Abstract
Zeolitic imidazole frameworks (ZIFs) are multifunctional and biocompatible material for biomedical applications. Herein we report for the first time Calotropis gigantea latex encapsulated ZIF-L nanoframeworks one-pot synthesis approach. The present study focuses on synthesis of latex encapsulated zeolitic imidazole frameworks (CG@ZIF-L) for elucidation of bio-film, mosquito and midge fly larvae. The physicochemical characteristic of as prepared CG@ZIF-L nanoframework was systematically investigated by microscopic and spectroscopic techniques. Encapsulation of Calotropis gigantea latex inside ZIF-L nanoframeworks shows notable toxicity against E. coli, S. epidermis. The larvicidal results revealed that CG@ZIF-L nanoframeworks beneficially kill the Aedes aegypti mosquito and midge fly (blood worm) larvae in a dose dependant manner. Over all the present study highlights the possibility of the multifunctional nature of CG@ZIF-L nanocomposite, which highly suitable material for biomedical applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Plants are first resource for human utilized from the environment to cure ailments. Plants provide many leading compounds for the development of the new medicines [1,2,3,4,5]. Calotropis gigantea is a perennial herb which has long history in Indian Ayurvedic treatments. A wide range of phytochemicals from whole plant including flavonoids, tannins, cardiac glycosides, terpenoids has been used for various disease control and prevention techniques such as elephantiasis, cancer, ulcer and leprosy [6,7,8,9]. The latex is most prominent product and it has special chemical compounds like as calotropin, uscharidin, lupeol and calotoxin [10]. The emergence of life threatening diseases and drug resistant pathogens diverted the attention of researchers to plant compounds for drug development [11,12,13,14]. In the present scenario, pathogenic bacterial strains develop into multidrug resistance bacterial strain adapting to climatic changes [15]. Biofilm formation is one of the primary and notorious mechanisms of bacterial adaptation in environmental condition. Formation of biofilm, provides more advantages to survival and adopt with environment [16, 17]. As bacterial infection and bio-film causing diseases are widely reported in animals and humans, it’s a great challenge to maintain public health and hygiene [18, 19]. On a global scale, a mosquito has greatest concern about transmission of contagious diseases such as chickungunya, malaria, dengue and yellow fever [20,21,22]. In this context, Bacillus thuringiensis (Bti) is the most abundant pesticide used for mosquito and some pest control programs. The continuous use of Bti causes food-web related effects in wetlands and nature conservation areas [23, 24]. On the other hand, some mosquitos such as Culex quinquefasciatus and Aedes aegypti have developed resistance to Bti pesticide [25, 26]. These emergences of mosquito control have gained much attention on development of effective pesticides to control dengue virus transmitting vector Aedes aegypti mosquito [27, 28]. Furthermore, over population of midge fly larvae has developed into major problem for fresh water ponds and agriculture lands causing toxic shock syndrome (TSS) to humans. The control of mosquito vector and midge fly larvae is very difficult process for many countries [29, 30]. Biting midges are blood-sucking flies belonging to the family Ceratopogonidae, which includes more than 6,000 species available worldwide. The most important consideration of biting midges is medical and veterinary interest [31,32,33]. They transmit a number of protozoans, pathogens, viruses and filarial nematodes. In particular, veterinary research and wildlife conservation, they transmit many viral diseases to animals such bluetongue disease, African horse sickness and epizootic hemorrhagic disease [34,35,36]. Because of the vast biodiversity of fauna and flora, plants are considered as an important source material for novel pesticides production. Furthermore, the natural components of plant extracts can provide an advantage to efficiently fight against pesticide resistance insects [37,38,39].
Recently, Plant derived compounds and nanomaterials play a major role in pest control with the help of nanotechnology. Plant compounds encapsulated nanomaterials are efficient in controlling the mosquito and some pest populations. Plant components together with nanomaterials exhibits, synergistic effect towards several molecular targets in pest control [39]. Zeolitic imdiazole framework (ZIF-L) has emerged into promising class of nanocarrier in the field of biomedicine due to its unique physiochemical properties such as crystalline structure with exceptional thermal and chemical stability, high surface area with tunable pore size, good adsorption capacity and ease of functionalization. In addition, the biocompatibility, biogenic mode of synthesis and pH responsiveness has made it a suitable candidate for drug delivery specifically for cancer theranostics and as antimicrobial and pest control agents [18, 19, 39]. Hence, the present study focused on the fabrication of Calotropis gigantea latex encapsulated ZIF-L nanoframeworks and evaluate its mosquito, midge fly larvicidal activities and anti-biofilm potentials.
