Abstract
In the present investigation, a series of (polyvinyl pyrrolidone/acrylic acid) (PVP/AAc) hydrogels were synthesized using gamma irradiation as super clean source for polymerization and crosslinking. Silver nanoparticles were deposited within (PVP/AAc) hydrogels as supporting matrices by means of in situ reduction of silver nitrate (AgNO3) as Ag+ ions precursor using sodium borohydride (NaBH4) as a reducing agent. UV–Vis spectroscopy and TEM image analysis confirmed the nanoscale size of the Ag° nanoparticles (NPs). (PVP/AAc)-Ag° nanocomposites were systematically characterized using XRD, EDX, and TGA techniques. The presence of Ag NPs increases the thermal stability of the obtained nanocomposite as confirmed by TGA studies. The developed nanocomposites show enhanced catalytic activity toward the reduction of 4-Nitrophenol as a model of hazardous anthropogenic materials in the presence of NaBH4 as a reducing agent. The catalytic performance proceeds with conversion yield exceeding 99% almost within 5 min depending on the amount of the loaded Ag NPs. Additionally, (PVP/AAc)-Ag° nanocomposites show efficient antimicrobial activity against different microbial strains which suggesting their use as potential disinfection during waste water treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Environmental pollution is the most global menace facing humanity and other life forms on our planet today. Since eighteenth century with the emergence of industrial revolution, anthropogenic pollution began to be visible due to the replacement of physical energy and start using machines in the industries [1]. Anthropogenic pollution has contributed to the environmental burden since they learned to control fire and smelt metals. Many anthropogenic contaminants such as industrial organics, pesticides, and trace metals have become widely distributed around the globe [2].
Nitrophenols are a class of anthropogenic; they are toxic, inhibitory and biorefractory organic compounds used extensively in the production of explosives, agrochemicals, dyes and pharmaceuticals [3]. In particular, 4-Nitrophenol (4NP) is a toxic derivative of the parathion insecticide and fungicide agent [4]. It considered hazardous materials and priority toxic pollutants by US Environmental Protection Agency (EPA) [5]. A short-term inhalation of 4NP causes disturbing symptoms such as headaches, drowsiness, nausea, and cyanosis in human. From the bright side, 4NP is a prevalent precursor for 4-aminophenol (4AP) synthesis which is a potent intermediate for the manufacture of many analgesic and antipyretic drugs. Also, it can be used as photographic developer, drying agent, corrosion inhibitor, anticorrosion lubricant, and hair-dyeing agent [6].
Catalytic reduction of (4NP) to profitable (4AP) is considered to be the most efficient economical approach for environmental remediation and resources regeneration [7]. Among different strategies, applying metal nanoparticles as efficient catalysts for the reduction of 4NP into 4AP assisted by sodium borohydride (NaBH4) as a reducing agent seems promising due to their high surface to volume ratio, unique electronic and surface properties, ease of fabrication and selectivity for specific reactions [8]. Moreover, using metal nanoparticles as a catalyst lowers the reduction potential and facilitates the electron transfer reactions [9]. Of all metal nanoparticles, silver nanoparticles (Ag NPs) attracted more attention in industrial applications. As a catalyst, Ag NPs act as mediator for electron transfer between organic pollutants and NaBH4 molecules. However, the low stability and difficulty of separation of the nano materials from the reaction medium are among the common obstacle involves nanoparticles applications as catalysts [10].
Recently, nanocomposite hydrogels in which the inorganic nanoparticles have been immobilized onto the hydrogel matrix have gained much attention [11]. Immobilization of NPs onto hydrogel matrices as polymeric supports could significantly enhance the stability and the activity of the NPs as a catalyst. Nanocomposite hydrogels are 3D crosslinked polymeric networks containing nanoparticles have the ability to absorb, swell and retain large amount of water and solutes in their crosslinked networks. They possess distinct superior properties such as enhanced swellability, more elasticity, high strength, good modulus and high heat resistance [12].
Nanocomposite hydrogels contained inorganic moieties are promising candidates to inhibit microbial growth, making them attractive in biomedical, environment and biotechnological fields [13]. Ag° nanocomposites are effective biocides against numerous kinds of bacteria, fungi, and viruses by releasing Ag+ that can inactivate the microorganism cells by destroying the cell membrane and replication ability of DNA [14, 15]. Due to their promising antibacterial capability, Ag NPs are often used as pharmaceutical gents, antiseptic, and disinfectant [16].
