Abstract
In present study Fe3O4 magnetic nanoparticles (MNPs) were prepared using co-precipitation method. Prepared MNPs were coated by SiO2 layer via sol–gel process. Subsequent Michel-addition reaction and amidation were used to synthesize G3 polyamidoamine dendrimer on prepared Fe3O4@SiO2. Fe3O4@SiO2@ PAMAM dendrimer was reacted with monochloroacetic acid for preparation of final Fe3O4@SiO2@Carboxyl-terminated PAMAM dendrimer nanocomposite. Successful synthesis of Fe3O4@SiO2@Carboxyl-terminated PAMAM dendrimer nanocomposite was confirmed by FTIR spectroscopy and CHN analysis. The nanostructure of prepared composite MNPs was investigated using TEM. X-ray diffraction pattern and thermal stability of pure MNPs and composite MNPs were studied using XRD and TGA analysis respectively. Unmodified and modified MNPs were used as adsorbent for the removal of Cu(II), Cd(II) and Pb(II) form aqueous solutions. It was observed that the modification of MPNs enhances the ability of MPNs for removal of these heavy metals significantly. Also it was shown that this modification enhances the accessibility of MNPs to heavy metal ions at low concentrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Population growth and industrialization are two most influential factors for increasing the rate of water pollution. Contamination of water resources with heavy metal ions which originated from a variety of industrial plants such as mining operation, recycling plants etc. is become the most important concern of environmental specialists because, these pollutants are non-degradable and toxic even at low concentrations, also they accumulate in plants, animals and human body and can result in a range of disorders and diseases [1,2,3,4,5]. Hence the elimination of heavy metal must be the top priority for any waste water treatment plant [1].
Eliminating heavy metal ions from aqueous media have been carried out with the aim of different techniques including precipitation, reverse osmosis, ion exchange and adsorption. Adsorption technique possesses different advantages such as simplicity, high efficiency, flexibility and low cost which make it an appropriate and preferred method for removal of heavy metal from waste water [1, 6].
Nanoparticle adsorbents due to their high surface area and subsequently high capacity are likely to be a convenient choice for the removal of heavy metals, although the problem of separation of these materials after application limits them to being used in water treatment plants [7, 8]. The using magnetic adsorbents, which can easily be removed by an external magnetic field, is a smart method for overcoming mentioned problem of nanoparticle adsorbents [7,8,9]. Considerable advantages of Fe3O4 MPNs including ease of separation, chemical stability, nontoxicity, biocompatibility and high efficiency make it a proper adsorbent for removal of heavy metals form waste water [6, 10].
In several studies, it has been reported that the modification of NPs surface plays a significant role in improving their stability and properties. One popular method in order to modify the surface of NPs is the synthesis of organic–inorganic nano composites. It means that the surface of Fe3O4 MNPs is covered by a layer of polymer. Takafouji et al. have reported the synthesis of poly vinil imidazole grafted to MNPs and investigated their applications for eliminating heavy metals like Cu2+, Ni2+ and Co2+ [11].
Chang et al. prepared Fe3O4 MNPs and poly(γ-glutamic acid)-coated Fe3O4 MNPs and investigated their average size, specific surface and their capacity for removal of Cr3+, Cu2+, Pb2+, and Ni2+. They reported that poly (γ-glutamic acid)-coated Fe3O4 has smaller size, larger surface area and higher metal removal capacity in comparison with unmodified Fe3O4 MNPs [12]. Ghasemi et al. synthesized magnetic Fe3O4@EDTA nanoparticles for removal of Ag(I), Hg(II), Mn(II), Zn(II), Pb(II) and Cd(II) at different pH, different adsorption times and variable amounts of nanoparticle. With the aim of response surface methodology and optimization, they simultaneously eliminated more than 99% of mentioned heavy metals [13].
