Abstract
Poly (cyclotriphosphazene-co-4,4′-diaminodiphenyl sulfone) (PZD) microspheres were prepared by precipitation polymerization with simple scheme and convenient operation, and used as a special effect adsorbent for adsorbing cationic dye, which is methylene blue (MB) in water. The synthesized PZD microspheres were characterized by SEM, TEM and XPS techniques. The influences of temperature, adsorption time, pH initial MB solution concentration and adsorbent dosage on MB adsorption were studied. The experimental results show that the adsorption of MB dye by PZD microspheres is more in line with the pseudo-second-order kinetics model than the pseudo-first-order kinetic model. In addition, the adsorption process was further resolved using Weber’s intraparticle diffusion model. Thermodynamic experiments show that the adsorption process is spontaneously carried out by endotherm. The mechanism of adsorption of MB dye by PZD microspheres may be related to the existence of a lot of electron-rich atoms N, P, S in the adsorbent, the electrostatic attraction and π–π stacking effect between PZD microspheres and MB dye.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
With the rapid speedy development of modern industry, dyes are widely applied to textiles, food, cosmetics, printing and other industries [1, 2]. However, the illegal discharge of dye wastewater will cause serious water pollution problems, and endanger human health and the entire ecological environment. This is a matter of concern. So, it has been widely concerned by scholars in China at home [3, 4] and elsewhere abroad [5, 6]. Because these dyes contain aniline organic compounds and they are characterized by complex composition, difficult biochemical degradation, and high biological toxicity [7, 8], the direct discharge of dyes will reduce dissolved oxygen in water and affect the normal growth of aquatic organisms [9, 10]. What’s worse is that excessive intake of dyes can damage the body’s digestive system and central nervous system [11], and cause mutations [12] and cancer [13]. Therefore, printing and dyeing wastewater must be treated through certain means and techniques.
At present, many methods for treating methylene blue (MB) have been developed, such as adsorption method [14,15,16,17], chemical oxidation method [18, 19], membrane separation method [20], ultrasonic catalytic degradation method [21] and biodegradation method [22], etc. Among them, the adsorption method has attracted the attention of researchers because of its low cost, low energy consumption, simple operation and good treatment effect [23]. The most common adsorbents are activated carbon [24], zeolite [25], agricultural waste [24], nanohybrid materials [26], etc., but these adsorbent materials generally have shortcomings such as low adsorption capacity and poor adsorption efficiency. They cannot meet the actual needs of people. In recent years, with the continuous expansion and application of nanotechnology to various fields, an increasing number of people are working on the preparation of functional polyphosphazene nanomaterials. The material is an organic–inorganic hybrid polymer composed of a lot of electron-rich N, P, and S atoms. Due to its designability of chemical structure and diversity of side groups, the new polyphosphazene materials have shown incomparable advantages over traditional polymers [27]. Therefore, the new polyphosphazene materials have wider applications in the fields of batteries, tissue engineering, drug carriers, new flame retardant materials, and new adsorbents. The PZS nanotubes prepared by Wang can effectively remove RhB dye in water in a short time and it is a highly efficient cationic adsorbent [28]. The heteroatom-containing porous carbon microspheres prepared by Fu et al. [29, 30] have high hydrogen storage capacity. Chen et al. [31] prepared a new flame retardant through the synthetic flame retardant surface modifier, which can effectively flame retard. It can be seen that the new polyphosphazene material is a very promising raw material.
Recently, we have prepared a new poly (cyclotriphosphazene-co-4,4′-diaminodiphenyl sulfone) microsphere (PZD), which is an organic–inorganic hybrid polymer being rich in multiple Heteroatoms (N, P, S, etc.). PZD is obtained by hexachlorocyclotriphosphazene (HCCP) and 4,4-diaminodiphenyl sulfone (DDS) as comonomers, with triethylamine (TEA) as an acid binding agent. The product, which is precipitated and undergoes polymerized under mild conditions. Per the unique morphology of PZD, was shown to be our group used it as a high-efficiency adsorbent to wipe off cationic dyes in aqueous solution. The adsorption capacity for methylene blue under the conditions of pH, initial concentration of methylene blue, temperature, a quantity of adsorbents and time of action were examined. The influence of the amount and the in-depth study on the adsorption kinetics and adsorption thermodynamics mechanism are so. According to the experimental results, PZD microspheres can be used as a highly efficient special adsorbent.