2 Materials and Methods
Calotropis gigantea latex, Nutrient agar, streptomycin, Zinc nitrate hexahytdrate, 2-Methyl imidazole, Mueller Hinton agar, penicillin and Crystal violet were purchased in Himedia, India. Artemia salina cysts were purchased in aquarium, Chennai, India. All the chemicals and reagents used are of analytical grade.
2.1 Collection of Latex from the Plant
The fresh latex was collected from exudation of aerial part of the plant in the natural population of Calotropis gigantea around Alagappa University, Science campus, Tamilnadu, India (10°05′32.6″ N 78°47′17.2″ E). The liquid latex was dried in hot air oven at 60 °C for 5 h. Dried latex was grinded by mortar and the powdered sample were stored at refrigerator for further uses.
2.2 Synthesis of CG@ZIF-L Nanoframeworks
Calotropis cigantea latex encapsulated ZIF-L nanocomposite was prepared with slight modification based on the previous report of [18]. Briefly, 200 mg of zinc nitrate hexahydrate and 600 mg of 2-methyl imidazole dissolved in separate 45 ml of double distilled water. 5 mg of latex powder dissolved in 10 ml of methanol (0.5 mg/ml). Under magnetic agitation latex solution was slowly added into 2-methyl imidazole solution and the stirring was continued for 30 min. Zinc nitrate solution was slowly added into latex and 2-methyl imidazole mixture under magnetic agitation for another one hour until pure white colored colloid is formed which indicates the formation of CG@ ZIF-L framework. Finally, the excess amount of precursors was removed by washing with DD water by centrifugation at 8000 rpm speed. The hydrated sample was dried at 60 ºC for 6 h.
2.3 Collection of Mosquito Larvae
The fourth instar stage of Aedes aegypti larvae were collected from the stagnant rain water drain in Peraiyur village, Ramanathapuram district, Tamilnadu, India (9°21′24.0″ N 78°27′12.8″ E). The collected larvae were maintained in plastic enamel trays containing dechlorinated tap water at room temperature for the further studies.
2.4 Collection of Midge Fly Larvae
The third instars stage of midge fly larvae were collected from field of sorghum cultivation ground in Peraiyur village, Ramanathapuram dist, Tamilnadu, India ((9°21′24.0″ N 78°27′12.8″ E). The collected midge fly larvae were maintained in plastic beaker containing decholorinated tap water at room temperature for further experimental studies.
2.5 Mosquito and Midge Fly Larvicidal Bioassay
Mosquitocidal potential of CG@ZIF-L nanoframeworks against Aedes aegypti was evaluated based on OECD guideline standard method [40]. Five different groups of 20 mosquito larvae were transferred into glass beakers containing 200 ml of sterile double distilled water with various concentrations of CG@ZIF-L nanoframeworks (25–125 μg/ml). Double distilled water alone was used as negative control without addition of nanoframeworks. The 16:8 h light/dark cycle trials were used for assessment of larvicidal activity of CG@ZIF-L nanoframeworks. A Morphological and behavior change of treated groups was keenly watched at distinct time intervals. Mortality rate was quantified after 24 h post exposure of treated larvae of Aedes aegypti. The biting midge fly larvicidal potential of CG@ZIF-L nanoframeworks was assessed by same above mentioned procedure and same concentrations. The lethality of larvae was quantified using the standard formula.
X: Survival in the untreated control and Y: Survival in the treated sample.
2.6 Anti-biofilm Potential of CG@ZIF-L Nanoframeworks
Anti-biofilm efficacy of the CG@ZIF-L nanoframeworks were investigated against gram positive and negative bacterial strains (E.coli, S.epidermis) based on methodology of [19] with some modifications. Bacterial strains was allowed to grow over night and the O/N culture was subjected to dilution (108 CFU/ml) in nutrient broth containing 0.5% glucose followed by treatment with different concentrations of nanoframeworks (25–100 µg/ml), for 24 h at 37 °C. The bacterial culture was coated on the surface of 1 × 1 cm glass slides. A free floating liquid bacterial suspension was removed by phosphate buffer solution. The slides were stained with 0.5% crystal violet stain. Excess amount of stain was removed by washing thrice with sterile distilled water for 10 min; bacterial inhibition was measured as color intensityat 570 nm using multi plate reader.
Architecture and morphological changes of CG@ZIF-L nanoframework treated groups was observed and images were taken by using light microscope at ×40 magnification.
2.7 Statistical Analysis
The IC50, LD50 and LD90 values are calculated using Probit analysis software and comparison of variance in control and treated groups were carried out using one-way analysis of ANOVA software. Results were considered significant at the level of p < 0.05. A result was expressed as Mean ± SD of triplicates.
