Abstract
The ring-opening polymerization (ROP) of tetrahydrofuran (THF) and ε-caprolactone (CL) was performed at 160 °C for 2 h under N2 ambiance using two different dyes such as fluorescein (Flur) and rhodamin6G (R6G) as a novel initiator. The structure of the dye-centered diblock copolymers was confirmed by analyzing the NMR spectra. The melt transition temperature and thermal stability of the polymers were determined by DSC and TGA. The FT-IR spectra indicated the functional groups present in the dye grafted homopolymer and diblock copolymers. The increase in molecular weight (Mw) of the dye-centered diblock copolymer was confirmed by the GPC measurement. The conjugation of dye with the polymer backbone was carried out by a single-step method without using any hazardous solvents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Recently, biomedical polymers such as poly(tetrahydrofuran) (PTHF) and poly(ɛ-caprolactone) (PCL) have received much attention because of their biocompatibility and less cytotoxicity. They are prepared by the ROP technique in the presence of a catalyst and an initiator. Generally, Sn salts are used as a catalyst in the presence of various initiators. The catalytic efficiency of FeCl3 on the ROP of THF was studied by Yang et al. [1]. The chloromethylaryloyl was employed as an initiator for the ROP of THF by Kohsaka et al. [2]. Nomura and his co-workers [3] reported the ROP of THF using vanadium complexes as a catalyst [4]. Zhang et al. [5] investigated the initiating efficiency of alkoxides towards the anionic ROP of THF. Bodulla and his research team [6] studied the ROP of THF using reusable poly(aniline) as a catalyst. Marchetti et al. [7] examined the initiating efficiency of Niobium and Tantalum halides towards the ROP of THF. The kaolin was used as an acid catalyst in the preparation of the ROP of THF [8]. The metal ion was employed as an initiator for the charge-transfer photopolymerization of THF by Woodhouse et al. [9]. Olah et al. [10] studied the cationic ROP of THF using the Al and gallium tristriflate as a catalyst. The reports on Flur and R6G initiated ROP of THF are scarce in the literature. The significance of this present work is to prepare the fluorescent dye tagged PTHF without using any hazardous solvents during the synthesis process.
PCL is an important biomedical polymer. It is used as a drug carrier material [11]. The PCL decorated with nano ZnO was suggested as a promising antimicrobial agent by Snigdha et al. [12]. The PCL/graphene nanocomposites were made as electrospun fibers to enhance their mechanical properties [13]. The PCL/bentonite composite was used as an adsorbent material towards the removal of Pb2+ [14]. Dlamini et al. [15] studied the thermal and mechanical properties of PCL-organoclay nanocomposite. The synthesis of PCL is performed in the presence of various catalysts and initiator systems. Here also, SO plays a significant role in the ROP of CL as a catalyst. Initiators like pullulan [16], heparin [17], chitin [18], Ag-kaolinite [19] and palygorskite were used for the preparation of PCL [20]. Park et al. [21] investigated the ROP of CL using lignin as an initiator. Similarly, α-amino acids [22], Fe3O4 nanoparticles [23], n-butanol [24], l-proline [25] and cellulose [26] were employed as an initiator for the ROP of CL [27]. Barium mercaptoacetate had been used as a lone chemical initiator for the polymerization of CL [28]. The Fe3O4/congo red nanohybrid was used as an initiator for the polymerization of CL by ROP [29]. Agathian et al. [30] studied the ROP of CL using the cotton fiber as an initiator. The reports on the dye conjugated PCL are scarce in the literature. This study intends to prepare the dye grafted PCL without using any hazardous solvents during its synthesis by a single-step process.
