Abstract
The medical field remains challenged with many unmet needs, prompting rigorous research for solutions. Dendrimers are emerging as innovative solutions to many unmet clinical needs. Indeed, commercial dendrimer-enabled gene transfection technologies exist and work is ongoing to translate many other dendrimer products into the market to assist patients. Here, we review the ongoing work on dendrimer-enabled drug delivery technologies, diagnostic platforms, and antimicrobial agents. Our goal is to inspire the continuous exploration of the dendritic scaffold to address many issues in the biomedical landscape.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
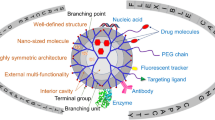
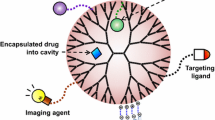
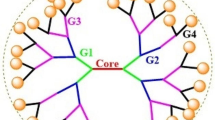
In 1985, Newkome et al. [1] and Tomalia et al. [2] independently reported the synthesis of “monocascade spheres” and “starburst dendritic macromolecules,” respectively, introducing an intriguing class of macromolecules, now known as dendrimers. Interest in dendrimers remains intense with over 2000 scientific reports published since January 2017 (SciFinder database search on 31st October 2017). This interest stems from dendrimer’s engineerable structure, which incorporates a core, well-defined branched monomers, and multiple termini (Fig. 1). Over the years, advances in chemistry have created a broad chemical space that offers versatile structures, diverse functionalities, and a rich synthetic toolbox to engineer the core, branches, and termini, endowing dendrimers with properties that address many fundamental and translational questions [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29]. Also, the nanoscopic dimension of dendrimers fosters their exploration as innovative solutions in nanotechnology and nanomedicine [30,31,32,33,34,35,36]. For instance, as nanovectors in drug delivery, a well-thought-out synthetic strategy permits control of critical nanoscale design parameters including size, shape, and surface chemistry to optimize relevant translational questions such as cellular uptake, transport, bioavailability, elimination, and toxicity [37, 38]. Their nanoscopic element is unique among biomaterials such as polymeric micelles and liposomes, which are limited by their in vivo instability, non-uniform dispersity, and lack of control over shape and size below the critical micelle concentration [38]. Dendrimers, on the other hand, are stable unimolecular micelles with well-defined size and shape that is independent of concentration [39,40,41,42]. Also, especially at higher generations, their physical properties differ from those of linear polymers [43,44,45,46,47], hallmarks that attract researchers to explore the dendritic scaffold as an attractive alternative to traditional polymer frameworks. Together, these features open new opportunities beyond the development of micelles initially proposed by Newkome et al. in their seminal report [1]. Indeed, dendrimer research is now mostly application-oriented with proven successes in catalysis [48,49,50], sensing [51, 52], and imaging [53, 54], as well as in drugs, genes and agrochemicals delivery [31, 55,56,57,58].
Application-driven investigation of dendrimers is a vibrant and growing field with numerous breathtaking developments in many areas (Fig. 2), biomedicine included. To put these advances in perspective, many experts have written excellent reviews on dendrimers, but the prolific contributions of researchers in field continually push the frontiers into new landscapes, opening new opportunities and challenges, and therefore, creating a need for frequent reviews and perspectives. Here, it is our goal to present application-driven investigations that exploit the dendritic structure to implement novel concepts, with the goal of addressing biomedical needs. Using selected research contributions, we aim to inspire continuous exploration of dendrimers as platforms to address many unmet needs in biomedicine.
2 Dendrimers in Biomedicine
2.1 Gene, Drug, and Vaccine Delivery
To address unmet clinical needs, researchers are exploring many fields including dendrimers for new diagnostic, prophylactic, and therapeutic platforms. Indeed, many commercial medical technologies incorporate dendrimers as functional units for advanced performance. For example, Dendritic Nanotechnologies, founded by Tomalia, leveraged the amenability of dendrimers to multi-functionalization to engineer and modify their proprietary dendrimer, Priostar®, into a dendrimer-enabled technology, PrioFect® Reagent, for DNA and siRNA transfection [59]. SuperFect®, another commercially available dendrimer-enabled medical technology, transfects nucleic acids into cells using a cationic dendrimer that interacts with the anionic phosphate groups of nucleic acid to form compact dendrimer/DNA polyplexes, which then enter cells via non-specific endocytosis [59].