3 Results and Discussion
3.1 Powder XRD Analysis
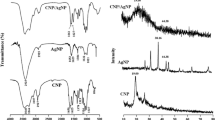
The crystal structure of CG@ZIF-L nanoframeworks was evaluated by powder XRD spectroscopy technique. PXRD spectra (Fig. 1) shows sharp diffraction peaks at around 14.32º, 15.63º, 17.07º, 21.24º, 25.38º, 27.04º, 29.87º, 30.34º, 31.56º, 32.93º, 36.58º and 38.75º corresponding to (100), (002), (101), (102), (110), (103), (200), (201), (004), (202), (104) and (203) Braggs reflection planes. Characteristic X-ray diffraction peaks at 2θ values and crystal planes confirmed that the hexagonal crystal structure according to JCPDS No. 01–1136. The crystalline size of CG@ZIF-L nanoframeworks was measured by Scherer’s formula to be around 46.38 nm respectively.
3.2 UV–Vis Analysis
The characteristic UV- Vis absorption spectra of bare ZIF-L showed peak at 210 nm. Latex encapsulated CG@ZIF-L nanoframeworks (Fig. 2) showed absorption peak at 201 nm respectively. Shifting in the absorption spectra of CG@ZIF-L nanoframeworks confirmed, that the encapsulation of latex within ZIF-L frameworks. Band gap energy of ZIF-L and CG@ZIF-L nanoframeworks was calculated to be 5.90 eV and 6.16 eV respectively. The band gap energy of synthesized nanoframeworks was increased by the encapsulation of latex into ZIF-L frameworks.
3.3 FTIR Analysis
The characteristic functional groups of synthesized CG@ZIF-L nanoframeworks were identified by FTIR spectroscopy. The spectra (Fig. 3) showed the intense peaks at around 683, 714, 949 and 1005 cm−1 which attributes to C–H aliphatic stretching vibration of imidazole. Small peaks at around 1145, 1505 cm−1 represented that the N–H stretching vibration of imidazole linker from zeolitic imidazole frameworks. The mild peak at around 1647 and 1756 cm−1 corresponding to the axial deformation of C–O bending to C=O stretching vibrations of imidazole molecules. Large peaks at around 3298, 3718 cm−1 confirmed that the bending vibration of hydroxyl group in the CG@ZIF-L nanoframeworks.
3.4 FE-SEM and EDX Spectroscopy Analysis
The crystal morphology and chemical composition of synthesized CG@ZIF-L nanoframeworks was studied by using FE-SEM and EDX spectroscopy analysis techniques. The results reveal that the encapsulation of Calotropis gigantea latex within the ZIF-L frameworks disturbed the crystal growth and leads lattice mismatch. The microscopic images concluded that the crystals are agglomerated (Fig. 4A). The results of EDX spectra of CG@ZIF-L nanoframeworks (Fig. 4B) shows higher amount of carbon and zinc ions which corresponds to the presence of zinc and organic components from 2-methyl imidazole and Calotropis gigantea latex. The notable amount of nitrogen represented that the formation of zinc and imidazole covalent bonding in ZIF-L frameworks.
3.5 TEM Analysis
The average particle size and crystal morphology was assessed by using HR-TEM imaging technique (Fig. 5A, B). Images of CG@ZIF-L nanoframeworks exhibited two dimensional spongy flakes and agglomerated spheres. The average particle size of CG@ZIF-L nanoframeworks was calculated to be 58.4 nm. The nano sized frameworks can be easily internalized to living cells either by endocytosis mechanism. As the prepared nanoframeworks are less than 100 nm sizes which gives more advantages to uses of biomedical applications.
3.6 Assessment of Particle Size and Thermal Stability Analysis of CG@ZIF-L
Size of the CG@ZIF-L was evaluated using DLS analysis and the results were shown in Fig. 6A. Results revealed the average particle size of CG@ZIF-L was observed to be 163 nm, which in accordance to the previous reports [41, 42] is suitable for cellular uptake and internalization of nanocomposite either by endocytosis or adsorption mechanism.
Thermal stability of ZIF-L and CG@ZIF-L was evaluated by thermo gravimetric analysis and the results are shown in Fig. 6B. Results revealed that ZIF-L is thermally stable up to 500 °C with no significant weight loss. ZIF-L alone showed mild weight loss of 4.04% at 100–500 ºC due to loss of water and second plateau with 25.94% weight loss between 500–700 °C indicating the degradation of ZIF-L frameworks as reported by Prabhu et al., [18]. TGA of CG@ZIF-L showed weight loss of 19.5% in the region 250–500 °C exemplifying the decomposition of Calotropis gigantea latex from CG@ZIF-L nanocomposite. Further reduction in total mass by 49.9% was observed at temperature range 500–800 °C indicating complete degradation of ZIF-L frameworks.