The proper choice of appropriate polymers and monomers used during the hydrogel synthesis is a milestone step. Poly acrylic acid- based hydrogels and nanocomposites have been introduced as one of the most efficient matrices for the production of homogenously stable Ag NPs. In addition, they control the size and shape of the produced AgNPs in the presence of reducing agent as well as their release at a site of action [17].
Herein, attempts have been made to fabricate a series of polyvinyl pyrrolidone and acrylic acid hydrogels (PVP/AAc) using γ-irradiation as clean source for initiation and crosslinking. Ag NPs were immobilized onto (PVP/AAc) as supporting matrices using in situ reduction method. The applicability of (PVP/AAc)-Ag° nanocomposites for the efficient reduction of (4NP) into nontoxic (4AP) using NaBH4 as well as their antimicrobial potential was demonstrated. Furthermore, the reusability and the corresponding kinetic and thermodynamic properties were also investigated.
2 Materials and Methods
2.1 Materials
Polyvinylpyrrolidone (PVP) of M.Wt. 1,300,000 (Acros, Belgium) and acrylic acid (AAc) of purity 99.9% (Merck, Germany) were used as received. 4NP and 4-amino phenol (4AP) were purchased from Sigma-Aldrich. Sliver nitrate (AgNO3; 99.0%) (Gamma, Laboratory Chemical), and Sodium borohydride, (NaBH4; 97%) (Loba Chemie). All other chemicals used were purchased from El-Gomhouria Co., Egypt and used as received.
2.2 Radiation Synthesis of (PVP/AAc) Hydrogels
A series of (PVP/AAc) hydrogels were prepared using radiation-induced copolymerization of an aqueous mixture from PVP and AAc of different concentration and compositions in wt%. Mixtures were mixed well and irradiated at 20 and 30 kGy in small vials using 60Co gamma rays at dose rate of 2 kGy/h. After copolymerization process, the obtained cylindrical hydrogels were cut into disks of 2 mm thickness and 5 mm diameter. All samples were washed in excess water to remove the unreacted component and air-dried at room temperature up to constant weight.
2.3 Preparation of (PVP/AAc)-Ag° Nanocomposites
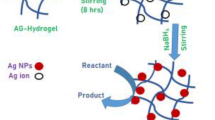
In situ reduction method was used to prepare (PVP/AAc)-Ag° nanocomposites. A known weight of the dried (PVP/AAc) (20:80) hydrogels were immersed in AgNO3 solution of different concentrations (0, 25, 50, 125 and 250 mmol/L) (named as: PAg0, PAg1, PAg2, PAg3 and PAg4, respectively) and allowed to equilibrate for 24 h. The Ag+ loaded hydrogels were washed using distilled water to remove the unbound Ag+ ions. Finally, they were transferred into a beaker containing 50 mL of 0.1 M NaBH4 and kept for 5 h to achieve complete reduction of Ag+ ions. The homogenous brown colour of the obtained Ag°-nanocomposites confirms the formation of Ag NPs along the (PVP/AAc) hydrogel network. The obtained (PVP/AAc)-Ag° nanocomposites were washed with distilled water then air-dried at room temperature and kept for further investigation.
2.4 Gel Fraction
(PVP/AAc) hydrogels and their Ag° nanocomposites were soaked in distilled water at 80 °C for 24 h to remove the sol fraction. Then after, samples were washed, re-dried to constant weight. The gel fraction was determined using the following equation:
where Wb is the initial weight before extraction and Wa is the dry weight after extraction.
2.5 Swelling Degree
Pre-weighed dried (PVP/AAc) hydrogels and their Ag° nanocomposites samples were allowed to swell in buffer solution of known pH values at room temperature until constant weight was reached. The swelling degree (S%) was calculated according to the following equation:
where Ws and Wd are the weights of the swollen and the dried samples, respectively.