Mahmoud et al. immobilized triethylenetetramine on nanoFe3O4@SiO2 and used Fe3O4, Fe3O4@SiO2, and Fe3O4@SiO2@triethylenetetramine as a sorbent for removal of Cu(II) and Pb(II) at different pH, contact times and sorbent dosages. They found that Fe3O4@SiO2@triethylenetetramine possess higher affinity for Cu(II), while Fe3O4 and Fe3O4@SiO2 have larger capacity for Pb(II) [14]. Xin et al. produced amine-functionalized Fe3O4 MPNs for eliminating Pb(II), Cd(II), and Cu(II). After optimization, they achieved maximum removal of 98% for Cu(II), Cd(II) and Pb(II) at the concentration of 5 ppm of above metal ions and solution volume of 50 ml [15]. Wang et al. prepared amino-functionalized Fe3O4@SiO2 MPNs for adsorption of Cu(II), Pb(II), and Cd(II) ions. They reported that their synthesized amino-functionalized Fe3O4@SiO2 MPNs could be regenerated easily using acid treatment [16].
Masoumi et al. [17] prepared Fe3O4@APTMS@Poly(MMA-co-MA) in order to use as nano adsorbent for Cd2+, Cr3+, Co2+, and Zn2+. They reported the maximum adsorption capacity of 90.09 mg g−1 for Co2+, 90.91 mg g−1 for Cr3+, 109.89 mg g−1 for Zn2+, and 111.11 mg g−1 for Cd2+ mg g−1 for their synthesized MPNs.
Chou et al. synthesized, poly amidoamine dendrimer coated magnetic nanoparticle in order to use as adsorbent for the removal of Zn(II). They investigated the adsorption and desorption behavior of synthesized nanocomposite material. They reported that the adsorption and desorption of this material are totally dependent on pH [18].
PAMAM dendrimers with three dimensional structures, from their amine groups, have the ability to act as a ligand for metal ions; hence they possess high metal adsorption capacity. The adsorption capacity of PAMAM dendrimers is dependent on pH so, at low pH, adsorbed ions can be released with high concentration of H+ and PAMAM dendrimers can be recycled and reused [18].
As a clear fact, EDTA has the ability to form stable coordination complexes with a broad range of metal ions. So it can be used as a surface modifier to enhance the adsorption capacity of metal adsorbents [13].
In this work with the goal of combining the advantages of Fe3O4 MPNs, polyamidoamine dendrimers and EDTA in heavy metal removal; we prepared Fe3O4@SiO2@Carboxyl-terminated PAMAM dendrimer nanocomposite in order to investigate its capacity for adsorption of heavy metal ions including Pb(II), Cd(II), and Cu(II) form aqueous solutions. Using FT-IR and CHN analysis it was confirmed that Fe3O4@SiO2@Carboxyl-terminated PAMAM dendrimer is successfully produced and TEM images exhibited the nanostructure of neat Fe3O4 and Fe3O4@SiO2@Carboxyl-terminated PAMAM dendrimer. It was shown that modification of Fe3O4 using carboxyl-terminated PAMAM dendrimer enhances its capacity for eliminating heavy metals.
2 Materials and Methods
2.1 Materials
The following materials were purchased from Sigma-Aldrich: FeCl3·6H2O–FeCl2·4H2O, Pb(NO3)2, CuCl2·4H2O, Cd(NO3)2·6H2O, ammonia, distilled water, tetraethyl orthosilicate, ethanol, ethylene diamine, methyl acrylate, chloroacetic acid, and (3-aminopropyl)triethoxysilane.
2.2 Instrumental Analysis
The FT-IR spectrum was taken by IR-Perkin Elmer-Spectrum X1 in KBr pellets. To determine the concentration of metals, AAS-GFAAS3260 Perkin Elmer atomic absorption spectrophotometer was used.
2.3 Preparation of Fe3O4@SiO2@Carboxyl-Terminated PAMAM Dendrimer
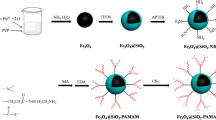
The synthesis procedure of Fe3O4@SiO2@Carboxyl-terminated PAMAM dendrimer is shown in Fig. 1.