3.7 Anti-biofilm Efficacy of CG@ZIF-L Nanoframeworks
Escherichia coli and Staphylococcus epidermis are more dreadful nosocomial pathogen infecting humans which causing life threatening disorders. A bacterial bio-film formed by the aggregation of bacterial cells adjoining by self-production polymer matrixes adhered to surface of medical tools and tissues causing serious health problems to humans and animals [19, 43]. The biofilm inhibitory potential of synthesized CG@ZIF-L nanoframeworks was assessed by crystal violet staining assay. Results revealed that, the encapsulation of Calotropis gigantea latex efficiently control the bacterial growth compared to control sample and ZIF-L alone. Figure 7B illustrated that CG@ZIF-L nanoframeworks controlled the growth and eradicated the biofilm matrix in dose dependant manner, while negligible activity was observed in ZIF-L alone treated group. Furthermore quantitative study of Crystal violet binding assay results (Fig. 7A) revealed that CG@ZIF-L nanoframeworks inhibited biofilm forming bacteria E.coli and S.epidermis growth 92% at 78 ± 1.35 μg/ml with IC50 value of 38.57 ± 0.94 μg/ml concentrations. ZIF-L alone exhibited 24% biofilm inhibition at maximum dose of 100 μg/ml. The presence of zinc and biochemical constituents of Calotropis gigantea latex is leading factors of enhanced anti-biofilm potential of CG@ZIF-L nanoframeworks.
3.8 Mosquito Larvicidal Efficacy of CG@ZIF-L Nanofameworks
Results of mosquito larvicidal activity of CG@ZIF-L and ZIF-L alone showed maximum mortality in CG@ZIF-L at the dose of 60 μg/ml, while ZIF-L showed no mortality effect even at the highest dose of 125 μg/ml. The LC50 and LC90 values of CG@ZIF-L against Aedes aegypti larvae were observed to be 39. 43 ± 1.16 and 72.53 ± 1.84 μg/ml respectively. Results reveal that CG@ZIF-L showed significantly effective larvicidal activity at low concentration when compared to CA@ZIF-L reported in our previous studies [18] The mosquitocidal potential of synthesized CG@ZIF-L nanoframeworks increased in higher concentration with reliable lethality and morphological changes in treated larvae groups. Most of the pesticides working mechanism are based on ROS mediated apoptosis leading to insects death [20, 24, 44]. Here, the presence of zinc ions in ZIF-L nanoframeworks, Calotropin and Calotoxin present in the CG latex might have induced ROS generation leading to apoptosis mediated cell death in mosquito larvae [45, 46]. The nanoscale CG@ZIF-L frameworks easily penetrate into the epithelial cells of treated larvae, which reduce the growth, leading to malformation based death. Figure 8 exhibit significant morphological changes and growth control in treated larvae compared with control group. The organelle damage and growth retardation such as head, thorax was observed in treated larvae in accordance to the reports of Prabhu et al., [18]. Microscopic observation of treated larvae revealed loss of hair, epithelial cells and thorax damage.
3.9 Larvicidal Effects of CG@ZIF-L Nanoframeworks Against Midge Fly Larvae
Biting midge flies is a type of biting pests, their blood sucking habits raises great concern about transmission of disease agents in humans and animals [47, 48]. Biting midges are annoying species, but no report in transmission of human disease. They have a greater impact on animal disease transmission through biting [49, 50]. In India, especially Tamil Nadu biting and non-biting midges multiply in the rainy season (July—December). They inflict a burning sensation in humans and small reddish welt at the bite site [51]. Furthermore the over population of non-biting midge larvae may cause water contamination and air filtration blockage. The midge fly is one of the non-biting insect in genus Culicoides, larvae feed the active plankton in water, which leads to oxygen demand and provide opportunity for the growth of pathogenic bacteria [29, 52]. Some species of non-biting midges are highly vulnerable in the initial stages of crop growth [53,54,55]. Globally sorghum midges create major shoot damage affecting 85% of crop yield in Sorghum bicolor L. plants [56,57,58,59]. There is no more data and research available about biting midges in Tamil Nadu, India. This is first report regarding the control of non-biting midge flies (blood worm) larvae by using nanomaterial. The larvicidal bioassay results revealed that Calotropis gigantea latex encapsulated ZIF-L nanoframeworks effectively control the growth of midge fly larvae. The quantitative results concluded that the maximum inhibition appeared at 80.25 ± 1.05 μg/ml concentrations and LC50 value against midge fly larvae was observed to be at 39.68 ± 1.23 μg/ml. ZIF-L alone showed negligible activity against midge fly larvae at the maximum concentration of 125 μg/ml. Microscopic observation of treated larvae groups (Fig. 9) showed morphological changes and hemoglobin degradation increased with increased dose of CG@ZIF-L nanoframeworks.