Flur is an organic dye that exhibits the monomeric and dimeric structure in an aqueous medium. Flur is used as a fluorescent probe in the biomedical sectors [31]. R6G is a dye belonging to a rhodamine family. It is soluble in alcohol and water. It is used as a laser dye with a λmax value of 532 nm [32]. In the present work, two different dyes are used as an initiator towards the ROP of THF and CL. The initiating efficiency of the proposed dyes on the Mw, absorbance (UV–visible), emission (Fluorescence), melt transition temperature (Tm), degradation temperature (Td) and surface morphology of prepared PCL was studied and compared. The -OH is an active group in Flur dye, whereas the –NH is an active group in R6G towards the ROP reaction. Sivabalan et al. [33] studied the ROP efficiency of various functional groups towards the polymerization of CL by ROP. It was confirmed that the –OH is a highly active group for the polymerization of CL. For this purpose, the Flur and R6G dyes were selected as an initiator. In biomedical engineering, the dyes are generally used as fluorescent probes. The chemical conjugation of dye with the polymer backbone is a two-step process, and it is also a time-consuming one. In this work, the dye molecule is acting as an initiator for the ring opening polymerization of CL. Hence, the dye is acting as a fluorescent probe and an initiator. Moreover, PCL and PTHF are biodegradable and biocompatible polymers. The main aim of this work is to synthesis the fluorescent probe containing diblock copolymer without using any hazardous solvents in a cost-effective manner. In the bio-medical industry, a biodegradable polymer-based fluorescent probe is prepared by the multi-step process using hazardous solvents. The novelty of the present work is to prepare the fluorescent probe containing polymer by a single step ROP reaction without using any hazardous solvents.
2 Experimental
2.1 Materials
Tetrahydrofuran (THF, co-monomer), stannous octoate (SO, catalyst) and ε-caprolactone (CL, monomer) were received from Merck, India. Fluorescein (Flur, initiator), rhodamine6G (R6G, initiator) and phthalic anhydride (PAH, accelerator), chloroform (CHCl3, solvent) and diethyl ether (non-solvent) were obtained from Aldrich, India. All the purchased chemicals were of AR grade with 99% purity.
2.2 Procedure
The preparation of dye grafted PTHF and PCL was carried out in two consecutive steps. A homopolymer was prepared by using dye as a chemical initiator in the first step. In the next step, a diblock copolymer was synthesized by using the prepared homopolymer as a chemical initiator.
2.3 Preparation of Homopolymer (Dye-PCL) (P1 and P3 Systems)
The homopolymer of dye grafted PCL was synthesized by using a standard literature procedure [33]. The procedure is described briefly: 2 g of CL (M), 0.001 g of SO (C) and 0.01 g of dye (I) were taken together in a 25 mL RB flask. This mixture was heated at 160 °C for 2 h in an oil bath under the N2 ambiance. The monomer to catalyst [M/C] and monomer to initiator [M/I] ratios were kept as 1000 and 100 respectively. The liquid monomer, catalyst and solid initiator are miscible with each other completely under this experimental condition. In the absence of either catalyst or initiator, the ROP of CL is absent. This proved the requirement of a functional group-containing initiator for the polymerization of CL. The SO accelerates the reaction via the co-ordination insertion mechanism, which yields a highly viscous product. This proves the polymerization of CL by ROP in the presence of a catalyst (C) and an initiator (I). The resultant highly viscous product was dried under a fume hood overnight. The finally obtained products are named as P1 for Flur dye end-capped system and P3 for the R6G dye end-capped system. The reaction mechanism for the same is illustrated in Scheme-1 and 2. In this experimental work, the purification of PCL is not necessary because the PCL was prepared by the bulk polymerization method and yielded the purest form of PCL.
2.4 Preparation of Diblock Copolymer (PCL-Dye-PTHF) (P2 and P4 Systems)
The diblock copolymer of PCL-Dye-PTHF was also prepared as follows: 0.50 g of homopolymer (PCL-dye) was taken along with 0.01 g of PAH in an RB flask (100 mL). Further, this mixture was added with 20 mL of THF and heated at 45 °C under N2 ambiance for 6 h. After the completion of reaction dil. HCl (100 mL) was added. Finally, the brown-colored diblock copolymer was obtained by the freeze-drying method [11]. The obtained final products are named as P2 (PCL-Flur-PTHF) and P4 (PCL-R6G-PTHF) systems. For further purification, the obtained diblock copolymer was dissolved in 10 ml of CHCl3 and dried under a fume hood overnight and stored in a zipper-lock cover under N2 atmosphere. The reaction scheme is illustrated in Scheme 1 and 2.