Some cationic dendrimers have been successfully translated into marketable gene vectors, but their low transfection efficiency, as well as the poor post-transfection cell viability, create a need for a better technology. Toward this, Wang et al. functionalized poly(amidoamine) (PAMAM) dendrimer with hydrophobic and lipophilic fluoro groups to increase affinity for and permeation through the cell membrane [60]. As fluorination lowers surface energy, the fluorinated dendrimers aggregate at low concentrations, triggering the condensation of plasmid DNA into polyplexes at very low nitrogen to phosphorus (N/P) ratio. Achieving gene transfection at a low N/P ratio mitigates toxicity and ensures cell viability. The fluorinated dendrimer outperforms commercial transfection agents such as SuperFect® and Lipofectamine 2000 in transfecting enhanced green fluorescent protein plasmid into the human embryonic kidney and human cervical carcinoma (HeLa) cells (Fig. 3). The fluorinated dendrimer is inactive to fetal bovine serum proteins, retaining up to 55% DNA transfection efficiency in HeLa cells in contrast to Lipofectamine 2000 that almost loses its efficacy. Compared with the parent PAMAM dendrimer, fluorination enhances cellular uptake (~ 100%) of DNA into HeLa cells and promotes endosomal escape from the polyplexes [60].

Reproduced from Ref. [60] with permission of Macmillan Publishers Limited
Depending on the N/P ratio, fluorinated PAMAM dendrimers can outperform commercial Lipofectamine in transfecting enhanced green fluorescence protein plasmid into (a, c) HEK293 and (b, d) HeLa cells.
Delivery systems are critical to therapeutic efficacy [61], and therefore, must be designed to ensure the biocompatibility and bioavailability of their cargoes. Solubility-limited bioavailability renders 40–70% drug candidates ineffective [62], and new strategies are intensively pursued to bolster drugs’ solubility, as well as to enhance drugs’ permeation through physiological barriers. An approach tunes the dendritic scaffold via functionalization to enhance solubility, permeation and cellular uptake. For instance, Gao et al. modified the water solubility and bioavailability of a PAMAM dendrimer that encapsulates baicalin, a flavonoid reported for antitumor activity, by conjugating folic acid to the dendrimer’s termini [63]. The presence of the folic acid on the dendrimer enhances baicalin’s solubility and endowed the dendrimer–drug complex with target-specificity, leading to increased drug uptake by the folate receptor-positive HeLa cancer cells. As another approach, the Gu group used an enzyme-cleavable peptide linker, glycylphenylalanylleucylglycine, to conjugate an anticancer drug, doxorubicin or gemcitabine, to PEGylated peptide-based dendrimers [64,65,66]. The conjugates self-assembled into nanoparticles that release the drug in the presence of enzymes such as cathepsin B. For example, dendrimer–gemcitabine conjugate releases over 80% of gemcitabine in a cathepsin B-secreting cancer cells compared to non-secreting cells [66] (Fig. 4). The nanoparticles accumulate within tumor tissues, localizing the drug to enhance efficacy. Indeed, compared with the free gemcitabine hydrochloride, the conjugate more effectively suppresses tumor growth in mice with 4T1 murine breast cancer model without side effects (Fig. 4) [66].

Reproduced from Ref. [66] with permission from Acta Materialia Inc. Published by Elsevier Ltd
a Gemcitabine conjugated to dendrimer via enzyme cleavable bond. b Enzyme, cathepsin B, triggers the release of gemcitabine from dendrimer. c Gemcitabine–dendrimer conjugate is more active than free gemcitabine hydrochloride against 4T1 murine breast cancer cells.