2.6 Characterization of (PVP/AAc)-Ag° Nanocomposites
UV–Vis absorption spectrum of the extracted Ag NPs was measured at room temperature on a spectrophotometer model UV-Analytic Jena AG specord 210 plus (Germany) using quartz cuvette over a 250–700 nm wavelength. TEM examination of the extracted Ag NPs was performed using a transmission electron microscope (TEM) JEOL: JEM-100cx, Japan. X-ray diffraction (XRD) measurements were performed using XRD 6000 diffractometer with Cu target. The XRD runs were carried out over 2θ ranging from 10° to 80° at a scan speed of 8°/min. EDX analysis was carried out using energy dispersive X-ray unit microprobe (Oxford, England-ISIS). Thermal stability evaluation was studied using Shimadzu TGA system of type TGA-30 under nitrogen atmosphere (20 ml/min) at temperature range from ambient to 600 °C at a heating rate of 10 °C/min.
2.7 Catalytic Activity Evaluation
Briefly, 0.1 g of (PVP/AAc)-Ag° nanocomposites was added to an aqueous solution contain 4NP and NaBH4 of different concentrations and at different temperature. The vanish of the yellow color characteristic for 4NP that appeared at 416 nm is accompanied by synchronous appearance of a new peak at 350 nm which is characterized for 4AP. For the reusability experiment, (PVP/AAc)-Ago nanocomposites were collected and washed several times with deionized water, and then reused.
2.8 Antimicrobial Activity Evaluation
Staphylococcus aureus (S. aureus), Escherichia coli (E. coli) and Candida albicans (C. albicans) as representatives of (Gram-positive), (Gram-negative) bacteria and fungi, respectively; were previously isolated and characterized at Microbiology Department, NCRRT. Cultures were grown on nutrient agar (NA) plates for S. aureus and E. coli and Sabouraud-Dextrose agar (SDA) for Candida albicans and maintained in the agar slants at 4 °C. The antimicrobial activity was assayed using disc diffusion technique. (PVP/AAc) and its Ag° nanocomposites swollen disks were placed in intimate contact onto the surface of nutrient agar plates previously smeared with freshly prepared bacterial solution. After incubation for 24 h at 37 °C, the diameter of the clear zone of inhibition underneath and along the side of swollen samples was determined visually (in mm).
3 Results and Discussion
3.1 Radiation Synthesis of (PVP/AAc) Hydrogels
Hydrogels are crosslinkable hydrophilic 3D network structure have the capability to absorb, swell and retain a large amount of different fluids. Gamma ray as an eco-friendliness ionizing radiation seems a proficient tool for hydrogels synthesis. Subjecting polymers and/or monomer aqueous solution into ionizing radiation generates radicals on the monomer and polymer in addition to the production of ·OH and H· radicals as the primary products of water radiolysis. The recombination of such macroradicals "crosslinking" yielding gel-like materials is called "gelation process". There are many factors affecting the gelation degree such as, feed solution concentration and compositions as well as the irradiation doses.
3.1.1 Effect of Feed Solution Concentration and Irradiation Dose
Table 1 shows the effect of initial feed solution total concentration on the gelation degree of (PVP/AAc) hydrogels prepared at 20 and 30 kGy. It can be observed that the gelation degree increased with the increase of the total concentration of the initial feed solution as well as the irradiation dose. At higher polymer/monomer concentration and by increasing the exposure dose, a higher number of free radicals are produced and the increased probability for more cross-links resulted in formation of high crosslinked network structure thence higher gelation degree for the developed (PVP/AAc) hydrogels [18].
3.1.2 Effect of Feed Solution Composition
Table 2 shows the effect of AAc content in the (PVP/AAc) feed solution on the gelation degree of the obtained hydrogels. It is clear that the gelation degree of (PVP/AAc) hydrogels increases with the increase of AAc content in the feed solution as well as irradiation dose to reach a maximum value ~ 99% at (30)kGy. The higher gelation degree could be attributed to the high tendency of AAc, as a vinyl monomer, for copolymerization and crosslinking under the investigated irradiation doses. The higher gelation degree of the prepared (PVP/AAc) hydrogels makes them used safely.