2.5 Characterizations
The structural analysis was performed for the prepared samples by recording the NMR (Bruker K 8600 instrument, USA) and FT-IR spectra (Shimadzu 8400S, Japan) respectively. The morphology of the sample was investigated by analyzing the recorded SEM micrograph on the JEOL JSM 6300 SEM instrument and FE-SEM micrographs on FE-SEM-Hitachi S4800 Japan, respectively. The thermal degradation temperature (Td) of the samples was examined by recording the TG thermograms on a Universal V4.4A TA thermal analyzer within the heating range of 30–800 °C@10 °C min−1 in air ambiance. The melt transition temperature (Tm) of the polymer samples were studied by recording the DSC thermograms on a Toledo DSC 822E instrument within the heating range of 30–150 °C under N2 ambiance. The electronic transitions present in the polymer sample were examined by capturing the UV–visible spectra on a Thermo Nicolet Avatar 370 spectrometer. The fluorescence property of the polymer sample was inspected using the Elico SL 172, India instrument.
3 Results and Discussion
It is well known that Flur is a xanthene type dye that consists of hydroxyl, carboxyl, ketone, ether, aromatic C = C like functionalities. In the present investigation, the ROP of CL and THF in the presence of PAH is analyzed. The various functional groups of R6G are ester, ether and secondary amine cation, etc. However, the secondary amine group is highly active for the polymerization of CL by ROP.
3.1 FT-IR Spectroscopy
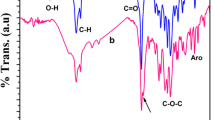
The FT-IR spectra of P1 and P2 systems are illustrated in Fig. 1a, b respectively. The symmetric and anti-symmetric modes of aliphatic C–H are seen at 2868 and 2942 cm−1 respectively. The peaks corresponding to symmetric and anti-symmetric modes of aromatic C–H are noticed at 2524 and 2650 cm−1 respectively. A peak at 1730 cm−1 is linked to the stretching of the carbonyl group of PCL [30, 34]. The out-of-plane bending of C-H vibration and linkage of C–O–C are seen at 733 cm−1 and 1183 cm−1 respectively. After the diblock copolymer formation, some new peaks are assigned for THF and PAH segments. The important peaks are encircled in the spectrum. The stretching of aromatic symmetric and anti-symmetric modes is noted. The stretching of the carbonyl group is assigned as a doublet peak. The short humps at 1693 cm−1 and 1730 cm−1 are corresponding to the stretching of the carbonyl group of PAH and PCL [30]. The stretching of tetrahydrofuronium ion appeared as a short peak at 1582 cm−1. The aromatic C-H deformations are seen at 666 and 799 cm−1 which concluded the diblock copolymer formation. The FT-IR spectrum of the P3 system exhibits the stretching modes of –OH (3440 cm−1), C-H (2872 and 2949 cm−1), carbonyl (1730 cm−1) [30], C-N (1366 cm−1), C–O–C (1196 cm−1) and out of plane bending of C–H vibration (734 cm−1) as demonstrated in Fig. 1c. The P4 system exhibits some new peaks corresponding to aromatic C–H stretching (2528 cm−1 and 2652 cm−1), C = O stretching (1686 cm−1 corresponding to the C = O stretching of PAH) and aromatic C–H deformation (665 and 802 cm−1) as illustrated in Fig. 1d. Hence, the occurrence of new peaks proved the ROP of THF while using PAH as an accelerator.