4 Conclusion
The present study successfully fabricated Calotropis gigantea latex encapsulated ZIF-L nanoframeworks by one-pot synthesis method. The synthesized CG@ZIF-L nanoframeworks showed excellent potent antibiofilm activity against Escherichia coli and Staphylococcus epidermis bacterial strains. Results indicates the growth inhibition of microcolonies and architectural deformation. Furthermore, CG@ZIF-L nanoframeworks exhibit reliable toxicity against Aedes aegypti and midge fly larvae in dose dependent manner. The larvicidal results concluded that the CG@ZIF-L nanoframeworks damaged the epithelial cells of mosquito and midge fly larvae. In midge fly larvicidal bioassay, CG@ZIF-L nanoframeworks induced the hemoglobin degradation and organelle malformation which leads to death of larvae. These findings are very new and provided initial evidence that CG@ZIF-L nanoframewroks might act as promising material for the designing of antibiotics and pesticides.
References
M.S. Butler, The role of natural product chemistry in drug discovery. J. Nat. Prod. 67, 2141–2153 (2004). https://doi.org/10.1021/np040106y
H. Khan, Medicinal plants in light of history: recognized therapeutic modality. J. Evid. Based Complement Altern. Med. 19(3), 216–219 (2014). https://doi.org/10.1177/2156587214533346
S.Z. Husain, R.N. Malik, M. Javaid et al., Ethanobotnical properties and uses of medicinal plants of Morgah biodiversity park, Rawalpindi. Pak. J. Bot. 40, 1897–1911 (2008)
S. Rates, Plants as source of drugs. Toxicon 39, 603–613 (2001). https://doi.org/10.1016/s0041-0101(00)00154-9
Z. Shinwari, Medicinal plants research in Pakistan. J. Med. Plant Res. 4, 161–176 (2010)
G. Kumar, L. Karthik, K.V. Bhaskara Rao, Antibacterial activity of aqueous extract of Calotropis gigantea leaves—an in vitro study. Int. J. Pharm. Sci. Rev. Res. 4(2), 141–144 (2010)
R. Rajesh, C.D. Raghavendra Gowda, A. Nataraju, B.L. Dhananjaya, K. Kemparaju, B.S. Vishwanath, Procoagulant activity of Calotropis gigantea latex associated with fibrin(ogen)olytic activity. Toxicon 46(1), 84–92 (2005). https://doi.org/10.1016/j.toxicon.2005.03.012
V. Saratha, S. Subramanian, S. Sivakumar, Evaluation of wound healing potential of Calotropis gigantea latex studied on excision wounds in experimental animals. Med. Chem. Res. 19(8), 936–947 (2009). https://doi.org/10.1007/s00044-009-9240-6
A.K. Pathak, A. Argal, CNS activity of Calotropis gigantea roots. J. Ethnopharmacol. 106(1), 142–145 (2006). https://doi.org/10.1016/j.jep.2005.12.024
N. Singh, N.K. Jain, P. Kannojia, N. Garud, A.K. Pathak, S.C. Mehta, In vitro antioxidant activity of Calotropis gigantea hydroalcohlic leaves extract. Der Pharmacia Lettr. 2(3), 95–100 (2010)
K.S. Lam, New aspects of natural products in drug discovery. Trends Microbiol. 15, 279–289 (2007). https://doi.org/10.1016/j.tim.2007.04.001
M. Ahmad, S.H. Kamran, A. Mobasher, Protective effect of crude Curcuma longa and its methanolic extract in alloxanized rabbits. Pak. J. Pharm. Sci. 27, 121–128 (2014)
M.M. Pandey, S. Rastogi, A.K.S. Rawat, Indian traditional ayurvedic system of medicine and nutritional supplementation. J. Evid. Based Complement Altern. Med. 23, 2013 (2013). https://doi.org/10.1155/2013/376327
A. Narayana, V. Subhose V, Standardization of Ayurvedic formulations: a scientific review. Bull Indian Inst Hist Med. 35(1), 21–32 (2005)
M.A. Alam, M.R. Habib, R. Nikkon, M. Rahman, M.R. Karim, Antimicrobial activity of akanda (Calotropis gigantea L.) on some pathogenic bacteria. Bangladesh J. Sci. Ind. Res. 43(3), 397–404 (2008). https://doi.org/10.3329/bjsir.v43i3.1156
G. Andrea, L. Graham, MRSA—‘bug-bear’ of a surgical practice: reducing the incidence of MRSA surgical site infections. Ann. R. Coll. Surg. Engl. 88, 222–223 (2006)
J.P. Motta, J.L. Wallace, A.G. Buret, C. Deraison, N. Vergnolle, Gastrointestinal biofilms in health and disease. Nat. Rev. Gastroenterol. Hepatol. 18(5), 314–334 (2021). https://doi.org/10.1038/s41575-020-00397-y
