Soc. Rev. 40, 1081–1106 (2011)
A.M. Kermani, V. Mahmoodi, M. Ghahramaninezhad, A. Ahmadpour, Highly efficient and green catalyst of Mo132 nanoballs supported on ionic liquid-functionalized magnetic silica nanoparticles for oxidative desulfurization of dibenzothiophene. Sep. Purif. Technol. 258, 117960 (2021)
S. Hosseinzadegan, N. Hazeri, M.T. Maghsoodlou, M. Moghaddam-Manesh, M. Shirzaei, Synthesis and evaluation of biological activity of novel chromeno [4, 3-b] quinolin-6-one derivatives by SO 3 H-tryptamine supported on Fe 3 O 4@ SiO 2@ CPS as recyclable and bioactive magnetic nanocatalyst. J. Iran. Chem. Soc. 17, 3271–3284 (2020)
L. Shiri, H. Narimani, M. Kazemi, Synthesis and characterization of sulfamic acid supported on Fe3O4 nanoparticles: a green, versatile and magnetically separable acidic catalyst for oxidation reactions and Knoevenagel condensation. Appl. Organomet. Chem. 32, e3927 (2018)
F. Mohamadpour, Solvent-free synthesis of pyrano [2, 3-d] pyrimidine scaffolds using per-6-NH2-β-CD as a reusable supramolecular host. Org. Prep. Proced. Int. 54, 277–283 (2022)
F. Mohamadpour, Catalyst-free synthesis of pyrano [2, 3-d] pyrimidine scaffolds via Knoevenagel-Michael cyclocondensation Using PEG-400 as a green promoting medium. Org. Prep. Proced. Int. 52, 503–509 (2020)
M. Bakherad, G. Bagherian, A. Rezaeifard, F. Mosayebi, B. Shokoohi, A. Keivanloo, Synthesis of pyrano [2, 3-d] pyrimidines and pyrido [2, 3-d] pyrimidines in the magnetized deionized water based on UV–visible study. J. Iran. Chem. Soc. 18, 839–852 (2021)
P.G. Patil, Y. Satkar, D.H. More, L-Proline based ionic liquid: A highly efficient and homogenous catalyst for synthesis of 5-benzylidene-1, 3-dimethylpyrimidine-2, 4, 6 (1H, 3H, 5H)-trione and pyrano [2, 3-d] pyrimidine diones under ultrasonic irradiation. Synth. Commun. 50, 3804–3819 (2020)
S.N. Maddila, S. Maddila, W.E. van Zyl, S.B. Jonnalagadda, Mn doped ZrO2 as a green, efficient and reusable heterogeneous catalyst for the multicomponent synthesis of pyrano [2, 3-d]-pyrimidine derivatives. RSC Adv. 5, 37360–37366 (2015)
M.A. Zolfigol, R. Ayazi-Nasrabadi, S. Baghery, The first urea-based ionic liquid-stabilized magnetic nanoparticles: an efficient catalyst for the synthesis of bis (indolyl) methanes and pyrano [2, 3-d] pyrimidinone derivatives. Appl. Organomet. Chem. 30, 273–281 (2016)
M. Abaszadeh, S.J. Roudbaraki, M. Ghashang, Effect of liquid glass composition on the catalytic preparation of pyrano [2, 3-d] pyrimidine derivatives. Org. Prep. Proced. Int. 51, 255–269 (2019)
S. Azimzadeh-Sadeghi, I. Yavari, Choline chloride/pentaerythritol: a deep eutectic solvent for the synthesis of pyran and chromene derivatives. J. Iran. Chem. Soc. 18, 1261–1267 (2021)
A. Khazaei, A. Ranjbaran, F. Abbasi, M. Khazaei, A.R. Moosavi-Zare, Synthesis, characterization and application of ZnFe2 O4 nanoparticles as a heterogeneous ditopic catalyst for the synthesis of pyrano [2, 3-d] pyrimidines. RSC Adv. 5, 13643–13647 (2015)
A. Khazaei, H.A.A. Nik, A.R. Moosavi-Zare, Water mediated domino knoevenagel-michael-cyclocondensation reaction of malononitrile, Various aldehydes and barbituric acid derivatives using boric acid aqueous solution system compared with nano-titania sulfuric acid. J. Chin. Chem. Soc. 62, 675–679 (2015)
N. Sheikhan-Shamsabadi, M. Ghashang, Nano-basic silica as an efficient catalyst for the multi-component preparation of pyrano [2, 3-d] pyrimidine derivatives, Main Group Metal. Chemistry 40, 19–25 (2017)
T. Farahmand, S. Hashemian, A. Sheibani, Efficient one-pot synthesis of pyrano [2, 3-d] pyrimidinone and pyrido [2, 3-d] pyrimidine derivatives by using of Mn-ZIF-8@ ZnTiO3 nanocatalyst. J. Mol. Struct. 1206, 127667 (2020)
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
The authors confirm contribution to the paper as follows: study conception and design: Yassine Riadi, Kadhum Al-Majdi, Rahman S. Zabibah; data collection: Tarik Hafdhi Abdtawfeeq, Salema K. Hadrawi; analysis and interpretation of results: Yassine Riadi, Ameen AL-Alwany; draft manuscript preparation: Zainab A. Farhan, Mohammed Abed Jawad, Marwah A. Shams. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no affiliation with any organization with a direct or indirect financial interest in the subject matter discussed in the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abdtawfeeq, T.H., Farhan, Z.A., Al-Majdi, K. et al. Ultrasound-Assisted and One-Pot Synthesis of New Fe3O4/Mo-MOF Magnetic Nano Polymer as a Strong Antimicrobial Agent and Efficient Nanocatalyst in the Multicomponent Synthesis of Novel Pyrano[2,3-d]pyrimidines Derivatives. J Inorg Organomet Polym 33, 472–483 (2023). https://doi.org/10.1007/s10904-022-02514-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-022-02514-7