2.3.1 The Preparation of Fe3O4 MNPs
In a 500 ml balloon equipped with a magnetic stirrer and nitrogen input, 3.1736 g of FeCl2·4H2O in addition to 7.5684 g of FeCl3·6H2O were dissolved in 350 ml of distilled water at 80 °C. The solution was then purged with nitrogen for 10 min. Finally, aqueous ammonia (28%) was added to the solution while it was stirring at 80 °C under the nitrogen atmosphere. As ammonia was added, black Fe3O4 MNPs were formed immediately. To complete the reaction, it was stirred at 80 °C for an hour. The reaction mixture was then cooled to room temperature and the MNPs were separated using a magnet (neobedium). Subsequently, the separated MNPs were washed several times with distilled water and 96% ethanol. Finally, they were dried at room temperature in order to gain 3.7 g of black Fe3O4 MNPs. They were, then, rinsed with methanol several times and dried at room temperature.
2.3.2 The Preparations of Fe3O4@SiO2 MNPs
In order to synthesize Fe3O4@SiO2 nanoparticles, sol–gel technique and Stöber method [19] were employed. 6 g of synthesized Fe3O4, from previous stage, with 200 ml ethanol and 10 ml distilled water were stirred and then sonicated for 10 min. Following that, 3 ml of tetraethoxsysylane was added to the reaction vessel. Afterwards, 10 ml of ammonia solution was added to the reaction, being stirred for 24 h. Finally, it was separated with the magnet and washed three times with methanol.
2.3.3 The Preparation of Fe3O4@SiO2@NH2
Three grams of Fe3O4@SiO2 was sonicated in 50 ml of toluene for 20 min. Then, 10 ml of (3-aminopropyl)triethoxysilane was added and the reaction was stirred at 50 °C for about 24 h. Finally, the MNPs were separated with the magnet and the final product was dried at room temperature.
2.3.4 The Preparation of G1 Generation of Polyamido Amine Dendrimer
Three grams of Fe3O4@SiO2@NH2 was sonicated in 50 ml methanol for 20 min until the MNPs could be totally dispersed in methanol. Afterwards, 10 ml of methyl acrylate was added to the reaction and it was stirred at room temperature under nitrogen atmosphere for 3 days. Following that, the MNPs were separated using the magnet and the product was washed by diethyl ether for several times, and then it was dried at room temperature.
In a 100 ml balloon, 2.9 g of the Fe3O4@SiO2@acrylate1 product of the third step in 50 ml of methanol was dispersed, and then 10 ml of ethylenediamine was added. Next, the suspension was stirred about 72 h at room temperature. Consequently, MNPs were precipitated by the magnet, and finally the product was washed several times by methanol and diethyl ether and dried at room temperature.
2.3.5 The Preparation of G2 Generation of Polyamidoamine Dendrimer
In a 100 ml balloon, 2.8 g of G1 dendrimer was dispersed in 50 ml of methanol and sonicated for 20 min. Afterwards, 10 ml of methyl acrylate was added to the reaction and the suspension was stirred under nitrogen atmosphere for 3 days at room temperature. As a result, the NPs were intercepted, washed several times with diethyl ether and they were dried at room temperature. In a 100 ml balloon, 2.7 g of previous product was dispersed in 50 ml of methanol and 10 ml of ethylenediamine was added to the suspension. Later, it was stirred at room temperature for 3 days. Subsequently, the product was separated with the magnet and washed several times by methanol and diethyl ether and dried at room temperature.
2.3.6 The Preparation of G3 Generation of Polyamidoamine Dendrimer
In a 100 ml balloon, 2.6 g of G2 generation product was sonicated in 50 ml of methanol for 20 min so that the MNPs could be totally dispersed in methanol. Then 10 ml of methyl acrylate was added to the reaction and it was stirred under nitrogen atmosphere for 3 days at room temperature. Subsequently, the MNPs were intercepted suing the magnet and washed several times by diethyl ether and were dried. In a 100 ml balloon, 2.5 g of the product was stirred in 50 ml methanol and then 10 ml Ethylenediamine was added. It was then stirred at room temperature for 3 days. Subsequently, the MNPs were separated by the magnet and washed several times by methanol and diethyl ether and finally dried at room temperature.
