Abstract
Five undoped and transition metal doped lithium borate glasses, were prepared by melting annealing method at low temperature range (650–750 °C). X-ray diffractograms confirm the amorphous natures of the prepared glasses. Some of their properties such as density, Infrared absorption spectra (FTIR) and electrical conductivity have been studied before and after being subjected to gamma radiation. Optical UV absorbance and calculations of optical energy gap have been also studied on some selected glasses. The variation in density, electrical and spectroscopic parameters was discussed in terms of both valence state and ionic radius of each doped transition metal modifier. FTIR studies demonstrate that these glasses consist of BO3 and BO4 constructing groups with an obvious increase in their IR band intensities after irradiation with 20 kGy. The electrical properties of glasses were studied under the effect of both gamma radiation and temperature range from 298 to 473 K. A significant variation in electrical conductivity values was obvious when the temperature exceeds 413 K in the dose range from 5 to 20 kGy of gamma radiation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Experimentally, Borate glasses have two important advantages; (1) the easily preparation method especially in presence of flux materials such as sodium carbonates that reduces the required melting temperatures. (2) They are good hosts for modifier oxides like transition metal oxides (TMO). For instance; TM doped lithium borate glasses are of great interest in many industrial applications. Cathode materials with interesting semiconducting properties in Li+ ion battery are popular example of their applications [1]. They can also be used in laser and infrared detection applications [2] because they have fast conductive properties. As well as their using in radiation dosemetric applications because of having an atomic number very near to that of human tissues [3]. Boron oxide B2O3 is a good glass former, it has two types of boron coordination; three and four coordinated boron BO3 and [BO4]− respectively. BO3 groups are only found in the pure B2O3 glass according to Nattapol et al. [1]. These units convert to [BO4]− with an addition of alkali or modifier oxide due to the formation of more non-bridging oxygen (NBO). Presence of TM ions incorporated in borate glasses caused unusual coordination environment due to their different oxidizing states. Some authors assumed that the TM ions occupy the network former sites while others showed that they are surrounded by modifying cations because the network former polyhedra cannot deal with their charge [4]. Incorporation of tellurium ions in borate glassy systems together with TM ions acquire glasses special physical properties such as low annealing temperature, relatively low electrical resistivity and, quite good infrared transmission properties e.g. vanadium tellurite glasses [5].
Studying effects and defects caused by gamma radiation on lithium borate glasses is very important since stronger lamps and lasers which are working at very shorter wavelengths become more widespread. Many developed research have been studied the effect of gamma radiation on lithium diborate glasses containing different TM ions [6]. Other researchers indicated also the dosimetric structures of lithium borate glasses doped with strontium/copper ions [7]. They confirmed that the number of NBO in glass affected greatly by the interaction of gamma radiation. Generally the main result of gamma radiation on glasses is to form induced point defects [8]. It was accepted that the response of glass to gamma radiation depends mainly on the structure of the glassy network as well as the type of radiation (i.e. ionizing or particle) and the energy of radiation impinging on the glass [9]. The nature of radiation destruction can be divided into three classes: (1) atomic displacement and energy transfer (2) ionization and charge trapping or (3) radiolytic and photochemical effects. In most cases gamma irradiation includes the second and third effects since the particle radiation creates displacements [10,11,12,13,14].
The main goal of this work is to prepare mixed glassy systems of alkali lithium borate glasses containing tellrium oxide and doped with different TM ions. A characteristic comparative study between the prepared glasses towards successive gamma radiation doses was discussed in a wide dose range starts from 1 to 100 kGy. Some physical properties were examined to test their sensitivity to different doses of gamma radiation such as density, FTIR spectra, electrical conductivity (EC), optical UV absorbance and calculations of optical energy gap before and after being subjected to gamma radiation.
2 Materials and Methods
2.1 Preparation of Glasses
Five glass batches were prepared with the chemical composition (in wt%): 56% B2O3 + 25% Li2O3 + 10%Na2O + 5% CaO + 2% Al2O3 + 2% SrO doped with 0.5% TeO2 + (x); where x = 0 for G1 and x = 1% from V2O5, ZrO2, NiO or CoO for G2, G3, G4 and G5, respectively. Chemically pure H3BO3 and analytical reagent grade quality of Li2CO3, Na2CO3, CaCO3, SrCO3, TeO2, V2O5, ZrO2, NiO and CoO were purchased from Sigma-Aldrich company and used to prepare the investigated glasses according to each composition. Table 1 shows obviously the compositions of the five prepared glasses. The five batches were precisely weighed by an electronic balance, stirred carefully and converted into a fine powder. By using porcelain crucibles they were melted in an electric furnace at 650–750 °C for 90 min with stirring of the melts to achieve homogeneity. The melts were then casted into preheated stainless steel molds and immediately transferred to a muffle furnace regulated at 350–380 °C for annealing. After 1 h, the muffle was switched off and its temperature decreased to room temperature with a rate 25 °C/h.