R. Prabhu, A. Pugazhendhi, N. Suganthy, One-pot fabrication of multifunctional catechin@ZIF-L nanocomposite assessment of antibiofilm, larvicidal and photocatalytic activities. J. Photochem. Photobiol. B 203, 111774 (2020). https://doi.org/10.1016/j.jphotobiol.2019.111774
R. Prabhu, N. Suganthy, Anticancer, antibiofilm and antimicrobial activity of fucoidan loaded zeolitic imidazole framework fabricated by one -pot synthesis method (Nanosci, Appl, 2021). https://doi.org/10.1007/s13204-021-01881-w
N.K. Arjunan, K. Murugan, C. Rejeeth, P. Madhiyazhagan, D.R. Barnard, Green synthesis of silver nanoparticles for the control of mosquito vectors of malaria, filariasis, and dengue. Vector Borne Zoonotic Dis. 12(3), 262–268 (2011). https://doi.org/10.1089/vbz.2011.0661
C. Kamaraj, A. Bagavan, A.A. Rahuman, A.A. Zahir, G. Elango, G. Pandiyan, Larvicidal potential of medicinal plant extracts against Anopheles subpictus Grassi and Culex tritaeniorhynchus Giles (Diptera: Culicidae). Parasitol. Res. 104, 1163–1171 (2009). https://doi.org/10.1007/s00436-008-1306-8
R.B. Salunkhe, S.V. Patil, C.D. Patil, B.K. Salunke, Larvicidal potential of silver nanoparticles synthesized using fungus Cochliobolus lunatus against Aedes aegypti and Anopheles stephensi Liston (Diptera; Culicidae). Parasitol. Res. 109(3), 823–831 (2011). https://doi.org/10.1007/s00436-011-2328-1
S. Allgeier, B. Frombold, V. Mingo, C.A. Bruhl, European common frog Rana temporaria (Anura: Ranidae) larvae show subcellular responses under field-relevant Bacillus thuringiensis var. israelensis (Bti) exposure levels. Environ. Res. 162, 271–279 (2018). https://doi.org/10.1016/j.envres.2018.01.010
S. Allgeier, A. Kastel, C.A. Bruhl, Adverse effects of mosquito control using Bacillus thuringiensis varisraelensis reduced chironomid abundances in mesocosm, semifield and field studies. Ecotoxicol. Environ. Saf. 169, 786–796 (2019). https://doi.org/10.1016/j.ecoenv.2018.11.050
S.P. Wraight, D.P. Molloy, H. Jamnback, P. McCoy, Effects of temperature and lnstar on the efficacy of Bacillus thuringiensis var. israelensis and Bacillus sphaericus strain 1593 against Aedes stimulans larvae. J. Invertebr. Pathol. 38, 78–87 (1981)
Q. Yang, S. Tang, J. Rang, M. Zuo, X. Ding, Y. Sun et al., Detection of toxin proteins from Bacillus thuringiensis strain 4.0718 by strategy of 2D-LC-MS/MS. Curr. Microbiol. 70, 457–463 (2015). https://doi.org/10.1007/s00284-014-0747-9
M.C. Wirth, W.E. Walton, B.A. Federici, Inheritance patterns, dominance, stability, and Allelism of insecticide resistance and cross-resistance in two colonies of Culex quinquefasciatus (Diptera: Culicidae) selected with cry toxins from Bacillus thuringiensis subsp. israelensis. J Med Entomol. 47, 814–822 (2010). https://doi.org/10.1603/me09227
M.C. Wirth, W.E. Walton, B.A. Federici, Inheritance, stability, and dominance of cry resistance in Culex quinquefasciatus (Diptera: Culicidae) selected with the three toxins of Bacillus thuringiensis subsp. israelensis. J. Med. Entomol. 49, 886–894 (2012). https://doi.org/10.1603/me11192
V. Baranov, J. Lewandowski, P. Romeijn et al., Effects of bioirrigation of non-biting midges (Diptera: Chironomidae) on lake sediment respiration. Sci. Rep. 6, 27329 (2016). https://doi.org/10.1038/srep27329
H. Chen, Y. Zhang, L. Ma et al., Change of water consumption and its potential influential factors in Shanghai: a cross-sectional study. BMC Public Health 12, 450 (2012). https://doi.org/10.1186/1471-2458-12-450
M. Semmler, F. Abdel-Ghaffar, K. Al-Rasheid, H. Mehlhorn, Nature helps from research to products against blood-sucking arthropods. Parasitol. Res. 105, 1483–1487 (2009). https://doi.org/10.1007/s00436-009-1634-3
E.A. Shaalan, D.V. Canyon, Aquatic insect predators and mosquito control. Trop Biomed. 26, 223–261 (2009)