2 Experimental Materials
Hexachlorocyclotriphosphazene (HCCP) and 4,4′-diaminodiphenyl sulfone (DDS) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. Triethylamine (TEA) was bought from Tianjin Tianli Chemical Reagent Co., Ltd. Ethanol and methylene blue dyes are all analytical grade reagents, purchased from Tianjin Kemiou Chemical Reagent Co., Ltd. Methylene blue powder and deionized water were used as raw materials to prepare different concentrations of methylene blue solution (1 g/L) according to a certain ratio.
3 Synthesis of the PZD Microspheres
The research group adopted a self-developed program to prepare polyphosphazene microspheres. Typically, 1.65 g of HCCP (4.75 mmol) and 4.78 g of DDS (19.25 mmol) were dissolved in 200 mL of acetonitrile, shaken to complete dissolution, and then 12 mL of triethylamine (TEA) was added. The molar ratio of 4,4′-diaminodiphenyl sulfone (DDS) to HCCP is about 4:1 (4,4′-diaminodiphenyl sulfone (DDS) exceeds HCCP). Then, it was stirred at 50 °C for 12 h, and then ultrasonicated (100 W, 40 kHz) for 12 h, and it was repeated three times. The entire reaction was carried out under the protection of nitrogen. At the end of the reaction, the product was separated by a centrifuge (rotation speed: 3500 r/min, time: 5 min) to obtain a precipitate, then the precipitated product was washed alternately with ethanol and deionized water, and repeated twice. The washed product was again dried in a vacuum oven (60 °C) to obtain a poly(cyclotriphosphazene-4,4′-diaminodiphenylsulfone) microsphere material in the form of a white powder.
4 Adsorption Experiments
The adsorption performance of poly(cyclotriphosphazene-4,4′-diaminodiphenyl sulfone) microspheres adsorbent on MB dye was studied by batch adsorption method.
For this experiment, 5 mg of microspheres were added to a 20 mL glass vial and 10 mL (50 mg/L) of methylene blue aqueous solution was added for ultrasonic dispersion. Then, the glass bottle was fixed in a constant temperature water bath shaker to oscillate. Adsorption experiments under different pH conditions (3, 5, 7, 9, 11, 12, 13, 14) were carried out at room temperature for 14 h. The supernatant was taken, and the concentration of methylene blue (MB) after adsorption was measured by a visible spectrophotometer. The adsorption amount(q) of methylene blue per unit mass of the microspheres and the removal rate(R) of methylene blue in the solution were calculated according to the Eqs. (A.1) and (A.2), respectively:
V represents the volume of the MB solution (L). C0 is the initial concentration of MB in aqueous solution (mg/L). Ct is the concentration after adsorption (mg/L). In the equation, m represents the amount of addition of the microspheres (mg).
In the kinetic experiment, 10 mL (50 mg/L) aqueous solution of methylene blue was added to the glass bottle, and then 5 mg of microspheres were added. After ultrasonic dispersion, the glass bottle was shaken in a constant temperature water bath oscillator, and the shaking time was 1 h, 2 h, 3 h, 4 h, 5 h, 6 h. By changing the number of microspheres which are used (2, 3, 4, 5 and 6 mg), the ultrasonic dispersion was shaken for 5 h. Besides we have studied the relationship between the amount of adsorbent added and the adsorption capacity, and the relationship between the amount of adsorbent added and the adsorption rate. Subsequent experiments used the same procedure to investigate the effect of PZD microspheres on the adsorption of methylene blue at different initial concentrations (40, 50, 60, 70 and 80 mg/L) and at different adsorption temperatures (25, 35, 45, 55 and 65 °C).