2.2 Gamma Irradiation
A Co60 gamma cell (2000 Ci) was used as a gamma ray source with a dose rate of 1.5 Gy/s at 30 °C. The glass samples were located into gamma cell in means that every sample was exposed to the required identical dose. The irradiation procedure has been achieved by putting the glass samples in the same place around a cylinder placed inside the chamber; the glass samples were irradiated for the necessary time interval to achieve the desired overall cumulative dose.
2.3 X-Ray Diffraction Measurements (XRD)
In order to identify the structural natures of the glass specimens, a Shimadzu XD-DI diffractometer was used. Measuring was worked at 30 mA and 40 kV. X- ray diffraction forms were studied at room temperature and in continuous functioning conditions.
2.4 Density
Density of the prepared glass samples before and after being gamma irradiated with 20 kGy was measured at room temperature, using the suspended weight method based on Archimeds principle. Xylene was used as immersion liquid. All the measurements were made three times, the maximum error 0.0002 g/cm3.
2.5 Infrared Absorption Measurements
Spectra of infrared absorption were examined at room temperature for all prepared glasses at 400–4000 cm−1 wave number range. Spectrometer type VERTEX 70, FT/IR-430, Japan was used in measuring. Glass samples were measured before and after being subjected to 1, 5, 20 and 100 kGy of gamma radiation.
2.6 UV–Visible Absorption Measurements
The optical absorption spectra of three highly polished samples of the TM doped glasses—V5+, Zr4+ and Ni2+ doped glasses—with the dimensions 1 \(\times\) 4 \(\times \hspace{0.17em}\)0.2 cm3 were recorded at room temperature before and after successive gamma irradiation with 20 kGy using a recording spectrophotometer in the range 200–1100 nm, type JASCO, Rel-00, Corp.,V-570. Japan.
2.7 Electrical Conductivity Measurements
Electrical conductivity measurements were performed for all glass samples before and after being subjected to 1, 5, 20 and 100 kGy of gamma radiation by using a locally constructed measuring system which is connected with an electronic temperature controller. For this purpose disc shaped samples were polished then rubbed with silver paste to give good contact with copper cell. A programmable digital electrometer/high resistance meter (KEITHLY 6517B) was used for the resistivity measurements at constant frequency and voltage with reliable fast response together with a high precion of power supply. All measurements take place with varying temperature range from 298 to 473 K. Glass samples were electrically measured after every raising of 20 °C.
3 Results and Discussion
Patterns of X-ray diffraction (XRD) are illustrated in Fig. 1 for the five prepared glasses. Where there is no sharp diffraction peaks are detected but a broad hump reflecting the characteristic of glassy amorphous natures.
3.1 Density
Density values of lithium borate glasses are listed in Table 2 where it can be noticed that the addition of TM ions V5+, Ni2+ and Co2+ leads to a slight increase in density values except when the glass is doped with Zr4+ ion, glass density decreases. The results also showed that all density values are decreased after irradiation with a dose of 20 kGy. Since the irradiation of doses 1, 5 and 100 kGy would not show a significate variation in the spectroscopic FTIR results as it will be later observed, but the dose of 20 kGy shows clear variations so the last dose would be taken as an example to explain the effect of irradiation on density values. Density was calculated according to the following formula:
where ρ is density of the glass sample, a and b are weights of the glass samples in the air and in xylene respectively, and 0.86 is the density of xylene at 20 °C.
Generally, density of glass is described as a contest between masses and volumes of their internal structural groups. Therefore, density is correlated to the chemical composition of glass constituents and how firmly the atomic groups of glassy network are interconnected altogether [15]. When the TM ions are doped in a glass they can behave as modifiers because they are housed in the interstices of the vitreous network causing an excess of oxygen and more NBO are produced. According to El-Alaily [16] the addition of ions to a glass changes the density ratios to the mass added ions corresponding to that of the ions already present in the glass. Density was previously defined as the weight per unit volume and it is thought that it depends mainly on masses of introduced ions so it may be expected that, the higher the atomic mass of introduced ion, the higher density value of its glass. But in fact this behavior does not take place when the atomic masses and ionic radii of these ions are high enough to retard their fitting inside the glass network interstices. The atomic mass order of the doped TM ion is: Zr91.22 > Co58.93 > Ni58.69 > V50 but according to our results the maximum value of density is given for G2 (V50) followed by G4 (Ni58.69) then G5 (Co58.93). Since the smaller the cation radius of doped TM ion, the easier to fit itself in the glass matrix. In other words it can be realized that the large ionic radius leads to an increase in the degree of distortion by increasing number of NBO to give more open structure with a large applied volume and then a decrease in density would take place. This can be interpreted the decrease in the density value by introducing Zr4+ ions as in the case of G3.