2.3.7 The Reaction of Fe3O4@PAMAM with Monochloroacetic Acid
In a 100 ml balloon, 10 g monochloroacetic acid was dissolved in 50 ml methanol. Next, in order to produce sodium chloro acetate 4.2 g of NaOH was added to the solution. Following that, 2.5 g Fe3O4@PAMAM was added to the solution and it was stirred for 3 days at room temperature. Finally, the MNPs were precipitated by the magnet and washed several times with methanol and dried at room temperature.
2.4 Batch Adsorption of Metal Ions
Solutions of copper(II), lead(II), and cadmium(II) ions with the concentrations of 160 and 480 ppm were prepared using CuCl2·4H2O, Pb(NO3)2 and Cd(NO3)2·6H2O respectively. Solutions of NaOH and HCl with the concentration of 0.01 mol/l were used to adjust the pH at 7.0 ± 0.1. For the adsorption experiments, 20 mg of final nanocomposite product was mixed with 50 ml of prepared metal solutions and stirred for 24 h (which is sufficient time for equilibrium [4]) at room temperature. After that the MPNs were separated using the magnet and the concentration of ions was determined using atomic absorption spectrometer. Following equations were used to calculate the amount of adsorbed metal ions:
where qe is equilibrium adsorption capacity (mg/g), R is removal efficiency, C0 (mg/l) is initial concentration of metal, Ce (mg/l) is equilibrium concentration of metal, V is solution volume (L), and m (g) is the adsorbent’s mass [20].
3 Results and Discussion
3.1 Characterization
3.1.1 FT-IR
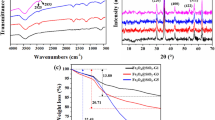
FT-IR as the most powerful technique was used to ensure successful synthesis of product in each step. Figure 2 shows FT-IR spectra of prepared Fe3O4 and different generation of synthesized polyamidoamine nanocomposite. In all spectra absorption bands at 3420, 1620 and 570 cm−1 are belong O–H stretching vibration of bound water, O–H deformed vibration of bound water, Fe–O bond vibration respectively [17, 18]. In all spectra absorption bands at 1080, 790 and 460 are attributed to Si–O symmetric vibration, Si–O bending and Si–O asymmetric vibration which confirms the successful formation of SiO2 layer on Fe3O4 MPNs [16, 17]. The appearance of esteric C=O absorption bands of methyl acrylate at 1730 cm−1 in spectrum (C) and (e) which are disappeared in spectrums (d) and (f) establishes the formation of G1 and G2 of polyamidoamine [21]. The absorption band at 1540 cm−1 in spectrum (g) attributed to carboxylate ion of final product of Fe3O4@SiO2@Carboxyl-terminated PAMAM dendrimer nanocomposite [22].
3.1.2 XRD Analysis
The change in crystalline structure of Fe3O4 during surface modification was investigated using XRD. Figure 3 shows XRD pattern of Fe3O4, Fe3O4@SiO2 and Fe3O4@SiO2@Carboxyl-terminated PAMAM dendrimer nanocomposite. As it is clear for Fe3O4 and Fe3O4@SiO2 the characteristic peaks of face-centered cubic lattice of Fe3O4 at 2θ(°) = 30.2, 35.5, 43, 53.5, 57.25 and 62.7 are clearly observed. Theses peaks assigned to (220), (311), (400), (422), (511) and (440) planes of inverse cubic spinel structure of Fe3O4 [10, 16, 17, 23].The resemblance of XRD pattern of Fe3O4 and Fe3O4@SiO2 and the appearance of all characteristic peaks of Fe3O4 in Fe3O4@SiO2 reveals that coating by SiO2 layer dosed not change the crystallinity structure of Fe3O4 [16, 24].The same observation about coating Fe3O4 by SiO2 layer reported by Wang et al. [16]. The broad peak at 2θ(°) = 24.5 in final product, which is attributed to PAMAM layer [21] is a confirmation for successful synthesis of PAMAM layer.