3.2 Synthesis and Characterization of (PVP/AAc)-Ag° Nanocomposites
Template hosting of silver cations (Ag+) along the swollen (PVP/AAc) hydrogel network followed by reduction using suitable reducing agent insure the homogenous dispersion of the entrapped Ag NPs and decrease the aggregates formation along the hydrogel network. Importantly, polymeric chains as a host template play a significant role in the reduction of the nanoparticle size with a narrow size distribution and well defined shape [19]. During the immersion of the (PVP/AAc) hydrogel matrices in the AgNO3 solution as (Ag+) precursors, the difference in the concentration between the precursor solution and the interior of the hydrogel network results in the diffusion of Ag+ ions into the hydrogel interior. The electrostatic interactions between the chelated Ag+ ions by the PAAc carboxylate anions (COO−) facilitates the uniform anchoring of Ag+ ions along the hydrogel network. Upon using NaBH4 as a reducing agent, Ag+ ions in the swollen hydrogel matrix were reduced to Ag° as can be described by the following equation:
Finally, Ag° aggregates results in atomic cluster–cluster formation because of the metal bonds. The pore size and hydrogel porous construction is responsible for dimension such atoms integration or nucleation [20].
3.2.1 UV–Vis Spectroscopy
The dark brown visual color change of the (PVP/AAc) hydrogels loaded Ag + ions after the addition of NaBH4 indicates the formation of Ag NPs within the hydrogel matrices. This was confirmed by the UV–Visible absorption spectrum of Ag NPs extracted from (PVP/AAc)-Ago nanocomposite that illustrated in Fig. 1. A typical absorbance maxima appeared at (410 nm) which is characteristic for surface Plasmon resonance (SPR) corresponding to Ag NPs. Such SPR band may be due to the collective oscillation or vibration of free electrons in the conduction band after excitation by incident light of particular wavelength [2, 21].
3.2.2 Transmission Electron Microscopy (TEM)
The nano-size of the Ag° nanoparticle immobilized in (PVP/AAc) hydrogel matrix was confirmed using TEM analysis as shown in Fig. 2. The micrograph of the extracted Ag° nanoparticles appeared as dark mostly spherical nanoparticles with an approximate average size of (7–10 nm).
3.2.3 X-ray Diffraction (XRD)
The XRD pattern for (PVP/AAc)-Ago (PAg4) nanocomposite hydrogel is shown in Fig. 3. A broad band appeared between 2θ = 10° and 30° is related to the amorphous nature of the (PVP/AAc) hydrogel. Four well-defined characteristic diffraction peaks appeared at 2θ = 38.28°, 44.45°, 64.35° and 77.42° corresponds to (111), (200), (220), and (311) planes of the face-centered cubic (fcc) Ag crystals, respectively [22]. The obtained XRD result confirmed the successful conversion of Ag + ions into metallic Ag during the in-situ reduction step.
3.2.4 Thermogravimetric analysis (TGA)
Figure 4 illustrates the thermal stability as well as the amount of nanoparticles loaded to the of (PVP/AAc)-Ag° nanocomposite hydrogels compared to its (PVP/AAc) parent hydrogel. As it can be seen, for (PVP/AAc) parent hydrogel (PAg0), the initial weight loss was in the range of 50–125 °C which may be corresponding to the evaporation of physically adsorbed water. The major weight loss occurred within the temperature range 250–470 °C which is probably due to the thermal decomposition of the polymeric chains of the (PVP/AAc) hydrogel network. Whereas, (PVP/AAc)-Ag° nanocomposites displayed the highest thermal stability with the lowest weight loss that decrease with increasing the immobilized Ag NPs content. The first weight-loss of below 150 °C which corresponds to the loss in moisture trapped within their network. At this stage, Ag NPs loaded nanocomposites undergo more weight loss as compared to their parent hydrogel and this may be due to the increased moisture content that decreased by increasing Ag NPs content. Thereafter, it is noticed that (PVP/AAc)-Ag° nanocomposites are more thermally stable. Such increase may be attributed to the relatively high thermal stability of Ag NPs. The presence of Ag NPs as metallic inorganic filler within the hydrogel network chains may restrict their mobility results in enhancement of their thermal stability [23, 24]. After the final thermal decomposition (at 600 °C), the percentage weight residual for the (PVP/AAc)-Ag° nanocomposites is typically related to the amount of the loaded nanoparticles within the hydrogel network. The percentage of the loaded Ag NPs of the (PVP-AAc)-Ag° nanocomposites was found to be 19, 26, 42 and 48 (wt%) for PAg1, PAg2, PAg3 and PAg4, respectively. The higher amount of Ag NPs in (PVP/AAc)-Ag° nanocomposites was attributed to more Ag ions chelated to the PAAc (–COOH) groups along the hydrogel network.