It can be also argued that the number of valance electrons of the doped ions can play an important role in changing the density value, where the number of accompanied oxygens differs according to its valence. E.g.by comparing the density value of glass containing V5+ and that containing either Ni2+ or Co2+ ions. V5+ ions are with more oxygen content and many oxidation states (V2+, V3+, V4+ or V5+) so they are able to change more of the NBO to BO or changing the trigonal BO3 to tetrahedral BO4 units. This behavior causes more compaction to the glass structure and gives in turn higher density values. From Table 2 it can be also observed that gamma irradiation with 20 kGy leads to a decrease in the density values which may be attributed to the growing of degree of distortion produced from the atomic movements and electronic defects caused by the ionizing radiation. An increase in the number of NBO would then take place giving more amorphous open structure, so the net result would be a lowering in density values [16, 17].
3.2 Infrared Absorption Spectra (FTIR)
Figures 2 and 3 elucidate the infrared spectra of undoped and TM doped lithium borate glasses before and after being exposed to different doses of gamma radiation. The vibrational types of borate network configurations are mainly concerned in three infrared spectral regions: (1) 1200–1400 cm−1 refers to B–O stretching of trigonal BO3 units; metaborates, pyroborates, and orthoborates [18]. (2) 850–1200 cm−1 refers to B–O stretching of tetrahedral BO4 units. (3) 600–800 cm−1 refers to the bending vibrations of various borate segments [6]. The observed small peaks in the range from 400 to 600 cm−1 may refer to the doped TM ions in octahedral units. The band at 470–490 cm−1 may assigned to specific vibration of Li–O bonds in two residing locates as bridging and non-bridging types as it was observed by Kamitsos [19]. As well as the band at 720 cm−1 may be produced due to B–O–B bend vibrations of borate network [20].
Figure 2 shows very insignificant effect on IR spectrum after doping with the TM ions. This manner may be due to the relatively low percent of the doped transition metal percent (1 wt%) so most of the structural groups remain unchanged giving their distinctive vibrations. The last realization may be granted with the concept of independent vibrations of different groups according to Tarte [21] and Condrate [22] which in sequence give multifaceted infrared spectra of lithium borate glasses owing to their widely overlapping bands. Consequently the high-frequency absorption 900–1100 cm−1 cannot easily be attributed to definite borate units because most of the borate groups absorb in this area. On the other hand, the manners of boron–oxygen triangular configurations (BO3 and BO2O−) are arising at 1200–1650 cm−1. The minor bands ranging from 2400 to 4000 cm−1 with the distinctive near-infrared absorption bands are attributable to OH, B–OH and H2O vibrations as it was ascribed by El Batal et al. [6]. Infrared absorption assignments of the bands attained from the investigated glasses are abbreviated in Table 3.
Gamma irradiation causes some alterations in the IR spectra of lithium borate glasses. This may be owing to the variation in bond angles and/or bond lengths in the B–O connecting groups so the progress or observation of certain peaks would be obtained. El Batal [23] assigned the possible changes caused by gamma irradiation in glasses to the changes in their constructing groups. Effects caused by irradiation on glass strongly depend on the glass matrix, dopants and impurity content as well as the irradiation dose. It is obvious from Fig. 3 that the dose of 20 kGy makes remarkable changes on the IR spectra for the five undoped and TM doped glasses. There is a large increase in the absorbance intensity and peaks become sharper, also there is a shift either to higher or lower wavenumber. The formed sharp peak at 1960–1720 cm−1 can be attributed to vibrations of B–O bonds. Also it can be noticed that there is a splitting in the band at 1200–1400 cm−1 that is attributed to B–O stretching of trigonal BO3 units. The band at 850–1200 cm−1 which is ascribed to B–O stretching of BO4 tetrahedral units is moved to a lower wavenumber. The last observations can be explained by two assumptions (1) at the dose of 20 kGy the energy given causes a tendency to more randomness or amorphicity to the structure due to the presence of many excited free electrons throughout the glass structure much more than the ability of the glass network to deal with. This means that the group arrangement becomes more asymmetric [24] and forms more NBO and more conversion of BO4 to BO3 that in turn leads to weakening of the network grouping vibration. (2) The ionizing process causes a relaxation in the network structure and reveals the electron hole pairs to provide paths for bond arrangements. While some of the spare energy kept in the structure are emitted for the relaxation process associated with a decrease in the bridging bond angel average [25, 26]. This effect continues only for G1 (the base glass) when irradiated as well by a dose of 50 kGy. While for other glasses containing TMO, as the irradiation dose increases the glass arrangement settled to give more stable structures because TM ions can absorb the released electrons. So the recombination process happens and causes the detected stability in the IR spectra as shown in Fig. 3.