3.1.3 TEM
In order to study the morphology of prepared neat Fe3O4 and Fe3O4@SiO2@Carboxyl-terminated PAMAM dendrimer nanocomposite, TEM was used. As can be seen in Fig. 4 both Fe3O4 and Fe3O4@SiO2@Carboxyl-terminated PAMAM dendrimer nanocomposite have uniform spherical shape. As implied by TEM images, the average size of neat Fe3O4 is about 10 nm which is in agreement with the average size calculated from Scherrer’s equation (12 nm) [17, 18] and what is reported by Wng et al. [16], Maesoumi et al. [17] and Qu et al. [25]. For Fe3O4@SiO2@Carboxyl-terminated PAMAM dendrimer the nanostructure is well preserved although the size of particles (22 nm) is larger than neat Fe3O4 which is totally expectable because SiO2 layer, a layer of (3-aminopropyl)triethoxysilane and three generations of polyamidoamine dendrimer and chloro acetic acid layer increase the radius of particles. Also as it is clear for final product the agglomeration of MPNs is less than that of neat Fe3O4 significantly because of electrostatic repulsion of anionic carboxylate terminals [10, 18].
3.1.4 CHN and TGA Analysis
The results of CHN analysis for G3 generation of Fe3O4@SiO2@ carboxy terminated PAMAM dendrimer are presented in Table 1. CHN analysis is another way to confirm successful formation of dendrimer layer on prepared magnetic nanoparticles. Based on the result of G3 generation of Fe3O4@SiO2@PAMAM dendrimer, it is concluded that the overall percentage of organic matter on prepared nanocomposite particles is 4.17%.
TGA thermograms of G1 and G3 generations of Fe3O4@SiO2@PAMAM dendrimer are shown in Fig. 5. In temperature range of 50–160 °C for both samples, the weight loss of 2.5 wt% is attributed to the evaporation of adsorbed water. The weight loss between 160 and 550 °C is related to decomposition of dendrimer layer. Considering the amount of adsorbed water, the percentage of dendrimer for G1 generation is 2.5 wt%; while for G3 generation is 4.5 wt%. The amount of dendrimer for G3 is in agreement with data obtained for organic matter percentage from CHN analysis [26,27,28,29].
3.2 Heavy Metal Adsorption
Cd2+, Cd(OH)+, Cd(OH)2, and Cd(OH)3 are different forms of Cd(II). The percentage of each form depends on the pH value of solution. At pH value lower than 8, the majority of Cd(II) is its Cd2+ form. For Pb(II), there are five different species including Pb2+, Pb(OH)+, Pb(OH)2, Pb(OH)3− and Pb(OH)42−. As the solution pH increases the percentage of Pb2+ decreases. Free Pb2+ and Cd2+ are preferred forms for adsorption [4]. Cu(II) can be present in different forms including Cu2+, Cu(OH)+, Cu(OH)2 and Cu(OH)3−. The percentage of each form depends on pH. As the pH value increases, hydroxyl forms will be more dominant [1].
There are different mechanisms for removal of heavy metals using unmodified and modified magnetic Fe3O4 nanoparticles including, physical adsorption, ion exchange and complexation reaction. Physical adsorption mechanism is influenced by surface charge of adsorbents and heavy metal species. As pH increases, negative surface charge of adsorbent increases and at the same time it may results in hydroxylation of heavy metals and creation of species with negative surface charge that decreases physical adsorption and heavy metal removal because of electrostatic repulsion. Hence, for highest physical adsorption an optimum pH must be chosen to increase the surface charge of adsorbent and at same time prevent precipitation or hydroxylation of heavy metal ions. When it comes to ion exchange and complexation, there is no doubt that protonation of adsorbent prevents effective ion exchange and complexation reaction. So, acidic pH declines heavy metal removal via these two mechanisms [1, 4, 18, 30].