The dendritic architecture is unique among polymers due to the presence of nanocavities (Fig. 1), which function as depots for diverse cargoes including therapeutics [67,68,69,70]. For instance, Nguyen et al. loaded cisplatin or 5-fluorouracil, well-known anticancer drugs, into carboxylated or PEGylated PAMAM dendrimers, respectively [71]. PAMAM dendrimers are a versatile platform to encapsulate drugs, for instance, their amines can coordinate to platinum, entrapping cisplatin or can hydrogen bond to 5-fluorouracil. Enabled by these interactions, the carboxylated and PEGylated dendrimers encapsulate up to 27% cisplatin and 35% 5-fluorouracil, respectively, within their nanocavities, and slowly release these cargoes [71]. As the electrostatic interaction between the cationic dendrimer’s termini and anionic cell membrane harm cells, it is essential to PEGylate or alkylate the termini to mitigate these interactions and enhance biocompatibility. Indeed, these modifications reduce the cytotoxicity of the dendrimer without compromising the therapeutic efficacy of the entrapped drugs, which remain active against NCI-H460 lung cancer cell and MCF-7 breast cancer cells [71].
Also, Conti et al. used an amine-terminated PAMAM dendrimer to complex and deliver siRNA to lung epithelium with up to 40% silencing efficiency [72]. In vitro, the N/P ratio has an insignificant effect on release profile, which strongly depends on pH. siRNA release is slower at pH 5 than at pH 7.4 (Fig. 5), a reasonable finding since acidic pH protonates the dendrimer’s amines, increasing binding affinity between cationic amines and the anionic siRNA, and ultimately reducing release rate. Conversely, physiological pH 7.4 deprotonates the tertiary amines, weakening the interaction between the dendrimer and the siRNA, and enhancing release rate. Also, the compact dendrimer–siRNA dendriplexes formed at pH 5 mitigates burst release, which is evident at pH 7.4 (Fig. 5). Conti et al. formulated these dendrimer–siRNA dendriplexes into a hydrofluoroalkane-based pressurized metered-dose inhaler to deliver siRNA to lungs [72].

Reproduced from Ref. [72] with permission of the American Chemical Society
Small interfering RNA release from dendrimer strongly depends on pH with little effect from N/P ratio. a N/P = 10; b N/P = 20; c N/P = 30.
Modern medical practice is advancing towards theranostic technologies that integrate drug delivery, target-specificity, and diagnostics in a single platform to localize and monitor treatment in real time [73, 74]. These platforms are particularly important in cancer therapy, where systemic toxicity and response to treatment are crucial to successful disease management. In this regard, dendrimers are an excellent candidate because of their flexibility towards multi- or hetero-functionalization. Zhang et al. leveraged this flexibility, designing a multifunctional aptamer sgc8-based DNA dendrimer that targets and images cancer cells as well as specifically delivers drugs to cancer cells [75]. The dendrimer achieves target specificity thanks to its aptamer sgc8 tags that recognize and enter human T-cell acute lymphoblastic leukemia cells via receptor PTK7-mediated endocytosis. Fluorescein isothiocyanate tags on the dendrimer enable real-time visualization of target and endocytosis of the DNA–dendrimer nanoparticles into the cells. Zhang and coworkers exploited the presence of a significant number of DNA base pairs in the dendrimer to intercalate doxorubicin between guanine–cytosine pairs. In vitro cytotoxicity assay proved the sgc8-based DNA dendrimer as an excellent technology to target cancer cells because unlike free doxorubicin, the intercalated drug specifically inhibits cell proliferation in human T-cell acute lymphoblastic leukemia cells significantly more than in control cells [69].