3.3 Optical UV Absorption Spectra
Optical methods like UV–Visible and infrared spectroscopy can give information about the average coordination number, local symmetry or covalent bonds between the metal ion and shell neighbors as well as the bond angels and lengths [15]. The presence of TM ions even at small concentrations causes an electron transfer mechanism including the transition of an electron from the orbital of a coordinating oxygen atom to an orbital of the metal ion. So that an obvious UV band on the UV spectrum appears.
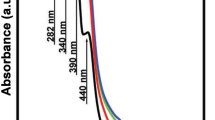
Figure 4a, b illustrates the UV–Visible absorption of three selected glasses G2, G3, G4 before and after being subjected to 20 kGy of gamma radiation. The UV absorbance was made by taking the base glass (G1) as a reference during measuring in order to study obviously the effect of doping such a transition metal. Since the dose of 20 kGy is the most effective dose as it was previously observed in density and IR results, it is selected to study the effect of radiation on the optical UV spectra. Figure 4a shows higher intensity absorption peaks for G4 than those of G2 and G3. It shows also a resolution of two absorption peaks at 320 and 485 nm in G4 or Ni2+ doped glass. The two peaks may originated from the combined sharing of both the divalent state of Ni2+ in octahedral coordination and the trace iron impurities Fe3+ present in the base host glass. From the figure it is obvious also that G2 and G3 that contain V5+ and Zr4+ ions respectively, have similar optical spectra with two predominant absorption peaks at 340 and 620 nm. The absorption spectra are in agreement with Nassar and Ghoneim [27] who postulated that the bands appear between 275 and 377 nm can be attributed to the charge transfer band of V5+ ions in the UV region, assuring the presence of only +5 oxidation state of vanadium ions in all glass. However The d–d transition absorption band of V4+ which contains one unpaired electron in d-orbital may appear at approximately 620 nm, which can be assigned to the transition of electron from 2B2g stage to 2Eg stage of VO2+ ions according to Rao et al. [28]. Likewise the d–d transition absorption band of Zr4+ which may also assigned to the electronic transition between the pervious stages of ZrO2+present in Li2O–CaO–B2O3 glasses containing ZrO2.
Figure 4b shows the UV spectra of glasses after being subjected to gamma radiation with a dose of 20 kGy, strong absorption bands are appeared with higher absorption intensities in the three doped glasses at 230 nm in G4 and 248 nm in G2 and G3 in addition to a peak at 337 nm in the three doped glasses. It is observed also that G2 and G3 have similar spectral behavior after irradiation.
The effect of gamma radiation on the glassy network depends on both the type of glass, the intrinsic defects already present within the glass before irradiation and the dose of irradiation [29]. The amorphous structure nature of the glassy network contains mainly of pre-existing intrinsic defects such as oxygen vacancies, NBO, or flaws created by the high energetic electrons. By subjecting glass to gamma radiation, it affects directly on the host glass itself by changing the number of NBO and/or breaking bonds. As shown in Fig. 4b where there is an increase in absorbance intensity of the three irradiated doped glasses. The observed induced absorption could be ascribed in accordance with the assumption of Mo¨ncke and Ehrt [30] who proposed that the formed extrinsic defects are owing to some photochemical reactions; photoreduction or photooxidation of the TM ions as a result of gamma irradiation. It may be also correlated with the formation of induced positive hole centers of the host borate network [31] which consists of mixed BO3 and BO4 groups with different structural combining units. When the TM ions doped glass exposed to UV radiation, the photons produce electrons and holes into the amorphous glassy matrix. Then they are trapped creating defect or color centers so an increase in absorbance of the UV photons would take place. These ions are not only able to change the intrinsic trapping sites of the glass but also affect both formation and recovery rates of the intrinsic color centers. The amount of higher valence ions caused by irradiation is directly proportionated to the amount of lower valence ions that already present before irradiation. So the photochemical reaction would be the photo-oxidation of the lower valence ions, where electrons produced from this process are trapped leading to the formation of electron centers. As well as the creation of hole centers is suppressed through the hole trapping which may lead to varying in some physical properties like transmittance intensity decrease or absorbance intensity increase on the UV spectrum [32].