Herein we modified magnetic nanoparticle of Fe3O4 with chloroacetic acid terminated PAMAM dendrimer in order to enhance the chemisorption via complexation through both amine and acetate functional groups. So it is important to adjust pH in a value that facilitate the complexation reaction and enhance heavy metal removal. Based on Pan et al. [31] report, the isoelectric pH of G3 PAMAM dendrimer is 6.3; so at pH > 6.3 the overall charge of PAMAM dendrimer is negative. Regarding the pKa = 4.7 of acetic and Henderson- Hasselbalch equation, at pH 7 the ratio of deprotonated form to protonated form of acetic acid is almost 200(CH3COO−/CH3COOH = 200).Hence by choosing pH 7, we are confident that we have enough acetate terminal group on surface for chelating, surface charge of PAMAM dendrimer is negative enough to participate in complexation reaction and adsorb positive free ions and finally this pH doesn’t result in precipitation or hydroxylation of metal ions. In other word in pH 7 all adsorption mechanism including electrostatic attraction, ion exchange and complexation reaction using amine and acetate functional groups are involved in removal of Pb(II), Cd(II) and Cu(II). The performance of prepared magnetic nanoparticles for heavy metal removal in the term of removal efficiency and equilibrium adsorption capacity is presented in Table 2.
For each initial concentration, by comparing the removal efficiency of neat Fe3O4 and modified Fe3O4 we can evaluate the efficiency of modification. As can be seen, the removal efficiency of neat Fe3O4 for Cu(II) with initial concentration of 16 ppm is 86.9% while for modified Fe3O4 is 99.2%, which means the modification enhances removal efficiency by 12.1%. For Cu(II) with initial concentration of 48 ppm removal efficiency of neat Fe3O4 is 92.16 and for modified Fe3O4 is 99.3%. This means modification improves the performance of Fe3O4 by 7%.
There are two facts; the first one is that in the term of removal efficiency, neat Fe3O4 shows better performance at higher initial concentration of Cu and the second one is that the difference between the performance of neat Fe3O4 and modified Fe3O4 for initial concentration of 48 ppm is less than that one for 16 ppm.
The interesting result is that although the removal of Cu at low concentration of 16 ppm is not easy as the removal of Cu at high concentration of 48 ppm; modified Fe3O4 enhances the accessibility of adsorbent to Cu ions. By comparing the performance of modified Fe3O4 at two initial concentrations of 16 and 48 ppm we understand that they are equal. This means that the modification of Fe3O4 with carboxy terminated PAMAM dendrimer enable us to removal Cu at very low concentrations. This fact implies that all those proposed mechanisms for removal of Cu using carboxy terminated PAMAM dendrimer act effectively.
For Pb(II), the differences between removal efficiency of modified and unmodified Fe3O4 are 12.41 and 7.31 for initial concentrations of 16 and 48 ppm respectively. So, for Pb(II), the same results are observed and the modification using carboxy terminated PAMAM dendrimer is more effective on the performance of Fe3O4 when the concentration of Pb(II) is 16 ppm. In other word, the modification of Fe3O4 using carboxy terminated PAMAM dendrimer enhances its accessibility to Pb(II) at low concentrations.
By comparing the performance of unmodified and modified Fe3O4 for removal of Cd, we can find out that for this heavy metal the performance of unmodified Fe3O4 is a bit better at initial concentration of 48 ppm. Again Cd is more available for modified Fe3O4 to adsorb.
Figure 6 illustrates the removal efficiency of both modified and unmodified Fe3O4 for Cu(II), Pb(II) and Cd(II).As it is clear, the performance of unmodified Fe3O4 for removal of Cu(II) is higher than both Pb(II) and Cd(II). Although minimum performance of unmodified Fe3O4 is observed for removal of Cd(II), interesting result is, that the modification of Fe3O4 enhances the removal efficiency of Cd(II) in a such way that it becomes larger than removal efficiency of unmodified Fe3O4 for both Cu(II) and Pb(II). In other words modification using carboxy terminated PAMAM dendrimer is a convenient way to overcome the weakness of Fe3O4 for removal of Cd(II).