3.2.5 Energy Dispersive X-ray Spectroscopy (EDX)
EDX analysis was employed to determine the purity constitution of the silver nanoparticles dispersed in (PVP/AAc)-Ag° nanocomposites. As can be seen from Fig. 5, the presence of high intense absorption peak at 3 keV revealed a typical characteristic of nanocrystalline silver [25].
3.2.6 Swelling Behavior
pH-dependent equilibrium swelling behavior of (PVP/AAc)-Ag° nanocomposites with different Ag NPs contents in comparison with their parent (PVP/AAc) hydrogel is illustrated in Fig. 6. As is clear, all the investigated samples show a pH-dependent swelling attitude and the swelling ability greatly influenced by the presence of Ag NPs. The swelling ratio increases with the increase of the pH value. At pH values lower than the dissociation constant of –COOH groups of the PAAc moieties(pKa > 4), the hydrogen bonding between the completely associated –COOH groups results in a restriction in the chain movement or relaxation of the gel network chains which consequently leads to collapsed network and the swelling degree is relatively very low.
A sudden change in the swelling degree was observed around pH 4, whereas the maximum extent of swelling was reached at pH 7 due to the complete dissociation of the acidic groups of PAAc. Dissociation of the carboxylic groups breaks hydrogen bonding and forming carboxylate ions. The electrostatic repulsion between the negatively charged carboxylate groups would results in the expansion of the network structure which consequently results in penetration of more water molecules either by hydration or within the free spaces formed along the network [26].
Also, at any pH value exceeds the dissociation constant of –COOH groups, the swelling degrees of (PVP/AAc)-Ag° nanocomposites are higher than that of their parent (PVP/AAc) hydrogel. It is clear that the swelling degree increases with increasing of the Ag NPs loaded content. The presence of Ag NPs affords more free spaces in the hydrogel network which in turn facilitates the penetration of more water molecules [27, 28].
3.3 Anti-microbial Potency
Disinfection of potable water and wastewater provides a degree of protection against the transmission of for waterborne diseases. Ag°-nanocomposites are efficiently playing a crucial role as antimicrobial agents for water purification [29]. The potency of Ag NPs as antimicrobial agent is mainly due to its large surface areas per volume ratio and high reactivity compared with the bulk solid. The proposed mechanism for the antimicrobial activity of Ag NPs may be due to: (1) damage cell membrane and intracellular components [30], (2) silver ions released from Ag NPs can be absorbed into the cell wall and cause lysis and death [31], and (3) the generation of highly reactive oxygen species (ROS) such as OH−, H2O2 and O2− on the surface of Ag NPs [32]. The antibacterial and antifungal efficiency of the (PVP/AAc)-Ag° nanocomposites were evaluated using disc diffusion assays against S. aureus, E. coli and C. albicans as representatives of (Gram-positive), (Gram-negative) bacteria and fungi, respectively under in vitro conditions, and the average inhibition zones were presented in Fig. 7. As is clear, the zone of inhibition obtained against the tested microbes clearly depicts the potent efficiency of the synthesized of (PVP/AAc)-Ag° nanocomposites. Such antimicrobial efficiency was enhanced by increasing Ag NPs content of the nanocomposite hydrogels.
3.4 Catalytic Reduction Performance of (PVP/AAc)-Ag° Nanocomposites
The catalytic performance of (PVP/AAc)-Ag° nanocomposites was evaluated using 4NP as one of the anthropogenic harmful pollutants. Despite of the reduction of 4NP to 4Ap using NaBH4 is thermodynamically favorable but it is kinetically limited. The potential difference between NaBH4 as electron donor molecules and 4NP as electron acceptor molecules reduce the probability of such reduction process [33]. Using Ag NPs as a catalyst can contribute to overcoming such kinetic barrier. Ag NPs catalyze this reaction by facilitating electron relay from the donor (BH4)− to the acceptor 4NP to overcome the kinetic barrier [2, 34].
3.4.1 Effect of Ag NPs Content
The catalytic reduction of 4NP into 4AP proceeds through the formation of intermediate 4-nitrophenolate ions. Since the absorption peak of 4NP underwent a red shift from 350 to 416 nm in the presence of NaBH4 as can be seen from Fig. 8, the change in the absorption spectra of 4-nitrophenolate ion at 416 nm was monitored to track the reaction process.