3.3.1 Optical Band Gap
In amorphous glassy systems, energy gap confined between valence and conduction bands can be stated by defining the optical band gap (Eopt). The base recognized in this performance is that the photon has higher energy than the absorbed band gap energy [33]. Figure 5 shows the optical band gap of G2, G3 and G4 (a) before and (b) after gamma irradiation by plotting (αhν)n against the incident photon energy (hν) as given by Eq. (2) [34, 35].
where α(ν) is the absorption coefficient, αo is a constant, n is a constant depends on the mechanism of electron transition (direct transition or indirect transition) and depending on whether of the transition is allowed or forbidden [20]. The results shown in Fig. 5 give an agreement with clarity that glasses with high absorbance intensity—see Fig. 4—should have a lower Eopt. Table 4 indicates Eopt values of glasses where the values are ranged from 0.464 to 1.408 eV.
In homogeneous materials, the lone–pair (LP) electron states produce the valence band whereas antibonding states produce the conduction band. As Feifei et al. [36] have reported, an increase in the bond strengths causes a larger splitting between the valence band and conduction band which leads to an increase in Eopt. This concept is agreed with Abo-Naf et al. [37] who assumed that, the more covalent bonds inside the glassy system network, not only reinforces it but also rises the number of bridging oxygens which in turn responsible for the higher values of Eopt. For example introducing of V5+ ions as in G2 glass may cause an increase in the Eopt as shown in Fig. 5 where these ions cause a compaction to the glass structure by two ways. The first is by filling the vacancies found in the glass structure, the second is by using the excess of oxygen in changing BO3 to BO4. This in turn gives higher density—see Table 2—and higher Eopt values as listed in Table 4. However by comparing Eopt values of G2 and G3, we found that G3 has lower Eopt values than G2 because G3 where Zr4+ ions are introduced in; has higher atomic weight and ionic radius. Therefore a disruption in the network connectivity would take place and forms more open structure with a decrease in the B–O–B linkages or bridging oxygens (BO). So a progressive increase in NBO concentration occurs then the chance for more electronic transitions between localized states becomes valid; and the net result will be a small Eopt values [38]. This postulation is agreed with density results listed in Table 2 as the glass with high density (e.g. G2) gives low absorbance intensity and high Eopt values and vice versa in case of G3. However G4 glass gives more stability in its Eopt values as like as its density values. Generally it is observed that there is a decrease in the Eopt values for the three tested glasses after irradiation. This may be due to the increase in the spin density of unpaired electrons or the number of energetic electrons inside the glassy matrix [37]. This performance causes more electronic transitions therefore the band tailing is definite in the form of a reduction in the forbidden energy gap.
3.4 Electrical Conductivity (EC)
Electrical resistance values were measured at a temperature range from 298 to 473 K. The specific electrical conductivity (σ) of each glass sample can be calculated as follows:
where (σ) is the electrical conductivity of the glass sample, (A) is its cross sectional area in cm2 and (L) is its thickness in cm and (r) is its measured resistance in ohm. The dependence of the electrical conductivity on temperature can usually be expressed according to Arrhenius equation as follows:
where (σ) is the electrical conductivity of the glass sample, (σo) is a constant namely frequency factor or pre-exponential factor, E is the activation energy of electrical conduction, (T) is the absolute temperature and (R) is the universal gas constant. The effect of temperature on electrical conductivity is expressed by plotting log σ on the y-axis against 1000/T on the x-axis and results were illustrated in Figs. 6 and 7 before and after gamma irradiation, respectively.
Previously, it was reported by Doremus [39] that the moving of alkali ions present in the interstitial positions within the glassy network is responsible for the electrical conduction in almost alkali oxide containing glasses. In marketable glasses the conducting species are sodium and lithium ions which are also relatively mobile in several oxide glasses according to Ezz El-Din et al. [40]. They concluded that in ordinary borate and silicate systems containing no transition metal ions, alkali cations are supposed to be the main carriers of the electrical current. So if a glass is free of network modifiers, then it must possess a very low conductivity or electrically resistant like the traditional silicate glasses. However for glasses containing TM ions conduction is attended where the TM ions can lose or accept electrons and therefore change their valence states that acquire glasses an electrical conducting behavior [41]. Conduction in oxide glasses is essentially ionic since anions are not observed to move however cations are considered to be the charge carriers. Consequently the electrical conductivity is directly proportionated to some factors: valence and size of charge carriers and their concentrations in addition to their ability to movement or diffusion under the effect of an external electric field. Migration of mobile ions is also affected greatly by temperature because at certain temperatures, the resistance of ions mobility decreases where they begin to move more freely causing an increase in the EC values as shown in Figs. 6 and 7 in the temperature range 433–473 K due to the thermally activated hopping of charge carriers.