5 Characterization
Scanning electron microscopy (SEM) with a JEOL JSM-7401Ffield-emission microscope was used to observe the size and morphology of PZD microspheres. And the acceleration voltage of Tecnai-G20 field emission microscope is 2.0 kV. The microstructure of the PZD microspheres was observed clearly by transmission electron microscopy (TEM). Fourier transform infrared spectroscopy (FT-IR) of PZD microspheres was measured on a Shimadu IR Prestige-21 infrared spectrometer manufactured by Shimadzu Infrared. The X-ray photoelectron spectroscopy of PZD microspheres was measured by Escalab 250 xi X-ray photoelectron spectroscopy, and its chemical composition was analyzed. According to measurement of methylene blue residual concentration in supernatant after adsorption using a visible spectrophotometer (Shanghai INESA Scientific Instruments Co., Ltd., Ltd., 751G), the maximum absorption wavelength of the MB dye was 665 nm. Before the start of the adsorption experiment, a certain concentration gradient of methylene blue solution (0–45 mg/L) should be configured to determine the standard curve of the methylene blue solution. The pH value of the methylene blue solution was measured using a pHS-25 pH meter (Shanghai INESA Scientific Instruments Co., Ltd.).
6 Results and Discussion
6.1 Characterization of the Adsorbent
The SEM and TEM images of PZD microspheres prepared by precipitation polymerization using HCCP and DDS as raw materials are shown in Fig. 1a, b. And the images show that most of the microspheres exhibit a nearly regular spherical structure. The microspheres has a micro-sized spherical outer surface, which can achieve rapid mass diffusion of the MB dye and rapid action of the adsorbent and the adsorbate [32, 33].
Figure 2a shows the FT-IR of monomeric DDS, HCCP and PZD microspheres. The absorption bands at 3361 cm−1 and 3215 cm−1 were designated as tensile vibrations of the –NH2 band. Two strong absorption peaks of 1593 and 1500 cm−1 were designated as C=C double bonds of the aromatic ring. 1300 and 1103 cm−1 are O=S=O telescoping vibration characteristic absorption peaks of sulfonydiphenol groups. 1143 cm−1 is the characteristic absorption peak of P=N stretching vibration. 880 cm−1 (the telescoping vibration of P–N) and 931 cm−1 (the telescoping vibration of Ar–P–N) demonstrate that the PZD microspheres are PZD microspheres are the product of precipitation polymerization of HCCP and DDS. The composition of the compound was analyzed by XPS. Figure 2b shows the XPS scans spectrum of the microspheres. The results show that the surface of the microspheres consists of C, N, O, P, and S elements, wherein the N,P, and S elements account for 17.45, 9.76, and 5.42%, respectively. Since the molar proportion of N, P and S accords with the theoretical value of the highly-crosslinked microspheres, it is indicated that the PZD microspheres are successfully prepared. Figure 2c shows the formation of highly crosslinked PZD microspheres by the condensation of HCCP and DDS as comonomers.
6.2 Effect of pH Value of Initial MB Dye on Adsorption Performance
The initial pH of the solution affects the binding site, surface charge and spatial structure of the dye on the surface of the adsorbent. The effect of adsorption of MB dye by microspheres was investigated by setting different pH values. As shown in Fig. 3, when the pH varies between 3 and 11, the adsorption amount of the microspheres and the removal rate of the MB dye slowly increase as the amount of the adsorbent increases. When the pH value varies between 11 and 13, the removal rate of MB increases, and the adsorption reaches the maximum value (the removal rate and qe reach 96. 55% (96. 548 mg/g) respectively. There was no significant change in the adsorption of PZD microspheres when the pH increases from 13 to 14. At a certain H+ concentration, H+ in the solution causes protonation of the amino group, which results in positive charges on the surface of the adsorbents. The positive charge is the main reason for the weakening of the adsorption effect. At the same time, the H+ and MB cationic dyes in the solution are adsorption competition relationships. With the reduction of H+ concentration, the degree of protonation of amino group decreases, and the competitive adsorption of H+ to MB dye weakens, so that the adsorption effect of PZD microspheres on MB dye is enhanced. In the interval of pH values 11–13, the removal rate of MB dye increases, indicating that the MB dye neutralizes the positive charge carried by the adsorbents, thereby reducing the Coulomb repulsion on the surface of the microspheres and the adsorption effect was enhanced. In conclusion, when the pH is raises, the concentration of OH− is increases, and the degree of neutralization of the protonated amino group is maximizes, so that the removal rate reaches a stable value and the optimum pH is selected to be 13.