As can be seen from Fig. 9a–d, upon the addition of 0.1 g (PVP/AAc)-Ag° nanocomposites, a noticeable rapid decrease in the 4-nitrophenolate ion absorption peak at (416 nm) with a concomitant appearance of a new band at 296 nm, corresponding to the formation of 4AP in the solution. The reaction proceeded with conversion yield exceeding 99.8% accompanied by colour change from deep yellow into colorless and the entire reaction was completed within 23, 5, 4, and 5 min for PAg1, PAg2, PAg3 and PAg4 nanocomposite, respectively.
Figure 10 shows a correlation between ln(A) (A is the absorbance at 416 nm) against the reduction time (t) per seconds for (PVP/AAc)-Ag° nanocomposites of different Ag° contents. It is observed that all catalytic systems show linear correlations which confirm that they followed pseudo first order reaction kinetics. The reaction rate constant (K) was determined from the slope was found to be 0.0187, 0.0219, 0.0251, and 0.0246 s−1 for PAg1, PAg2, PAg3 and PAg4 nanocomposites, respectively. These results mean that the catalytic activity of (PVP/AAc)-Ag° nanocomposite increased with the increase of the Ag NPs content. However, at higher Ag NPs content, PAg4 (48%), a slight decrease in the reactivity was observed. This may be due to the aggregation of Ag NPs along the network matrix results in Ag NPs surface activity reduction. The higher activity of the (PVP/AAc)-Ag° nanocomposites arises from the swelling characteristics of the polymer matrix leading to highly efficient contact between the interactive molecules. Such swelling behavior provides a high concentration of 4NP near to the Ag NPs immobilized on the (PVP/AAc) hydrogel network leading to highly efficient contact between them [35].
Correlation between ln(A) (A is the absorbance at 416 nm) against the reduction time(t) per seconds for (PVP/AAc)-Ag° nanocomposite of different loaded Ag content (wt%); (filled diamond) PAg1(19), (filled square) PAg2(26), (filled circle) PAg3(42) and (filled inverted triangle) PAg4(48), respectively using 50 mL (0.7 mmol/L) 4NP, NaBH4 ( 0.16 mol/L) at 30 °C
3.4.2 Effect of 4NP and NaBH4 Concentration
Using PAg3 nanocomposite sample (42% Ag NPs content), a correlation between the rate constant and the concentration of either 4NP and NaBH4 was illustrated in Fig. 11. As is clear, the catalytic reaction rate of 4NP significantly increased with increasing NaBH4 concentration. Whereas, an obvious decreases in the rate constant is observed by the increase of 4NP concentration. The increase in 4NP concentration results in its accumulation on the surface of (PVP/AAc)-Ag° nanocomposite results in surface poisoning which in turn slowing down the reaction rate.
3.4.3 Effect of Temperature
The effect of temperature on the catalytic reduction of 4NP was studied at various temperatures and the rate constant was calculated as shown in Table 3. As illustrated, the rate constants of the reaction increased by increasing the temperature of the catalytic reduction processes. This observation can be explained in terms of collision theory, i.e. particles not at 0 K tend to move around and therefore collide which increase reaction probabilities [36]. The higher the temperature, the faster the collision of these particles and hence the reaction will occur faster. The activation parameters can be calculated either Arrhenius Equation (1) or Eyring Equation (2)
where Ea is the activation energy, A is the Arrhenius factor, T is absolute temperature, kB is the Boltzmann constant, h is the Planck constant, ΔH*is activation enthalpy, ΔS* is activation entropy, and R is the ideal gas constant. The value of activation energy (Ea) was found to be 33.635 kJ/mol as calculated from the plot of lnk against 1/T with slope (− Ea/R) as shown in Fig. 12. The values of ΔH* and ΔS* were calculated from the slope and intercept of the plot of ln(k/T) against 1/T, respectively, where ΔH* was found to be 36.275 kJ/mol and of ΔS* was − 385.790 J/k/mol.