2.2 Antiseptic Agents
Microbial infections present a threat to our society as revealed by the World Health Organization global health statistics of 429,000 deaths from malaria, 1.1 million from HIV-related illnesses, 1.3 million from hepatitis, and 1.4 million from tuberculosis in 2015 [76]. In the same year, the economic burden was enormous considering that more than 1.6 billion people sought medical treatment for infectious diseases [70]. The prevalence of drug-resistant infections worsens this bleak scenario, emphasizing the need for an action plan [77]. Toward this, many research groups are developing potent antimicrobial agents to replenish the exhausted pipeline. The Abd-El-Aziz group developed a library of antimicrobial agents including those based on the dendritic scaffold (Figs. 6, 7, 8) [78,79,80,81]. They used the redox active organoiron sandwich complex, η6-dichloro-η5-cyclopentadienyliron(II) complex as a functional and structural motif to construct several dendrimers that exhibit potent activity against infection-causing microorganisms. The redox active iron centers trigger the formation of free radicals, such as reactive oxygen species (ROS), to harm the microorganisms [78, 79]. Field-emission scanning electron microscopy reveals that these positively charged dendrimers interact with and compromise the structural integrity of the negatively charged cell membrane of methicillin-resistant Staphylococcus aureus (MRSA) [78]. Capping the dendrimers with piperazine [80], 2-mercaptobenzothiazole [79] or quaternary ammonium group [79] improves the antimicrobial activity. Capping with piperazine, for instance, resulted in 1.3-, 2.6-, and 11.4-fold increase in activity against vancomycin-resistant Enterococcus faecium, MRSA, and Staphylococcus warneri, respectively. High generation piperazine-terminated dendrimers exhibit anticancer activity against MCF-7 and HTB-26 breast cancer cells [80]. Importantly, these activities are selective because the dendrimers are inactive towards human epidermal keratinocytes, and human foreskin BJ fibroblast cells at concentrations that exceed their antimicrobial IC50s [78,79,80]. Also, the dendrimers are inactive towards Gram-negative bacteria under the experimental conditions.
Structure permitting, cationic polymers, including dendrimers, are broad-spectrum antimicrobial agents, acting against Gram-positive and Gram-negative bacteria [82,83,84,85,86]. As an example, a cationic dendrimer designed by capping PAMAM dendrimer with a quaternary ammonium compound, 1-hexadecyl-azoniabicylo[2.2.2]octane, inhibits the growth of S. aureus, Bacillus cereus, Pseudomonas aeruginosa, and Escherichia coli at low micromolar MICs [87]. The activity of the dendrimer against these microorganisms is by an order-of-magnitude higher than that of 1-hexadecyl-azoniabicylo[2.2.2]octane due to multivalency [87], which also explains the over two order-of-magnitude increase in activity of 2-mercaptobenzothiazole against MRSA, VRE, and S. warneri when conjugated to a dendritic scaffold [79]. Multivalency also accounts for the reduced tendency of the dendrimer to trigger the development of resistance in E. coli and B. cereus towards 1-hexadecyl-azoniabicylo[2.2.2]octane [87]. Further, the dendrimer is inactive toward established biofilms due to its large size, but inhibits the growth of S. aureus biofilms on a dendrimer-treated surface (Fig. 9). Recently, photoactive cationic zinc or ruthenium phthalocyanine dendrimers photogenerate singlet oxygen to inactivate S. aureus, E. coli, and Candida albicans [88]. The zinc-containing dendrimer generates higher 1O2 and is more active against the microorganisms than the ruthenium congener. Increasing the positive charge from 4 to 8 has little impact on activity [88].

Reproduced from Ref. [87] with permission from the American Chemical Society
a Schematics of a 1-hexadecyl-azoniabicylo[2.2.2]octane-terminated dendrimer. b Pre-treatment of membrane with dendrimer inhibits growth of S. aureus biofilm.