3.2 NMR Analysis
The structure of homo and diblock copolymer was confirmed by NMR spectroscopy. Figure 2a, b denote the 1H- and 13C-NMR spectra of the P1 system. A signal for standard TMS is noticed at 0 ppm. The alkoxy proton signal of PCL is seen at 4.12 ppm. A signal at 2.3 ppm is due to the proton of –CO2CH2. A signal at 3.8 ppm is corresponding to the –CH2 proton of PCL [28, 35]. A peak at 7.3 ppm is owing to the CDCl3 solvent. The 13C-NMR spectrum of the P1 system exhibits a signal for carbonyl carbon at 173 ppm (Fig. 2b). A signal at 64 ppm is linked to the signal of alkoxy carbon. The signals for other carbon are assigned between 20 and 40 ppm. Thus the NMR spectra confirmed the structure of the P1 system. The 1H- and 13C-NMR spectra of the P2 system are demonstrated in Fig. 2c, d respectively. This system also showed the above-discussed peaks. However, no proton and carbon signals were associating with the THF segments. It is really due to the non-solvating behavior of the PTHF units in CDCl3 solvent. Figure 3a represents the 1H-NMR spectrum of the P3 sample. The signals for the aromatic protons of R6G are identified between 6.2 and 8.2 ppm. The sharp peaks at 4.1 ppm and 2.2 ppm are attributing to the –OCH2 and –CO2CH2 protons of PCL [27]. The other methylene protons are assigned between 1 and 1.8 ppm. A signal for CH2 protons of PCL occurs at 3.7 ppm. The occurrence of peaks associating with the aromatic and alkoxy protons proved the ROP of CL by R6G. The 13C-NMR spectrum of the P3 sample is illustrated in Fig. 3b. A signal at 172 ppm is related to the carbonyl carbon signal of PCL [28]. A peak at 62 ppm is assigned to the signal for alkoxy carbon. The other carbon signals are related to the structure of PCL. The 1H-NMR spectrum of the P4 system is represented in Fig. 3c. This system also showed the above-discussed peaks corresponding to the structure of PCL. The proton signals for THF units did not appear owing to the poor solvation effect. The 13C-NMR spectrum of the P4 sample is represented in Fig. 3d. The signals corresponding to THF segments are absent due to the poor salvation effect.
3.3 UV–visible Spectral Analysis
Figure 4a demonstrates the UV–visible spectrum of pristine Flur. It exhibited one absorption peak at 490 nm relating to the monomeric structure of Flur [36]. The absorbance peak was highly suppressed for the P1 (Fig. 4b) and P2 (Fig. 4c) systems. This is due to the degradation of Flur dye at 160 °C. The –OH group of Flur is active towards the ROP of CL. Moreover, the carboxyl group of Flur is active only for the polymerization of THF in the presence of PAH as an accelerator. But it is proved that Flur is degraded at a lower temperature since it is not having any halogen or nitro group. Figure 4d illustrates the UV–visible spectrum of pure R6G. The absorption peaks at 534 nm (monomeric) and 502 nm (dimeric) are assigned to the structure of R6G [37]. The P3 system (Fig. 4e) shows an absorption peak at 532 nm and a small hump at 499 nm associating with the structure of the R6G. The UV–visible absorption spectrum of the P4 system shows a peak at 528 nm due to the monomeric form of R6G (Fig. 4f). The absorption peak for R6G was blue-shifted during the ROP of THF and CL. This explains the decrease in the conjugation length of R6G. This can also be explained by the decrease in the size of R6G during the ROP of THF and CL. In comparison, the xanthene type dyes (Flur) are not suitable for the ROP of CL at 160 °C, whereas the R6G dye is stable at the same experimental conditions. In our previous publications [38], a similar effect was observed while using xanthene type dyes.