6.3 Influence of Initial MB Concentration on Adsorption
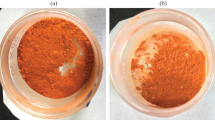
The trend of the removal rate of the final MB solution for different MB concentrations is shown in Fig. 4. To determining the optimal adsorption pH of the solution (close to 13), the concentration of the MB solution increases from 30 to 70 mg/L, and its concentration effect was studied in the greenhouse. As the concentration of MB dye increases, the removal rate decreases rapidly. The results indicate that the adsorbent of a certain quantities of microspheres (5 mg) has reached the optimum adsorption amount. However, at low concentrations, the adsorbent exhibits a rapid increase in adsorption capacity. At the initial concentration of 50 mg/L, the adsorption capacity reaches a maximum (92 mg/g). The experimental results show that the microspheres can be in a full contact with the high concentration of MB solution, while at high concentrations it exhibits a tendency to increase slowly. Considering the aboving two factors, the adsorption ratio of 50 mg/L is selected by comparing with the adsorption capacity of different concentrations. It is determined that 50 mg/L is the best adsorption condition, and the pattern before and after the adsorption is selected for comparison. Figure 4a shows a picture of the MB adsorption protocol and Fig. 4b shows the control of the microspheres before and after adsorption. The solution after MB adsorption in Fig. 4b almost becomes clear and transparent, thus indicating that PZD microspheres are a highly efficient adsorbent for MB.
6.4 Influence of Adsorbent Quality on Adsorption of MB Dyes
The initial concentration of MB solution is set at 50 mg/L, of which the pH value is 13 or so. The effect of the quantities of adsorbents on the adsorption effect of MB dye is shown in Fig. 5. As the quantitiy of microspheres increases from 2 to 6 mg, the adsorption rate of PZD to MB increases from 56.32 to 95.58%, indicating that PZD microspheres are a highly efficient adsorbent. It can be seen from the figure that when the quantitiy of microspheres is 5 mg, the removal rate of MB dye (50 mg/L) can reach a maximum value, but the adsorption capacity shows a downward trend. The reason is that when the mass of the microspheres is more than 5 mg, the driving force generated by the MB dye molecules in the solution cannot offset the repulsive force generated by the large amount of MB molecules adsorbed by the adsorbent. That is, the optimum amount of adsorbent is 5 mg, and the removal rate of MB reaches a maximum now. Therefore, in order to obtain the maximum removal rate without adding more adsorbents, in the experiment of other influencing factors, the best dosage of 5 mg as the adsorbent is selected.
6.5 Kinetic Studies
The effect of PZD microspheres on MB adsorption at different times was investigated by studying the adsorption kinetics, as shown in Fig. 6a. The experimental results show that after about 5 h, the MB removal rate reaches a maximum (the removal rate reaches 93.43%), and remains basically unchanged, which can be regarded as the adsorption equilibrium. This paper simulates a quasi-first-order dynamic model and a quasi-second-order dynamic model. The pseudo first-order kinetic model and the pseudo second-order kinetic model can be expressed by the linear formal Eqs. (B.1), (B.2), as follows:
Effect of oscillating time on the adsorption of MB by PZD microspheres. Experimental conditions: The concentration of MB dye was 50 mg/L, the amount of adsorbent was 5 mg, The graph represents the three separate experiments (a), the pseudo first-order kinetics (b) and pseudo second-order kinetics (c) of adsorbed MB dyes on PZD microspheres
In the equation, qt and qe (mg g−1) are the adsorption amount at times(h) and the adsorption equilibrium value, respectively. K1 (g mg−1 h−1) and K2 are expressed as the rate constants of the quasi-first-order model and the rate constants of the quasi-second-order model, respectively. The first-order kinetics and second-order kinetic fit curves are shown in Fig. 6b, c. The experimental results show that, as shown in Table 1, the theoretical value qe of the two models is close to the actual experimental measured value qe. However, the correlation coefficient of the quasi-second-order dynamic model is R2 = 0.9994, which is larger than the pseudo-first-order dynamic model. This indicates that the quasi-second-order model is better than the first-order adsorption dynamic model in explaining the adsorption kinetics, which provides a theoretical basis for further exploration and identification of the adsorption process of PZD microspheres for methylene solution.