2.3 Tissue Engineering and Regenerative Medicine
Tissue engineering and regenerative medicine promise revolutionary clinical therapies to meet patients’ needs [89]. Success in this area depends, among other critical factors, on the scaffolds used to engineer and promote cell proliferation and tissue regeneration. The dendritic scaffold is attractive in this regard because it provides a 3D platform for cells to grow and attach, as well as tunability to control biodegradation and mechanical properties, which are crucial to tissue regeneration. By conjugating hydrophilic polymers such as poly(ethylene glycol) or hyaluronic acid to the PAMAM dendrimer, hydrogels, hydrated 3D materials, are designed to support cell proliferation [90,91,92,93,94]. Wang and coworkers, for instance, constructed an arginylglycylaspartic acid-modified PAMAM dendrimer and crosslinked it with hyaluronic acid to form a hydrogel that promotes the proliferation of bone marrow stem cells [93]. In this design, the dendrimer provides the 3D scaffold, the hyaluronic acid contributes to the formation of the hydrogel network, which promotes cell migration, and the arginylglycylaspartic acid enhances cell attachment and proliferation. While PAMAM dendrimer–hyaluronic acid hydrogel support bone marrow stem cell proliferation, the presence of the arginylglycylaspartic acid prevents cell aggregation and enhances cell attachment and proliferation [93].
Beyond providing the 3D template, the dendritic scaffold tunes the mechanical properties by creating microenvironments with distinct swelling properties. Jia and coworkers advanced the mechanical property of poly(lactic acid)-b-poly(ethylene glycol)-b-poly(lactic acid) hydrogels by cross-linking them with PEGylated and peptide-modified PAMAM dendrimers [92]. Crosslinking the block copolymer with the dendrimer tunes the swelling, degradation, and mechanical properties of the hydrogel, and promotes cell attachment, differentiation, and proliferation. To illustrate, crosslinking with 2 or 20% PEGylated and peptide-modified PAMAM dendrimer decreases the swelling ratio from 1000 to 300 or 180%, respectively. The hydrogel degradation time and mechanical stiffness increase with the concentration of the dendrimer. Stiffness, for instance, increases until a critical point where a further increase in the dendrimer concentration yields a weaker network, lowering elastic moduli due to increasing intra- and intermolecular crosslinking among the dendrimers. At this critical point, the hydrogel withstands a broad range of deformation until 1000% strain, which is desirable for bone or tissue regeneration under conditions of high mechanical strain. Compared to the block copolymer-based hydrogel, crosslinking with the dendrimer ensures cell viability of mouse bone marrow mesenchymal stem cells, while the presence of the peptide, arginine-glycine-(aspartic acid)-(d-tyrosine)-cysteine, enables these cells to attach and proliferate better. The 3D dendritic framework creates a culture medium where cells maintain their natural morphology and interact with their environment.
The dendrimer generation affects cell viability as demonstrated by Murugan and Arumugam, who conjugated poly(propylene imine) (PPI) dendrimer to multi-walled carbon nanotubes to form nano-hybrids for bone tissue engineering [91]. Their results suggest that human bone cancer cells are less viable in the hybrid developed from a third-generation than a second-generation PPI dendrimer. Also, the orientation of the dendrimer and the conjugated peptide to the cells influences the degree of cell proliferation [90]. Perez-Inestrosa and coworkers, for instance, showed that while pre-treating a polystyrene surface with arginylglycylaspartic acid-modified PAMAM dendrimer enables mesenchymal stem cells to attach, the extent of proliferation depends on the orientation of the peptide–dendrimer complex to the surface [90]. A peptide–dendrimer orientation better promotes cell attachment and proliferation than a dendrimer–peptide one (Fig. 10). For instance, after 10 days, surfaces treated with peptide–dendrimer contained 6.8 ± 105 cells/mL, which are significantly more than the 4.6 ± 105 found with dendrimer–peptide.

Reproduced from Ref. [90] with permission from The Royal of Society of Chemistry
Orientation of peptide–dendrimer conjugate proliferation and attachment of mesenchymal stem cells; a untreated; b treated with dendrimer–peptide conjugate; c treated with peptide–dendrimer conjugate; d c after trypsin digestion.