3.4 Fluorescence Emission Spectrum (FES) Study
Flur is a well-known dye molecule that exhibits a fluorescence property. The FES of the P1 (Fig. 5a) and P2 systems (Fig. 5b) are given here for the sake of comparison. Both the spectra showed an emission peak at 521 nm [36]. Unfortunately, the peak’s intensity was found to be very low. This may be due to (i) the presence of a low quantity of dye molecules, (ii) degradation of Flur dye during the ROP of CL at 160 °C for 2 h. Generally, both homopolymer and diblock copolymer exhibited a very low intensity than the pristine Flur dye. This suggests that the degradation of Flur dye may occur during the polymerization of CL. The FES of the P3 sample exhibits an intensity of 80 cps at 563 nm [37] as illustrated in Fig. 5c. The peak is red-shifted to 568 nm for the P4 sample after the formation of the diblock copolymer (Fig. 5d). This confirmed the ROP of THF and CL in the presence of the R6G dye initiator. In comparison, the R6G dye is more stable than the Flur dye in the experimental conditions. Moreover, the –NH group in R6G is substituted with an ethyl group and the ortho position of the phenyl ring is substituted by electron releasing –CH3 group. Hence, the hydrogen transfer from the –NH group is somewhat difficult. In the case of Flur, the hydrogen transfer is very easy because of the electro-negativity of the phenolic group. Hence, the degradation of the Flur dye occurs during the polymerization reaction. The FES explained the structure–property relationship of the diblock copolymer.
3.5 DSC Analysis
PCL is a semi-crystalline polymer, and hence it exhibits a melt transition peak (Tm). The Tm was found to be 68.1 °C (Table 1) for the P1 system as demonstrated in Fig. 6a [37]. The Tm value of the P2 system decreased to 64.5 °C (Fig. 6b). This indicates that the Tm value of the PTHF is almost equal to the Tm value of PCL. It absorbs moisture due to its hydrophilic nature. Hence, there is a reduction in Tm value. The endothermic peak was observed at 63.8 °C for the P3 system [30] attributing to the Tm of PCL (Fig. 6c). The DSC thermogram of the P4 system exhibited the Tm at 61.2 °C (Fig. 6d). In comparison, the diblock copolymer exhibited a lower Tm value than the homo PCL. The appearance of a single Tm confirmed the homogeneity of the diblock copolymer. In the overall comparison, the homopolymer exhibited higher Tm values, and particularly the Flur dye yielded a higher Tm value. The P1 sample exhibited the highest Tm value of 68.1 °C owing to the hydrophobic nature of PCL. The higher Tm values are associated with the high molecular weight of PCL and it is supported with GPC in the forthcoming session.
3.6 TGA Study
The thermal properties of the P1 system were analyzed by TGA. The decomposition of the P1 system occurs in two-steps (Fig. 7a). The major mass loss at 410 °C (Table 1) is attributing to the decomposition of PCL [38]. The minor mass loss at 500 °C is responsible for the decomposition of Flur dye attached to the chain end of the PCL. The decomposition of the P2 sample occurs in two-steps (Fig. 7b). The major mass loss at 200 °C is attributing to the decomposition of PTHF units [27]. The minor mass loss at 385 °C is responsible for the decomposition of PCL. The thermal stability of hydrophobic PCL was higher than the hydrophilic PTHF segments. The decomposition of the P3 system occurs in two-steps (Fig. 7c). The major mass loss at 422 °C is attributing to the decomposition of PCL. The minor mass loss at 525 °C is related to the decomposition of the R6G dye. The decomposition of the P4 system occurs in three-steps (Fig. 7d). The minor mass loss at 214 °C is attributing to the decomposition of PTHF units. The major mass loss at 393 °C is due to the decomposition of PCL. The minor mass loss at 475 °C is ascribed to the decomposition of the R6G dye. The homo PCL showed higher thermal stability than the diblock copolymer. This is because of the attached hydrophilic PTHF units to the hydrophobic PCL backbone. In the overall comparison, the P3 sample revealed the highest Td value of 422 °C for the R6G end-capped PCL. It means that the R6G dye yielded the highest thermal stability for the PCL system. The higher thermal stability is associated with the molecular weight and hydrophobicity of PCL.