Glasses containing both alkali ions as well as TM ions have a special trend in their electrical properties as they have a mixed electronic–ionic, pure ionic or pure electronic conduction depending on glass composition constituents [42]. In this case the conduction behavior can be ascribed depending on both alkali ions and the electron hopping between different valence states of the doped TM ion. The alkali ions are present in high concentrations in the prepared glasses where they can act as network modifying ions leading to some structural changes in borate glass network. They can cause creation of more NBO and reconversion of BO4 to BO3 units therefore more open or weaker structure would be obtained. This trend allows the mobile ions or charge carriers to move more freely and give an increase in the conductivity values.
Pratula et al. [4] have showed a mixed valence states for the vanadium ions as Vreduced and Voxidized (V5+ ↔ V4+) where the ions can build a path or chain between those different valence states for the charge transfer process. They reported also that this type of chains looks to be interrupted by the presence of alkali or alkali-earth oxides. While Terny et al. [43] confirmed the independent migration path of alkali and TM ions on the electrical response of the glassy system x BaO (1 − x) (0.5V2O5 · 0.5MoO3) 2TeO2. They concluded that the active centers of vanadium ions take responsibility of polaron hopping mechanism while barium cations are responsible for the ionic transport regime. Moreover, according to Sen and Ghosh [44] glasses are ionic conductors when the ratio of TM to alkali metal oxides is lower than 1 (TMO/AMO < 1). In this case the hopping mechanism relates to the low mobility oxide semiconductors and the charge carriers are defined as small-polarons. This assumption can be applied on the prepared glasses where the TM ions are doped in low percent (only 1 wt%) but the alkali ions are present in high concentrations (25% Li+ + 10% Na+ + 2% Al3+ + 5% Ca2+ wt%). So it can be concluded that; if the alkali ions are present in high concentrations they will be the predominant species responsible for the conduction. Where the TM ions are coordinated to the free oxygens which are not part of the glass network but are attached to one end, these kinds of glass are called quasi-molecular complexes [41]. This interpretation explains that the undoped glass G1 has higher electrical conductivity values than the other TM doped glasses as shown in Fig. 6. From the figure it is also observed that G2 has relatively lower EC values than the other three TM doped glasses which may be agreed with its high density values listed in Table 2. Where the denser and more blocked structure can relatively retard the motion of alkali mobile ions or charge carriers that are responsible for the conduction. So we may expect that our glassy systems behave as ionic conductors and due to the high concentration of mixed alkali ions, a distribution of cations mobility can take place and their paths become more crowded. Therefore the electrical mechanism of the investigated systems of TM doped alkali glasses would be with an independent path way.
Figure 7 shows the effect of different gamma radiation doses ranged from 1 to 100 kGy on EC values of all examined glasses in the same previous temperature range. It can be observed that each glass gives certain stability in its electrical behavior with heating energy until the temperature reaches to ~433 K. However an obvious deviation in their EC values takes place with gamma irradiation doses. It is also observed that there is a slight increase in EC values with the dose range from 5 to 20 kGy and this range becomes more widely for the undoped glass G1 to extend to 50 kGy. Previously it was reported that the increase in DC conductivity and in polarization current is related to radiation-induced space charge as well as rate of electron creation [45]. Some researchers have attributed the observed EC changes caused by irradiation to the creation of glass matrix defects [46]. So it may be expected that EC of glasses increases with irradiation due to increasing number of either interstitials or vacancies travelling to the glass surface. This in turn allows alkali ions to emigrate through the glass matrix and causes a remarked increase in the EC values [47].
The absence of TM ions in G1 glass gives more availability for Li+ and Na+ ions to move more freely with the help of both radiation and heating energies without the electronic overcrowding caused by the TM ions. However, irradiation of glasses with the high doses 50 and/or 100 kGy leads to a slight stability—or decreasing in some cases—in EC values of glasses. At high doses of radiation, movement of vacancies becomes very slow or terminated because recombination between the formed holes or color centers may take place and reduce defect concentrations. In addition, the decaying of EC at high doses are produced by mobile ions which can move through the glassy network but have slightly long relaxation periods and finally become restricted [48].