Simultaneously, Weber’s intraparticle diffusion model has a good effect on describing the adsorption process, which is expressed by Eq. (C.1) [33], as follows:
In the formula, Ki (mg g−1 h−1/2) represents the intraparticle diffusion rate; c (mg/g) is expressed as a constant. The parameters of Weber’s intraparticle diffusion model are also given in Table 2.
From Fig. 7, we can see that the graph exhibits multiplicity, indicating the existence of two adsorption stages. The first stage is the diffusion of MB molecules through the solution onto the surface of the adsorbent PZD microspheres. The second stage is intraparticle diffusion from MB dye molecules through a large number of inter-crosslinked PZD microspheres voids. It is easy to find from Table 2 that the MB dye diffusion rate K1 (mg g−1 h1/2) in the first stage is 12 times larger than the second stage, which fully demonstrates that the internal diffusion of MB dyes is progressive. However, the linear equation of Fig. 7 does not pass through the origin, suggesting that the internal diffusion process of the dye molecules is not a quick-control step, and that other factors are likely to affect the diffusion rate as well. In addition, intraparticle diffusion and film diffusion may also occur simultaneously.
6.6 Influence of Solution Reaction Temperature on Adsorption Effect
Adsorption includes physical adsorption and chemical adsorption. Physical adsorption relies on the van der Waals force between the adsorbent and the adsorbate molecules. There is no selective adsorption, and the adsorption heat is low about 20–40 kJ/mol. Besides the adsorption is less affected by temperature. The chemical adsorption relies on the chemical bond between the molecules, and the heat of adsorption is close to the heat of reaction, which is about 40–400 kJ/mol. Under normal circumstances, when the temperature raises, the amount of physical adsorption will decrease. Nevertheless the chemical adsorption is difficult to reach equilibrium, because the adsorption equilibrium will reach the maximum value firstly during the heating process and the adsorption effect will decrease as the temperature increases. As shown in Fig. 8a, when the temperature is lower than room temperature (298.15 K), the removal rate of MB dye decreases as the temperature decreases. At room temperature (298. 15 K), PZD has the best adsorption effect on MB, and the removal rate reaches the maximum (about 96.55%). At temperatures above room temperature (298.15 K), the removal rate of methylene blue decreases with increasing temperature. This is consistent with previous kinetic studies.
For further study to determine whether the adsorption reaction proceeds spontaneously, Fig. 8 and Table 3 are the results of thermodynamic analysis of the adsorption process. The calculation formulas of each thermodynamic parameter (△G0, △H0 and △S0) are Eqs. (D.1), (D.2) and (D.3) as follows:
It can be seen from Table 3 that ΔG0 < 0 at different temperatures the adsorption of MB by the microspheres is a spontaneous irreversible process. When the temperature is 298.15 K, the absolute value of △G0 is the largest, signifying that the adsorption driving force reaches the maximum at this temperature. Concequently, in order to obtain the best adsorption effect and reduce the energy consumption of the adsorption process, the adsorption experiment needed to be carried out at room temperature (298.15 K). The △H0 result is a negative value, indicating that the adsorption reaction is an exothermic reaction. And that the elevated temperature has an adverse effect on the reaction. △S0 is a negative value as well. In that the MB dye ion changes from the free movement in the solution before adsorption to the two-dimensional movement on the surface of the PZD microspheres after adsorption, resulting in the degree of freedom after the adsorption reaction occurs and making the system less chaotic.
6.7 Adsorption Mechanism
Through the analysis of the chemical structure of PZD under the above different conditions, the adsorption mechanism of MB dye on PZD microspheres for optimal adsorption was proposed, as shown in Fig. 9. The cationic dye MB is a planar molecule owing an aromatic backbone [34]. A large number of amino groups in the PZD compound synthesized by our team (confirmed by infrared spectroscopy), Fig. 2, It can be easily protonated in a solution with a high hydrogen ion content, and the deprotonation process can be completed under alkaline conditions. Therefore, in the alkaline case, the amino group can provide an adsorption site which can be electrostatically bonded to the cation of the MB dye [35]. As the adsorption reaction proceeds, there may be the existence of π–π stacking between the aromatic ring of the PZD microspheres and the aromatic ring of the MB dye molecule. Additionally, PZD microspheres contain a large number of electron-rich atoms such as N, S, and P. These multi-electron-rich atoms facilitate the adsorption of MB dyes and can be used as their basic reaction center [36].