3.7 GPC Study
The initiating efficiency of the Flur and R6G dyes on the ROP of THF and CL was confirmed by GPC measurements. The P1 system exhibited the Mw, Mn and PD values of 12,763 g/mole, 7295 and 1.7 respectively (Table 1). For the P2 system, the Mw increased to 14,388 g/mole with the simultaneous increase of Mn (8688). This is indicated in Fig. 8a, b for the prepared P1 and P2 systems. The increase in Mw of the P2 system proved the polymerization of THF by the ROP method while using PAH as an accelerator and Flur as a chemical initiator. The GPC profile confirmed the polymerization of CL with R6G initiator. The Mw, Mn and PD values of the P3 system are 5516 g/mole, 3064 and 1.8 respectively (Fig. 8c). Similarly, the Mw, Mn and PD values of the P4 system were determined as 6348 g/mole, 3341, and 1.9 respectively (Fig. 8d). The increase in Mw proved the ROP of THF while using PAH and R6G as an initiator. As usual, the Mw of the diblock copolymer was higher than the homopolymer. The R6G initiator system yielded a low molecular weight than the Flur initiator system. This is due to the poor ionizing nature of –NH group. This proved a higher ring-opening capability of the –OH group than the N–H group. Sivabalan et al. [33] proved the ROP ability of different functional groups toward CL and also confirmed the same. The molecular weight of the polymers can be explained on the basis of a chemical structure and the functional group involved in the ROP of CL and THF. In the case of Flur, the –OH group was involved in the ROP of CL whereas the secondary amino group was involved in the ROP of CL for R6G. Moreover, the NH group was surrounded by the electron releasing methyl groups and hence the hydrogen transfer is a difficult one. As a result, the R6G-based dye grafted polymer yielded a polymer with lower molecular weight. Moreover, the R6G has a steric effect because of ethyl ester and –NH–CH2–CH3 groups. Due to the electron releasing nature of methyl or ethyl groups, the hydrogen transfer reaction is restricted to some extent. Therefore, the R6G dye-based initiator produced a somewhat low molecular weight polymer.
3.8 SEM and FE-SEM Study
The surface morphology of the P1 sample is illustrated in Fig. 9a. It seems to be like a dry-sky with some microvoids [38]. These voids can be accommodated by drug molecules while loading the sample with drugs. Hence, it proved that PCL is an ideal candidate for drug release applications. The FE-SEM micrograph of the P2 system is demonstrated in Fig. 9b. The morphology looks like a dried sky with some NPs, and this can be explained as follows. The formation of polymer NPs at the interface of the polymers is due to the combination of hydrophilic PTHF and hydrophobic PCL. The polymer NPs are the key elements for the active drug release process. The size of the particles was estimated as 50–80 nm. Figure 9c illustrates the SEM micrograph of the P3 system. The surface texture looks like a broken stone [38] with spherical particles of size about 800 nm. Here also, one can see the various structures like microvoids and micro rods. The presence of microvoids is very much useful for drug-carrying purposes. The surface texture of the P4 sample is analyzed using the FE-SEM image (Fig. 9d). It exhibited a broken stone-like texture with the homogeneously dispersed spherical NP of size approximately 90 nm. The formation of polymer NP at the interface is due to the interaction of hydrophobic and hydrophilic segments. Here the dye molecule acts as a hydrophilic region whereas the PCL acts as a hydrophobic region. In their interface region, there will be a formation of polymer NP.