4 Conclusion
We prepared a novel magnetic nano composite of Fe3O4 by subsequent modification of Fe3O4 with SiO2, G3 generation of PAMAM dendrimer and chloroacetic acid. Modification increases the size of Fe3O4 nanoparticles and decreases agglomeration. Performance of neat Fe3O4 for removal of Cu(II), Pb(II) and Cd(II) at initial concentration of 48 ppm is higher than that of initial concentration of 16 ppm. Modification of Fe3O4 with carboxy terminated PAMAM dendrimer improves its removal efficiency. Improving the accessibility of Fe3O4 to heavy meal ions at low concentrations is the most important advantage of modification with carboxy terminated PAMAM dendrimer.
References
L. Hui et al., Adsorption behavior and adsorption mechanism of Cu(II) ions on amino-functionalized magnetic nanoparticles. Trans. Nonferrous Met. Soc. China 23(9), 2657–2665 (2013)
A.N. Baghani et al., One-pot synthesis, characterization and adsorption studies of amine-functionalized magnetite nanoparticles for removal of Cr(VI) and Ni(II) ions from aqueous solution: kinetic, isotherm and thermodynamic studies. J. Environ. Health Sci. Eng. 14(1), 11 (2016)
F. Fu, Q. Wang, Removal of heavy metal ions from wastewaters: a review. J. Environ. Manage. 92(3), 407–418 (2011)
K. Chen et al., Removal of cadmium and lead ions from water by sulfonated magnetic nanoparticle adsorbents. J. Colloid Interface Sci. 494, 307–316 (2017)
X. Bai et al., Design of multi-N-functional magnetic PVA microspheres for the rapid removal of heavy metal ions with different valence. Desalin. Water Treat. 56(7), 1809–1819 (2015)
S. Hanif, A. Shahzad, Removal of chromium(VI) and dye Alizarin Red S (ARS) using polymer-coated iron oxide (Fe3O4) magnetic nanoparticles by co-precipitation method. J. Nanopart. Res. 16(6), 2429 (2014)
S. Zhang et al., Thiol modified Fe3O4@ SiO2 as a robust, high effective, and recycling magnetic sorbent for mercury removal. Chem. Eng. J. 226, 30–38 (2013)
S. Venkateswarlu, S.H. Kumar, N. Jyothi, Rapid removal of Ni(II) from aqueous solution using 3-mercaptopropionic acid functionalized bio magnetite nanoparticles. Water Resour. Ind. 12, 1–7 (2015)
M. Moazzen et al., Multi-walled carbon nanotubes modified with iron oxide and silver nanoparticles (MWCNT-Fe3O4/Ag) as a novel adsorbent for determining PAEs in carbonated soft drinks using magnetic SPE-GC/MS method. Arab. J. Chem. (2018). https://doi.org/10.1016/j.arabjc.2018.03.003
Y. Wei et al., Synthesis of Fe3O4 nanoparticles and their magnetic properties. Proced. Eng. 27, 632–637 (2012)
M. Takafuji et al., Preparation of poly(1-vinylimidazole)-grafted magnetic nanoparticles and their application for removal of metal ions. Chem. Mater. 16(10), 1977–1983 (2004)
J. CHANG et al., Fabrication of poly(γ-glutamic acid)-coated Fe3O4 magnetic nanoparticles and their application in heavy metal removal. Chin. J. Chem. Eng. 21(11), 1244–1250 (2013)
E. Ghasemi, A. Heydari, M. Sillanpää, Superparamagnetic Fe3O4@ EDTA nanoparticles as an efficient adsorbent for simultaneous removal of Ag(I), Hg(II), Mn(II), Zn(II), Pb(II) and Cd(II) from water and soil environmental samples. Microchem. J. 131, 51–56 (2017)
M.E. Mahmoud, M.S. Abdelwahab, E.M. Fathallah, Design of novel nano-sorbents based on nano-magnetic iron oxide–bound-nano-silicon oxide–immobilized-triethylenetetramine for implementation in water treatment of heavy metals. Chem. Eng. J. 223, 318–327 (2013)