4 Conclusion
Five amorphous undoped and TM doped lithium borate glasses are prepared economically at 650–750 °C. They are followed by some physical measurements before and after gamma irradiation such as density, FTIR spectra, electrical conductivity (EC), optical UV absorbance and calculations of optical energy gap. Results obtained can be concluded as follows.
Most of structural groups remain unchanged after doping of TM ions on IR spectrum depending on the concept of independent vibrations of different groups. The obvious sharp peak appears at 1690–1720 cm−1 after irradiating the base glass (G1) with 20 and 50 kGy is related to B–O bonds due to more disorders or amorphicity. While it appears only at the dose of 20 kGy for TM doped glasses because the relaxation process may be facilitated by doping of TM ions at higher doses. Density of glass are increased after doping with V5+, Ni2+ or Co2+ ions in the order of their atomic weights where a reducing of the free volume takes place to give more closed structure. However doping with the large ionic radius of Zr4+ ions tends to decrease the glass density because the accommodation of such large ions increases the glass B–O frame work. Gamma irradiation with 20 kGy causes a slightly lowering in density values because of more distortions or electronic defects. The UV optical spectra of glasses before irradiation reveals charge transfer bands of G4 or Ni2+doped glass at 320 and 485 nm originated from the combined sharing of the divalent states of Ni2+ in octahedral coordination. However the UV band appears at approximately 620 nm in both G2 and G3 may refer to the d-d transition absorption band of V4+ or Zr4+ ions respectively. More electronic transitions take place between valence and conduction bands gives lower Eopt because of increasing the electron spin density after irradiation. Electrical conductivity of the base glass G1 depends on the ionic conduction due to the hopping mechanism of alkali ions as charge carriers. However TM doped glasses follow a mixture of ionic and electronic mechanism. Temperature range from 433 to 473 K shows a clear change in EC results where the mobile ions move more freely due to the thermally activated hopping of charge carriers. EC values are increased under the effect of radiation because of the increase of vacancies and defects in the glass structure. However at higher irradiation doses 50 and/or 100 kGy, a recombination of vacancies takes place and causes a stability or decrease in EC values. The last assumption is obviously compatible with IR results where they give high stable spectra at the same high doses similar to their spectra before irradiation.
G2 glass or V5+ doped glass has higher density values among the prepared glasses. In sequence it gives lower UV absorbance intensity and higher Eopt with relatively smaller electrical conductivity values in opposite to G3 or Zr4+ doped glass. Results obtained can be interpreted briefly as; when the density of the glass decreased, a more open structure with more vacancies and high NBOs is formed. So more electronic transitions between localized states are available which in turn cause a decrease in Eopt values. Then the alkali ions or charge carriers that are responsible for the conduction would move more freely giving higher electrical conductivity values.
References
L. Nattapol, P. Panida, K. Pinit, K. Phetlada, T. Weerinradah, P. Ratchadaporn, Non-Cryst. Solids 453, 118 (2016)
M. Jewell, Non-Cryst. Solids 146, 145 (1992)
A. Ab Rasid, H. Wagiran, S. Hashim, Z. Ibrahim, H. Ali, Radiat. Phys. Chem. 112, 29 (2015)