3.9 Mechanism of ROP Reaction
The ROP of CL occurred via co-ordination-insertion mechanism [27, 28, 38]. In order to facilitate the ROP reaction, the hydrogen transfer reaction from the functional group must be an easy one. This depends on the nature of the chain extension group, resonance stabilization and the nature of substituents present on the phenyl ring. Flur dye consists of one aromatic phenolic group, one carboxyl group and one quinone group whereas the R6G has one secondary amino group, one ethyl ester group and one quaternary ammonium ion. Among them, the –NH is generally involved in the ROP of CL. Already our research team [33] proved that the –NH2 or –NH– or –SH groups are less favorable towards the ROP of CL. The ROP reaction occurs at 160 °C under N2 atmosphere with the mild stirring condition. At this high temperature, the oxidation reaction is possible in the presence of SO catalyst. The –OH and –NH groups are stable at the given experimental conditions but the ether linkage present in the middle ring is not stable due to the influence of the oxidized quinone ring of Flur dye. The oxidation of quaternary ammonium ion is not possible for R6G. Therefore, the structure of R6G was not degraded during the ROP reaction. Anbarasan and his research team [39] reported that the conjugation length was reduced due to the chemical grafting of PCL onto the eosin blue dye backbone. The present result is also in accordance with the literature report [39].
4 Conclusions
The FT-IR spectra revealed a doublet peak at 1730 cm−1 due to carbonyl stretching of PCL and PAH, which proved the formation of the diblock copolymer. The NMR study confirmed the chemical structure of the homopolymers. The absorption spectra of P1 and P3 systems were highly depressed due to the degradation of the Flur dye. The Tm of the diblock copolymer is lower than a homopolymer owing to the hydrophilic behavior of PTHF units. The diblock copolymer has a lower Td value than the homopolymer. Both the initiator systems exhibited a high molecular weight for the prepared diblock copolymers rather than the homopolymer. The FE-SEM image indicated the formation of polymer NPs during the diblock copolymer formation. This study proved that the xanthene type dyes are not suitable for the ROP of CL at 160 °C under a nitrogen atmosphere. At the same time, the R6G dye is an excellent candidate for the ROP of CL and THF under the same experimental conditions. In the future, the authors are going to investigate the detection of cancer cells with the help of fluorescent probe such as PCL-dye-PTHF diblock copolymer. PCL and PTHF are bio-degradable polymers, and the dye can also be induced the degradation of polymer chains.
References
J. Yang, S.W. Yaog, H.S. Park, K.H. Lee, N.H. Hur, J. Appl. Polym. Sci. 136, 47999 (2019)
Y. Kohsaka, N. Nagatsuka, Polym. J. 52, 75 (2020)
K. Nomura, K. Suzuki, S. Katao, Y. Matsumoto, Organomet. 31, 5114 (2012)
S. Kobayashi, H. Danda, T. Saegusa, Bull. Chem. Soc. Jap. 46, 33214 (1973)
X. Zhang, X. Fan, X. Zhou, Asian J. Chem. 26, 6391 (2014)
R. Bodulla, S.A. Palaniappan, Curr. Green Chem. 3, 346 (2020)
F. Marchetti, G. Pampaloni, T. Repo, Eur. J. Inorg. Chem. 12, 2107 (2008)
A. Moumen, Z. Hattab, N. Rebbani, Bull. Chem. React. Eng. Catal. 14, 294 (2019)