3.5 Reusability of (PVP/AAc)-Ag° Nanocomposites
The reusability of (PVP/AAc)-Ag° nanocomposite (PAg3) for the catalytic reduction of 4NP was investigated under the optimized reaction conditions four times repeatedly. As shown in Fig. 13, there is no significant decrease in the conversion percent after reuse for 4 cycles whereas; the activity was reduced to 85% after the fourth use. The activity is calculated based on the reduction of the reaction rate relative to the initial rate for each use. The obtained data revealed the relative stability of the (PVP/AAc)-Ag° nanocomposites.
4 Conclusion
(PVP/AAc) hydrogels were successfully prepared by gamma radiation then after were used for the in situ preparation of Ag° nanocomposites using silver nitrate as (Ag+) ion precursor and NaBH4 as a reducing agent. TEM image confirmed the nanoscale size of the Ag NPs. The XRD and EDX analysis studies affirm the hosting of Ag° along the (PVP/AAc) network constructer. Enhanced thermal stability was observed due to presence of Ag NPs as indicated by TGA studies. (PVP/AAc)-Ag° nanocomposites shows pH-dependent swelling where the swelling ratio increases with the increase of the Ag NPs content. The antimicrobial activity depicts the potent efficiency of the (PVP/AAc)-Ag° nanocomposites that enhanced by increasing the Ag NPs content. The catalytic reduction activity of (PVP/AAc)-Ag° nanocomposites against 4NP using excess NaBH4 increased with the increase of the loaded Ag NPs. The rate of the reduction reaction increased with the increase of temperature and decrease with the increase of 4NP. The values of Ea, ∆H, and ∆S were found to be 33.635 kJ/mol, 36.275 kJ/mol, and − 385.790 J/k/mol, respectively. The nanocomposites showed efficient reusability for four times, the conversion percent almost unchanged while the activity reduced to 85% in the fourth usage.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
M. Bilal, T. Rasheed, H.M.N. Iqbal, Y. Yan, Sci. Total Environ. 644, 1–13 (2018)
G. Arya, N. Sharma, J. Ahmed, N. Gupta, A. Kumar, R. Chandra, S. Nimesh, J. Photochem. Photobiol. B 174, 90–96 (2017)
A. Al Kahtani, T. Almuqati, N. Haqbani, T. Ahamad, M. Nausha, S. Alshehri, J. Clean. Prod. 191, 429–435 (2018)
A. Kumar, M. Belwal, R. Maurya, V. Mohan, V. Vishwanathan, Mater. Sci. Energy Technol. 2, 526–531 (2019)
N. San, A. Hatipoğlu , G. Koçtürk, Z. Cinar, J. Photochem. Photobiol. A 146(3), 189–197 (2002)
W. Shen, Y. Qu, X. Pei, S. Li, S. You, J. Wang, Z. Zhang, J. Zhou, J. Hazard. Mater. 321, 299–306 (2016).
Y.S. Seo, E.-Y. Ahn, J. Park, T.Y. Kim, J.E. Hong, K. Kim, Y. Park, Y. Park, Nanoscale Res. Lett. 12, 7 (2017)
K. Zhang, J.M. Suh, J.-W. Choi, H.W. Jang, M. Shokouhimehr, R.S. Varma, ACS Omega 4, 483–495 (2019)
C. Kästner, A.F. Thünemann, Langmuir 32, 7383–7391 (2016)
H. Karki, D. Ojha, M. Joshi, H. Kim, Appl. Surf. Sci. 435, 599–608 (2017)
P. Thoniyot, M.J. Tan, A.A. Karim, D.J. Young, X.J. Loh, Adv. Sci. 2, 1400010 (2015)
G. Sharma, B. Thakur, M. Naushad, A. Kumar, F. Stadler, S. Alfadul, G. Mola, Environ. Chem. Lett. 16(1), 34–146 (2018)