7 Conclusions
In summary, in this experiment, microspheres materials containing N, P, S atoms and multiple amino groups were prepared by precipitation polymerization. The PZD microspheres is an high-efficiency adsorbent for adsorption of MB dye solution. The experimental results showed that the maximum adsorption capacity of PZD microspheres can be as high as 96.548 mg/g. That is, the removal rate is 96. 55% at a pH of 13 and a temperature of 25 °C. Under alkaline conditions, the amino functional groups in the microspheres are prone to deprotonation, providing an adsorption site that allows the PZD microspheres to combine with the cationic dye (methylene blue) by electrostatic interaction to achieve adsorption removal. Experiments showed that the quantitiy of adsorbents, pH, contact time, temperature and initial concentration of MB have an important effect on MB in PZD adsorption solution, and its shows that the substance is a novel adsorbent capable of effectively separating MB dye from an aqueous solution. The results of kinetic experiments show that the adsorption of adsorbed MB dye by PZD microspheres can better conform to the quasi-second-order dynamic model. In the further analysis under the Weber particle diffusion model, the process of adsorbing MB dye by PZD microspheres can be divided into intraparticle diffusion and film diffusion. Besides, The model also shows that the main step in the rate of adsorption is not intramolecular diffusion. Thermodynamic experiments show that the adsorption of MB on PZD microspheres is spontaneously carried out by endotherm. Numerous N, P, S atoms are contained in PZD microspheres. As well as the electrostatic attraction and π–π stacking between PZD microspheres and MB dyes are the main reasons for efficient adsorption. The new PZD microspheres synthesized in this project are expected to be used for the treatment of cationic dyes in wastewater.
References
D. Robati, S. Bagheriyan, M. Rajabi, O. Moradi, A.A. Peyghan, Effect of electrostatic interaction on the methylene blue and methyl orange adsorption by the pristine and functionalized carbon nanotubes. Phys. E 83, 1–6 (2016)
P. Sharma, H. Kaur, M. Sharma, V. Sahore, A review on applicability of naturally available adsorbents for the removal of hazardous dyes from aqueous waste. Environ. Monit. Assess. 183, 151–195 (2011)
P. Sharma, B.K. Saikia, M.R. Das, Removal of methyl green dye molecule from aqueous system using reduced graphene oxide as an efficient adsorbent: kinetics, isotherm and thermodynamic parameters. Colloids Surf. A 457, 125–133 (2014)
T.A. Nguyen, C.C. Fu, R.S. Juang, Effective removal of sulfur dyes from water by biosorption and subsequent immobilized laccase degradation on crosslinked chitosan beads. Chem. Eng. J. 304, 313–324 (2016)
Y. Pan, Y. Wang, A. Zhou, A. Wang, Z. Wu, L. Lv, X. Li, K. Zhang, T. Zhu, Removal of azo dye in an up-flow membrane-less bioelectrochemical system integrated with bio-contact oxidation reactor. J. Chem. Eng. 326, 454–461 (2017)
J. Chen, L. Zhang, T. Huang, W. Li, W. Ying, Z. Wang, Decolorization of azo dye by peroxymonosulfate activated by carbon nanotube: radical versus non-radical mechanism. J. Hazard. Mater. 320, 571–580 (2016)
E. Forgiarini, A.A. de Souza, Toxicity of textile dyes and their degradation by the enzyme horseradish peroxidase (HRP). J. Hazard. Mater. 147, 1073–1078 (2007)
C.R. Holkar, A.J. Jadhav, D.V. Pinjari, N.M. Mahamuni, A.B. Pandit, A critical review on textile wastewater treatments: possible approaches. J. Environ. Manag. 182, 351–366 (2016)