V.P. Sharma, Health hazards of mosquito repellents and safe alternatives. Curr Sci. 80, 341–343 (2001)
J. Hemingway, H. Ranson, Insecticide resistance in insect vectors of human disease. Annu. Rev. Entomol. 45, 371–391 (2000). https://doi.org/10.1146/annurev.ento.45.1.371
G. Ceballos, P.R. Ehrlich, R. Dirzo, Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. Proc. Natl. Acad. Sci. 114, E6089-e6096 (2017). https://doi.org/10.1073/pnas.1704949114
G. Ceballos, P. Ehrlich, The sixth extinction crisis loss of animal populations and species. J. Cosmol. 8, 1821–1831 (2010)
C. Vidya, S. Hiremath, M.N. Chandraprabha, M.A.L. Antonyraj, G.I. Venu, A. Jain, B.K. Kokil, Green synthesis of ZnO nanoparticles by Calotropis gigantea. Int. J. Curr. Eng. Technol. 1, 118–120 (2013)
S.R. Srivastava, G. Keshri, B. Bhargavan, C. Singh, M.M. Singh, Pregnancy interceptive activity of the roots of Calotropis gigantea (Lin) In rats. Contraception 75, 318–322 (2007). https://doi.org/10.1016/j.contraception.2006.11.010
R. Prabhu, R. Mohamed Asik, R. Anjali, G. Archunan, N.M. Prabhu, A. Pugazhendhi, N. Suganthy, Ecofriendly one pot fabrication of methyl gallate@ZIF-L nanoscale hybrid as pH responsive drug delivery system for lung cancer therapy. Process. Biochem. 84, 39–52 (2019). https://doi.org/10.1016/j.procbio.2019.06.015
Report of the WHO informal consultation on the evaluation on the testing of insecticides. CTD/ WHO PES/IC/96.1. WHO, Geneva, p. 69, (1996).
P. Foroozandeh, A.A. Aziz, Insight into cellular uptake and intracellular trafficking of nanoparticles. Nanoscale Res. Lett. 13, 339 (2018). https://doi.org/10.1186/s11671-018-2728-6
N. Ma, C. Ma, C. Li, N. Hel, Influence of nanoparticle shape, size, and surface functionalization on cellular uptake. J. Nanosci. Nanotechnol. 13(10), 6485–6498 (2013)
H.B. Allen, N.D. Vaze, C. Choi, T. Hailu, B.H. Tulbert, C.A. Cusack, S.G. Joshi, The presence and impact of biofilm-producing staphylococci in atopic dermatitis. JAMA Dermatol. 150(3), 260–265 (2014). https://doi.org/10.1001/jamadermatol.2013.8627
P. Kumari, P.K. Panda, E. Jha, K. Kumari, K. Nisha, M.A. Mallick, S.K. Verma, Mechanistic insight to ROS and Apoptosis regulated cytotoxicity inferred by green synthesized CuO nanoparticles from Calotropis gigantea to embryonic Zebra fish. Sci. Rep. 7, 16284 (2017). https://doi.org/10.1038/s41598-017-16581-1
G. Kumar, K. Loganathan, B. Rao, V. Kirthi, J. Chidambaram, A. Rahuman, Phytochemical composition, mosquito larvicidal, ovicidal and repellent activity of Calotropis procera against Culex tritaeniorhynchus and Culex gelidus, Bangladesh. J. Pharmacol. 7, 63–69 (2012). https://doi.org/10.3329/bjp.v7i1.10156
M. Shahi, A.A. Hanafi-Bojd, M. Iranshahi, H. Vatandoost, M.Y. Hanafi-Bojd, Larvicidal efficacy of latex and extract of Calotropis procera (Gentianales: Asclepiadaceae) against Culex quinquefasciatus and Anopheles stephensi (Diptera: Culicidae). J Vector Borne Dis 47:185–188.Srivastava SR, Keshri G, Bhargavan B, Singh C, Singh MM (2007) Pregnancy interceptive activity of the roots of Calotropis gigantea (Lin) In rats. Contraception 75, 318–322 (2010). https://doi.org/10.1016/j.contraception.2006.11.010
S. Carpenter, M.H. Groschup, Garros et al., Culicoides biting midges, arboviruses and public health in Europe. Antivir Res. 100(1), 102–113 (2013). https://doi.org/10.1016/j.antiviral.2013.07.020
F. Sick, M. Beer, H. Kampen, K. Wernike, Culicoides biting midges underestimated vectors for arboviruses of public health and veterinary importance. Viruses 11(4), 376 (2019). https://doi.org/10.3390/v11040376
R.W. Elbers, C.J. Koenraadt, R. Meiswinkel, Mosquitoes and Culicoides biting midges: vector range and the influence of climate change. Rev. Sci. Technol. 34(1), 123–137 (2015)
Mullen GR, and Murphree CS (2019) Biting midges (Ceratopogonidae). Med. Vet. Entomol. 213–236.