R.F.N. Quadrado, G. Gohlke, R.S. Oliboni, A. Smaniotto, A.R. Fajardo, J. Ind. Eng. Chem. 79, 326–337 (2019)
W.K. Jung, H.C. Koo, K.W. Kim, S. Shin, S.H. Kim, Y.H. Park, Appl. Environ. Microbiol. 74, 2171–2178 (2008)
Q.L. Feng, J. Wu, G.Q. Chen, F.Z. Cui, T.N. Kim, J.O. Kim, J. Biomed. Mater. Res. 52, 662–668 (2000)
V.A. Oyanedel-Craver, J.A. Smith, Environ. Sci. Technol. 42, 927–933 (2008)
A. Raafat, G. Mahmoud, A. El-Hag Ali, N. Badawy, M. Elshahawy, J. Bioact. Compat. Polym. 33, 088391151771066 (2017)
A. El-HagAli, H. Shawky, H. Rehim, E.-S. Hegazy, Eur. Polym. J. 39, 2337–2344 (2003)
P. Dallas, V.K. Sharma, R. Zboril, Adv. Colloid Interface Sci. 166, 119–135 (2011)
L. Mulfinger, S.D. Solomon, M. Bahadory, A.V. Jeyarajasingam, S.A. Rutkowsky, C. Boritz, J. Chem. Educ. 84, 322 (2007)
V. Thomas, M.M. Yallapu, B. Sreedhar, S.K. Bajpai, J. Colloid Interface Sci. 315, 389–395 (2007)
M. Guo, Y. Zhang, F. Du, Y. Wu, Q. Zhang, C. Jiang, Mater. Chem. Phys. 225, 42–49 (2019)
V. Thomas, M.M. Yallapu, B. Sreedhar, S.K. Bajpai, J. Biomater. Sci. Polym. Ed. 20, 2129–2144 (2009)
J.W. Rhim, L.F. Wang, S.I. Hong, Food Hydrocoll. 33, 327–335 (2013)
V. Kiruba, A. Dakshinamurthy, P. Subramanian, M.S. Paulraj, J. Exp. Nanosci. 10, 532–544 (2014)
S.G. Abd Alla, H.M. Nizam El-Din, A.W.M. El-Naggar, Eur. Polym. J. 43, 2987–2998 (2007)
M. Yadollahi, S. Farhoudian, S. Barkhordari, I. Gholamali, H. Farhadnejad, H. Motasadizadeh, Int. J. Biol. Macromol. 82, 273–278 (2016)
A.I. Raafat, N.M. El-Sawy, N.A. Badawy, E.A. Mousa, A.M. Mohamed, Int. J. Biol. Macromol. 118, 1892–1902 (2018)
A. Alonso, X. Muñoz-Berbel, N. Vigués, R. Rodríguez-Rodríguez, J. Macanás, M. Muñoz, J. Mas, D.N. Muraviev, Adv. Funct. Mater. 23, 2450–2458 (2013)
X. Jin, M. Li, J. Wang, C. Marambio-Jones, F. Peng, X. Huang, R. Damoiseaux, E.M. Hoek, Environ. Sci. Technol. 44, 7321–7328 (2010)
H. Zhang, V. Oyanedel-Craver, J. Environ. Eng. 138, 58–66 (2011)
J.S. Kim, E. Kuk, K.N. Yu, J.-H. Kim, S.J. Park, H.J. Lee, S.H. Kim, Y.K. Park, Y.H. Park, C.-Y. Hwang, Y.-K. Kim, Y.-S. Lee, D.H. Jeong, M.-H. Cho, Nanomed. Nanotechnol. Biol. Med. 3, 95–101 (2007)
A. Vanaamudan, M. Sadhu, P. Pamidimukkala, J. Mol. Liq. 271, 202–208 (2018)
A.A. Al-Kahtani, T. Almuqati, N. Alhokbany, T. Ahamad, M. Naushad, S.M. Alshehri, J. Clean. Prod. 191, 429–435 (2018)
J. Li, C.-Y. Liu, Y. Liu, J. Mater. Chem. 22, 8426–8430 (2012)
N. Sahiner, A. Kaynak, S. Butun, J. Non-Cryst. Solids 358, 758–764 (2012)
Acknowledgements
The authors express their deep gratitude to Dr. Eman Araby, Associate Professor, Radiation Microbiology Department, National Center for Radiation Research and Technology, for antimicrobial assessment and her good interpretation and discussion.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Raafat, A.I., Mahmoud, G.A. & Mostafa, T.B. Efficient Catalytic Reduction of Hazardous Anthropogenic Pollutant, 4-Nitrophenol Using Radiation Synthesized (Polyvinyl Pyrrolidone/Acrylic Acid)-Silver Nanocomposite Hydrogels. J Inorg Organomet Polym 30, 3116–3125 (2020). https://doi.org/10.1007/s10904-020-01470-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-020-01470-4