O. Duman, S. Tunç, T.G. Polat, B.K. Bozoğlan, Synthesis of magnetic oxidized multiwalled carbon nanotube-κ-carrageenan-Fe3O4 nanocomposite adsorbent and its application in cationic Methylene Blue dye adsorption. Carbohydr. Polym. 147, 79–88 (2016)
P.M. Rowiński, Influence of selected fluorescent dyes on small aquatic organisms. Acta Geophys. 59, 91–109 (2011)
M.M. El-Zawahry, A. Fatma, R.A. Abdelghaffar, A.G. Hassabo, Equilibrium and kinetic models on the adsorption of reactive black 5 from aqueous solution using Eichhornia crassipes/chitosan composite. Carbohydr. Polym. 136, 507–515 (2016)
Z. Du, Z. Yue, Z. Li, C. Hui, W. Ying, G. Wang, Z. Ping, H. Chen, Y. Zhang, Facile one-pot fabrication of nano-Fe3O4/carboxyl-functionalized baker’s yeast composites and their application in methylene blue dye adsorption. Appl. Surf. Sci. 392, 312–320 (2017)
V.C. La, A. Tavani, Epidemiological evidence on hair dyes and the risk of cancer in humans. Eur. J. Cancer Prev. Organ. 4(1), 31–43 (1995)
N. Gupta, A.K. Kushwaha, M.C. Chattopadhyaya, Application of potato (Solanum tuberosum) plant wastes for the removal of methylene blue and malachite green dye from aqueous solution. J. Chem. 9, S707–S716 (2016)
H. Sayğılı, F. Güzel, High surface area mesoporous activated carbon from tomato processing solid waste by zinc chloride activation: process optimization, characterization and dyes adsorption. J. Clean. Prod. 113, 995–1004 (2016)
R. Das, M. Bhaumik, S. Giri, A. Maity, Sonocatalytic rapid degradation of Congo red dye from aqueous solution using magnetic Fe0/polyaniline nanofibers. Ultrason. Sonochem. 37, 600–613 (2017)
M. Perullini, M. Jobbã, M.L. Japas, S.A. Bilmes, New method for the simultaneous determination of diffusion and adsorption of dyes in silica hydrogels. J. Colloid Interface Sci. 425, 91–95 (2014)
N. Ertugay, F.N. Acar, Removal of COD and color from direct blue 71 azo dye wastewater by Fenton’s oxidation: kinetic study. J. Chem. 10, S1158–S1163 (2017)
D. Wiedmer, E. Sagstuen, H. K. Welch, J. Håvard, H. Tiainen, Oxidative power of aqueous non-irradiated TiO2 -H2O2 suspensions: methylene blue degradation and the role of reactive oxygen species. Appl Catal B-Environ. 198, 9–15 (2016)
J. Lin, W. Ye, M.C. Baltaru, Y.P. Tang, N.J. Bernstein, P. Gao, S. Balta, M. Vlad, A. Volodin, A. Sotto, Tight ultrafiltration membranes for enhanced separation of dyes and Na2SO4 during textile wastewater treatment. J. Membr. Sci. 514, 217–228 (2016)
K. Zare, V.K. Gupta, O. Moradi, A.S.H. Makhlouf, M. Sillanpää, M.N. Nadagouda, H. Sadegh, R. Shahryari-ghoshekandi, A. Pal, Z.J. Wang, A comparative study on the basis of adsorption capacity between CNTs and activated carbon as adsorbents for removal of noxious synthetic dyes: a review. J. Nanostruct. Chem. 5, 227–236 (2015)
K.C. Chan, C.Y.H. Chao, C.L. Wu, Measurement of properties and performance prediction of the new MWCNT-embedded zeolite 13X/CaCl2 composite adsorbents. J. Heat Mass Transf. 89, 308–319 (2015)
H.S. Jamwal, S. Kumari, G.S. Chauhan, N.S. Reddy, J.H. Ahn, Silica-polymer hybrid materials as methylene blue adsorbents. J. Env. Chem. Eng. 5, 103-113 (2017)
T. Maneerung, J. Liew, Y. Dai, S. Kawi, C. Chong, C.H. Wang, Activated carbon derived from carbon residue from biomass gasification and its application for dye adsorption: Kinetics, isotherms and thermodynamic studies. Bio. Tech. 200, 350–359 (2016)
B. Armağan, M. Turan, M.S. Ęlik, Equilibrium studies on the adsorption of reactive azo dyes into zeolite. Desalination 170, 33–39 (2004).