Y. Liu, H. Tao, Y. Yu, L. Yue, W. Xia et al., Molecular differentiation and species composition of genus Culicoides biting midges (Diptera: Ceratopogonidae) in different habitats in southern China. Vet. Parasitol. 254, 49–57 (2018). https://doi.org/10.1016/j.vetpar.2018.02.035
M.S. Fard, F. Pasmans, C. Adriaensen, G.D. Laing, G.P. Janssens, A. Martel, Chironomidae bloodworms larvae as aquatic amphibian food. Zoo Biol. 33(3), 221–227 (2014). https://doi.org/10.1002/zoo.21122
S. Althwab, T.P. Carr, C.L. Weller, I.M. Dweikat, V. Schlegel, Advances in grain sorghum and its co-products as a human health promoting dietary system. Food Res. Int. 77, 349–359 (2015). https://doi.org/10.1016/j.foodres.2015.08.011
J.S. Armstrong, W.L. Rooney, G.C. Peterson et al., Sugarcane aphid (Hemiptera: Aphididae) host range and sorghum resistance including cross-resistance from greenbug sources. J. Econ. Entomol. 108, 576–582 (2015). https://doi.org/10.1093/jee/tou065
F.P. Baxendale, C.L. Lippincott, G.L. Teetes, Biology and seasonal abundance of hymenopterous parasitoids of sorghum midge (Diptera: cidomyiidae). Environ. Entomol. 12, 871–877 (1983). https://doi.org/10.1093/ee/12.3.871
M.J. Brewer, J.W. Gordy, D.L. Kerns, J.B. Woolley, W.L. Rooney, R.D. Bowling, Sugarcane aphid population growth, plant injury, and natural enemies on selected grain sorghum hybrids in Texas and Louisiana. J. Econ. Entomol. 110, 2109–2118 (2017). https://doi.org/10.1093/jee/tox204
C. Guo, W. Cui, X. Feng, J. Zhao, G. Lu, Sorghum insect problems and management. J. Integr. Plant Biol. 53, 178–192 (2011). https://doi.org/10.1111/j.1744-7909.2010.01019.x
A.M. Malgwi, D.M. Dunuwel, Damage and control of panicle insect pests of sorghum (Sorghum bicolor (L.) Moench) in northeastern Nigeria. ARPN J Sci Technol. 3, 195–202 (2011)
M. Meena, G.G. Radadia, U.C. Shinde, Efficacy of different botanicals in the management of Rhyzopertha dominica (Fabricius) in stored sorghum. J. Eco-friendly Agric. 12, 56–58 (2017)
Acknowledgements
R. Prabhu and N.Suganthy thanks the RUSA-Phase 2.0 grant (No. F. 24-51/2014-U, Policy (TN Multi-Gen), Dept. of Edn. Govt.of India, (Dated: 09.10.2018).Authors also acknowledge the Department of Science and Technology, New Delhi for the financial support in general and infrastructure facilities sponsored under PURSE 2nd Phase programme (Order No. SR/PURSE Phase 2/38 (G) dated: 21.02.2017)
Funding
Funding was provided by rusa phase 2.0 [No. F. 24-51/2014-U, Policy (TN Multi-Gen), Dept. of Edn. Govt.of India, Dated 09.10.2018] and Department of Science and Technology (Order No. SR/PURSE Phase 2/38 (G) dated: 21.02.2017).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of the authors declared that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Raju, P., Natarajan, S. Investigation of Pesticidal and Anti-biofilm Potential of Calotropis gigantea Latex Encapsulated Zeolitic Imidazole Nanoframeworks. J Inorg Organomet Polym 32, 2771–2780 (2022). https://doi.org/10.1007/s10904-022-02298-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-022-02298-w