P.E. Pratula, S. Terny, E.C. Cardillo, M.A. Frechero, Solid State Sci. 49, 83 (2015)
E.P. Denton, H. Rawson, J.E. Stanworth, Nature 173, 1030 (1954)
F.H. El Batal, A.A. El Kheshen, M.A. Azooz, S.M. Abo-Naf, Opt. Mater. 30, 881 (2008)
H.K. Obayes, H. Wagiran, R. Hussin, M.A. Saeed, Luminescence 176, 202 (2016)
N.A. El-Alaily, E.M. Abou Hussein, Y.K. Abdel-Monem, T.D. Abd Elaziz, F.M. Ezz Eldin, Radioanal. Nucl. Chem. 299, 65 (2014)
A.M. Abdelghany, H.A. ElBatal, Non-Cryst. Solids 379, 214 (2013)
I. Ardelean, S. Cor, Mater. Sci. Mater. Electron. 19, 584 (2008)
H. Doweidar, Y.B. Saddeek, Non-Cryst. Solids 355, 348 (2009)
R.D. Husung, R.H. Doremus, Mater. Res. 5, 2209 (1990)
E.I. Kamitsos, A.P. Patsis, M.A. Karakaissides, G.D. Chryssikas, Non-Cryst. Solids 126, 52 (1990)
F.H. ElBatal, A.A. Elkheshen, M.A. Azooz, S.M. Abo-Naf, Opt. Mater. 30, 881 (2008)
M.A. Marzouk, J. Mol. Struct. 1019, 80 (2012)
N.A. El-Alaily, Glass Technol. 44, 30 (2003)
F.M. Ezz Eldin, N.A. El-Alaily, H.A. El Batal, Radioanal. Nucl. Chem. 163, 267 (1992)
B. Ashok, R.V. Kumar, P. Kistaiah, Non-Cryst. Solids 426, 47 (2015)
E.I. Kamitsos, Phys. Chem. Glasses 44, 79 (2003)
I. Kashif, A. Ratep, J. Mol. Struct. 1102, 1 (2015)
P. Tarte, Spectrochim. Acta 18, 467 (1962)
R. Condrate, Introduction to Glass Science (Plenum Press, New York, 1972)
F.H.E.L. Batal, Egypt J. Chem. 47, 101 (2004)
W. Primak, Appl. Phys. 43, 2745 (1972)
F. Piao, W.G. Oldham, E.E. Haller, Non-Cryst. Solids 276, 61 (2000)
F. Piao, W.G. Oldham, E.E. Haller, Appl. Phys. 87, 3287 (2000)
A.M.A. Nassar, N.A. Ghoneim, Non-Cryst. Solids 46, 181 (1982)
R.B. Rao, N.O. Gopal, N. Veeraiah, J. Alloys Compd. 368, 25 (2004)
A.M. Abdelghany, F.H. El Batal, H.A. El Batal, F.M. Ezz ElDin, J. Mol. Struct. 1074, 503 (2014)
D. Moncke, D. Ehrt, Opt. Mater. 25, 425 (2004)
A. Bishay, Non-Cryst. Solids 3, 54 (1970)
S.Y. El-Zaiat, M. Medhat, M.F. Omar, M.A. Shirif, Opt. Commun. 370, 176 (2016)
J.S. Kumar, J.L. Kumari, J.S. Rao, M.S. Cole, Opt. Mater. 35, 1320 (2013)
R. Stefan, E. Culea, P. Pascuta, Non-Cryst. Solids 358, 839 (2012)
B. Sumalatha, I. Omkaram, T. Rajavardhana, Rao, Ch. Linga Raju, Non-Cryst. Solids 357, 3143 (2011)
C. Feifei, D. Shixun, N. Qiuhua, X. Tiefeng, S. Xiang, W. Xunsi, Wuhan Univ. Technol. Mater. Sci. Ed. 24, 716 (2009)
S.M. Abo-Naf, R.L. Elwan, M.A. Marzouk, Mater. Sci. Mater. Electron. 23, 1022 (2012)
D. Saritha, Y. Markandeya, M. Salagram, M. Vithal, A.K. Singh, G. Bhikshamaiah, Non-Cryst. Solids 354, 5573 (2008)
R.H. Doremus, Glass Science, 2nd edn (Wiley, New York, 1994)
F.M. Ezz El-Din, A.H. Zahran, N.A. El-Alaily, H.A. El-Batal, Indian J. Pure Appl. Phys. 31, 481 (1993)
I. Kashif, S.A. Rahman, A.A. Soliman, E.M. Ibrahim, E.K. Abdel-Khalek, A.G. Mostafa, A.M. Sanad, Physica B 404, 3842 (2009)
S. A.Yadav, A. Khasa, M.S. Hooda, A.Agarwal Dahiya, P. Chandc, Spectrochim. Acta A 157, 129 (2016)
C.S. Terny, E.C. Cardillo, P.E. di Prátula, M.A. Villar, Non-Cryst. Solids 387, 107 (2014)
S. Sen, A. Ghosh, Appl. Phys. 87, 3355 (2000)
K. Yamamoto, M.J. Tsuchiya, Appl. Phys. 33, 3016 (1963)
N.A. El-Alaily, L.S. Ahmed, Eur. J. Glass Sci. Technol. 43, 80 (2002)
A.A. Soliman, S.A. Aly, H. Farouk, Y.M. Abo-Zeid, Radiat. Phys. Chem. 54, 499 (1999)
H.A. El-Batal, H. Farouk, F.M. Ezz-Eldin, Radiat. Phys. Chem. 47, 811 (1996)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abou Hussein, E.M., El-Alaily, N.A. Study on the Effect of Gamma Radiation on Some Spectroscopic and Electrical Properties of Lithium Borate Glasses. J Inorg Organomet Polym 28, 1214–1225 (2018). https://doi.org/10.1007/s10904-017-0776-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-017-0776-5