L. Jiang, Y. Liu, S. Liu, X. Hu, G. Zeng, H. Xi, S. Liu, S. Liu, B. Huang, M. Li, Fabrication of β-cyclodextrin/poly (L-glutamic acid) supported magnetic graphene oxide and its adsorption behavior for 17β-estradiol. J. Chem. Eng. 308, 597-605 (2017)
L. Hu, A. Zhang, Y. Yu, Z. Zheng, S. Du, X. Cheng, Synthesis of hybrid crosslinked polyphosphazenes and investigation of their properties. J. Iran. Polym. 23, 689–698 (2014)
M. Wang, J. Fu, Y. Zhang, Z. Chen, M. Wang, J. Zhu, W. Cui, J. Zhang, Q. Xu, Removal of Rhodamine B, a cationic dye from aqueous solution using poly(cyclotriphosphazene-co-4,4′-sulfonyldiphenol) nanotubes. J. Macromol. Sci: Part A - Chem. 52, 105–113 (2015)
J. Fu, M. Wang, C. Zhang, P. Zhang, Q. Xu, High hydrogen storage capacity of heteroatom-containing porous carbon nanospheres produced from cross-linked polyphosphazene nanospheres. Mater. Lett. 81, 215-218 (2012)
Z. Chen, J. Zhang, J. Fu, M. Wang, X. Wang, R. Han, Q. Xu, Adsorption of methylene blue onto poly(cyclotriphosphazene-co-4,4′-sulfonyldiphenol) nanotubes: Kinetics, isotherm and thermodynamics analysis, J. Hazard Mater. 273, 263–271 (2014)
Y. Chen, L. Li, W. Wang, L. Qian, Preparation and characterization of surface-modified ammonium polyphosphate and its effect on the flame retardancy of rigid polyurethane foam. J. Appl. Polym. Sci. 134, 45369 (2017)
L. Guo, L. Zhang, J. Zhang, J. Zhou, Q. He, S. Zeng, X. Cui, J. Shi, Hollow mesoporous carbon spheres--an excellent bilirubin adsorbent. Chem. Commun. 40, 6071–6073 (2009)
L. Ai, C. Zhang, F. Liao, Y. Wang, M. Li, L. Meng, J. Jiang, Removal of methylene blue from aqueous solution with magnetite loaded multi-wall carbon nanotube: kinetic, isotherm and mechanism analysis. J. Hazard. Mater. 198, 282-290 (2011)
T. Madrakian, A. Afkhami, M. Ahmadi, H. Bagheri, Removal of some cationic dyes from aqueous solutions using magnetic-modified multi-walled carbon nanotubes. J. Hazard. Mater. 196, 109–114 (2011)
E.C. Lima, B. Royer, V. Jcp, N.M. Simon, C.B. Da, F.A. Pavan, E.V. Benvenutti, R. Cataluna-Veses, C. Airoldi, Application of Brazilian pine-fruit shell as a biosorbent to removal of reactive red 194 textile dye from aqueous solution : Kinetics and equilibrium study. J. Hazard. Mater. 155, 536-550 (2008)
D.L. Guerra, A.C. Batista, R.R. Viana, C. Airoldi, Adsorption of methylene blue on raw and MTZ/imogolite hybrid surfaces: Effect of concentration and calorimetric investigation. J. Hazard. Mater. 183, 81-86 (2010)
Acknowledgements
We are grateful to the University of South China Student Research Learning and Innovative Experimental Project Fund (No. 2018XJXZ32).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
:All the authors do not have any possible conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zou, J., Liao, K., Xiang, L. et al. Synthesis of Poly(cyclotriphosphazene-co-4,4′-diaminodiphenysulfone) Microspheres and Their Adsorption Properties for Cationic Dyes (Methylene Blue). J Inorg Organomet Polym 30, 976–985 (2020). https://doi.org/10.1007/s10904-019-01235-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-019-01235-8