M.E. Woodhouse, F.D. Levis, T.J. Marks, J. Am. Chem. Soc. 104, 5586 (1982)
G.A. Olah, O. Farooq, G.H. Li, A. Akklonis, J. Appl. Polym. Sci. 45, 9 (1992)
S. Kailash, B. Meenarathi, R. Anbarasan, J. Polym. Res. 27, 355 (2020)
S. Snigdha, M. Rahul, N. Kalarikal, S. Thomas, E.K. Radhakrishnan, J. Inorg. Organomet. Polym. Mater. 29, 1503 (2019)
M. Bagheri, A. Mahmoodzadeh, J. Inorg. Organomet. Polym. Mater. 30, 1566 (2020)
D.S. Dlamini, A.K. Mishra, B.B. Mamba, J. Inorg. Organomet. Polym. Mater. 22, 342 (2012)
D.S. Dlamini, S.B. Mishra, A.K. Mishra, B.B. Mamba, J. Inorg. Organomet. Polym. Mater. 21, 229 (2011)
T. Carvalhoa, R.M. Moraesa, G.M. Alvesa, F. Medeino, Int. J. Biol. Macromol. 145, 701 (2020)
J. Liu, J. Wet, Y. Li, J. Biomater. Sci. Polym. Ed. 31, 1421 (2020)
R. Jeyakumar, H. Tamura, Int. J. Biol. Macromol. 43, 32 (2008)
F. Benhacine, A. Ouargh, A.S. Hadjtamel, Polym. Plast. Technol. Mater. 58, 328 (2019)
G. Wang, R. Ma, T. Chen, C. Yan, F. Bao, Polym. Plast. Technol. Mater. 52, 1193 (2013)
I.K. Park, H. Sun, S.H. Kim, G.E. Kim, H.R. Chen, Sci. Rep. 9, 7033 (2019)
E. Oledjka, K. Sokolowski, M. Sobczab, W. Kolodziejski, Polym. Int. 60, 787 (2011)
G.S. Wang, L. Wang, Z.Y. Wei, L. Sang, W.X. Zhang, Chin. J. Polym. Sci. 31, 1011 (2013)
D. Wu, Y. Lv, R. Guo, R. Lu, H. Wang, Z. Wei, Macromol. Res. 25, 1070 (2017)
J. Liu, L. Liu, Macromolecules 37, 2674 (2004)
H. Lonnberg, Q. Zhou, H. Brusser, T.T. Teen, E. Malmstrom, A. Hult, Biomacromol 7, 2178 (2006)
M. Jeyapriya, B. Meenarathi, R. Anbarasan, Polym. Bull. 77, 2631 (2020)
R. Anbarasan, L. Kannammal, B. Meenarathi, Polym. Bull. 76, 5381 (2019)
R. Anbarasan, V. Kohila, B. Meenarathi, G. Jeyalakshmi, A. Jancirani, S.N. Appl, Sci. 1, 602 (2019)
K. Agathian, L. Kannammal, B. Meenarathi, S. Kailash, R. Anbarasan, Int. J. Biol. Macromol. 107, 1102 (2018)
M. Thomas, Ann. Thorac. Surf. 97, 27 (2014)
E.A. Hermosilla, D. Muñoz, S. Orellana, A. Yáñez, A.F. Olea, F.O. Ampuero, I.M. Villoslad, React. Funct. Polym. 81, 14 (2014)
A. Sivabalan, R. Hariharasubramani, B. Meenarathi, S. Palanikumar, R. Anbarasan, Int. J. Sci. Res. Eng. Technol. 1, 9 (2014)
A.V. Ragu, G.S. Gadaginamata, N. Mallikarjuna, T.M. Aminabhavi, J. Appl. Polym. Sci. 100, 576 (2006)
A.V. Ragu, G.S. Gadaginamata, N. Mallikarjuna, T.M. Aminabhavi, J. Appl. Polym. Sci. 98, 2236 (2005)
R. Sjoback, J. Nygren, M. Kubist, Spectrochim Acta Part A Mol. Biomol. Spect. 51, l7 (1995)
P. Abinayasri, M. Nageswari, B. Meenarathi, R. Anbarasan, Bull. Mater. Sci. 40, 591 (2017)
B. Rajkumar, T. Dhanalakshmi, B. Meenarathi, R. Anbarasan, Polym. Bull. 73, 2147 (2016)
R. Anbarasan, B. Meenarathi, P. Senthilkumar, V. Parthasarathy, J. Macromol. Sci. Part A Pure Appl. Chem. (2020). https://doi.org/10.1080/10601325.2020.1866435
Acknowledgement
We express our sincere thanks to Dr. N. Sundararajan, Associate Professor, Department of English, KCET, Madurai for his valuable help during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Anbarasan, R., Meenarathi, B. & Parthasarathy, V. Structural and Thermal Studies of Fluorescein and Rhodamin6G Grafted Diblock Copolymers. J Inorg Organomet Polym 31, 3549–3561 (2021). https://doi.org/10.1007/s10904-021-01978-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-021-01978-3