X. Xin et al., Highly efficient removal of heavy metal ions by amine-functionalized mesoporous Fe3O4 nanoparticles. Chem. Eng. J. 184, 132–140 (2012)
J. Wang et al., Amino-functionalized Fe3O4@ SiO2 core–shell magnetic nanomaterial as a novel adsorbent for aqueous heavy metals removal. J. Colloid Interface Sci. 349(1), 293–299 (2010)
A. Masoumi, M. Ghaemy, A.N. Bakht, Removal of metal ions from water using poly (MMA-co-MA)/modified-Fe3O4 magnetic nanocomposite: isotherm and kinetic study. Ind. Eng. Chem. Res. 53(19), 8188–8197 (2014)
C.-M. Chou, H.-L. Lien, Dendrimer-conjugated magnetic nanoparticles for removal of zinc(II) from aqueous solutions. J. Nanopart. Res. 13(5), 2099–2107 (2011)
W. Stöber, A. Fink, E. Bohn, Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 26(1), 62–69 (1968)
Y. Huang, A.N. Fulton, A.A. Keller, Simultaneous removal of PAHs and metal contaminants from water using magnetic nanoparticle adsorbents. Sci. Total Environ. 571, 1029–1036 (2016)
F.E. Peer, N. Bahramifar, H. Younesi, Removal of Cd(II), Pb(II) and Cu(II) ions from aqueous solution by polyamidoamine dendrimer grafted magnetic graphene oxide nanosheets. J. Taiwan Inst. Chem. Eng. 87, 225–240 (2018)
A. Zhang et al., Preparation of anti-fouling silicone elastomers by covalent immobilization of carboxybetaine. RSC Adv. 5(107), 88456–88463 (2015)
L.P. Lingamdinne et al., Biogenic reductive preparation of magnetic inverse spinel iron oxide nanoparticles for the adsorption removal of heavy metals. Chem. Eng. J. 307, 74–84 (2017)
R.X. Li et al., Preparation and characterization of cross-linked β-cyclodextrin polymer/Fe3O4 composite nanoparticles with core-shell structures. Chin. Chem. Lett. 22(2), 217–220 (2011)
J. Qu et al., A novel sensor based on Fe3O4 nanoparticles–multiwalled carbon nanotubes composite film for determination of nitrite. Sens. Bio-Sens. Res. 3, 74–78 (2015)
A.S. Ertürk, G. Elmacı, PAMAM dendrimer functionalized manganese ferrite magnetic nanoparticles: microwave-assisted synthesis and characterization. J. Inorg. Organomet. Polym. Mater. 28, 1–8 (2018)
M. Huysal, M. Şenel, Dendrimer functional hydroxyapatite nanoparticles generated by functionalization with siloxane-cored PAMAM dendrons. J. Colloid Interface Sci. 500, 105–112 (2017)
U. Kurtan, A. Baykal, H. Sözeri, Synthesis and characterization of sulfamic-acid functionalized magnetic Fe3O4 nanoparticles coated by poly(amidoamine) dendrimer. J. Inorg. Organomet. Polym. Mater. 24(6), 948–953 (2014)
M. Tajabadi, M.E. Khosroshahi, S. Bonakdar, An efficient method of SPION synthesis coated with third generation PAMAM dendrimer. Colloids Surf. A 431, 18–26 (2013)
S. Venkateswarlu et al., A novel green synthesis of Fe3O4 magnetic nanorods using Punica Granatum rind extract and its application for removal of Pb(II) from aqueous environment. Arab. J. Chem. (2014). https://doi.org/10.1016/j.arabjc.2014.09.006
Pan, B.-f., F., Gao, H. Gu, Dendrimer modified magnetite nanoparticles for protein immobilization. J. Colloid Interface Sci. 284(1), 1–6 (2005)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zarei, A., Saedi, S. & seidi, F. Synthesis and Application of Fe3O4@SiO2@Carboxyl-Terminated PAMAM Dendrimer Nanocomposite for Heavy Metal Removal. J Inorg Organomet Polym 28, 2835–2843 (2018). https://doi.org/10.1007/s10904-018-0948-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-018-0948-y