2.4 Diagnostic
Diagnosis is an essential component of clinical procedures that provide information on disease conditions based on sensing or imaging modalities. The process is often challenging given the complexity of biological specimens, which affect sensitivity and specificity, as well as the non-specificity of many disease symptoms. Researchers are, therefore, developing new methodologies to enhance sensitivity and specificity. In this regard, dendrimers are an attractive platform to develop these methods, due to the flexibility of conjugating sensing or imaging probes at precise locations within the scaffold. The Abd-El-Aziz group demonstrated this flexibility by decorating η6-arene-η5-cyclopentadienyliron(II)-derived dendrimers with various photoactive molecules, such as naphthol [95], pyrene [96], and tetraphenylethene [97], molecules that are well-explored in sensing and imaging biological events [98,99,100,101,102]. The presence of η6-arene-η5-cyclopentadienyliron(II) moieties endows the dendrimers with redox activity, an electrochemical phenomenon that is well-utilized in many sensing protocols [103, 104]. For instance, the redox–active ferrocene mediates electron transport in many commercially available state-of-the-art electrochemical glucose sensors [105, 106].
Şenel et al. fabricated a glucose biosensor that incorporates an asymmetric ferrocene-cored PAMAM dendrimer (Fig. 11), immobilized with glucose oxidase (GOx) and 3-mercaptopropionic acid on a gold electrode [107]. In the absence of glucose, only the ferrocene redox process is evident (Fig. 11). On addition of glucose to the electrochemical cell, the oxidation current increases as GOx catalyzes the oxidation of glucose, while the redox–active ferrocene enables efficient transport of electrons between the enzyme and the electrode (Fig. 11). Changing the generation of the dendrimer tunes the sensitivity and the response time with these parameters increasing at higher generations. Apparently, binding sites for the enzyme multiply at higher generation, leading to increased electron transport, which eventually results in higher sensitivity. The fabricated electrode is robust, maintaining 90% of its activity after 20 days and reliably measuring glucose levels in a human serum sample.

Reproduced from Ref. [107] with permission from Elsevier B.V
a Schematic of a dendrimer-enabled glucose sensor. Cyclic voltammograms of dendrimer in b the absence, c the presence of glucose.
In addition to acting as an electron mediator, as demonstrated by Şenel et al. [107], the dendrimers can function in another capacity in sensing platforms. Zeng et al., for instance, designed a mediator-less glucose sensor that incorporates PAMAM dendrimers, which enhances the aqueous solubility of a carbon nanotubes (CNTs)-based sensing technology [108]. Xu et al. also reported a robust dendrimer–CNTs-based hydrogen peroxide sensor, where the dendrimer encapsulates electrocatalytic platinum nanoparticles, an excellent catalyst for the reduction of hydrogen peroxide [109].
Şenel et al. fabricated a ferrocene-cored PAMAM dendrimer-based immunosensor that detects prostate-specific antigen (PSA) [110] and a cytosensor that senses human gastric adenocarcinoma cells [111]. To assemble the immunosensor, they covalently conjugated a monoclonal antibody of prostate-specific antigen (anti-PSA) to the dendrimer, then immobilized it on a cysteamine-coated gold electrode [110]. The electrode response depended on the ability of PSA to bind anti-PSA, thereby insulating the electrode surface and precluding electron diffusion from the redox center to the electrode with a resultant decrease in current. Differential pulse voltammetry revealed that signal decreases as the concentration of PSA increases [110]. Compared with lower generation dendrimers, the higher congeners give lower current responses and higher noise levels [110]. Şenel et al. also fabricated a dendrimer-based cytosensor to detect human gastric adenocarcinoma cells (AGS) by leveraging the cells’ overexpressed folate receptors and sialic acid for selectivity [111]. Their approach involves immobilizing a folic or boronic acid-modified PAMAM dendrimer on a cysteamine-coated gold electrode with a fabricated electrode exhibiting detection limits of 20 cells/mL. Selectivity toward the cancer cells in the presence of human embryonic kidney cells was achieved due to the high affinity of folic acid for the receptors, or boronic acid for sialic acid [111].
Bioimaging has also benefited from the tunability of the dendritic scaffold to enhance performance. In magnetic resonance imaging (MRI), for example, the low signal associated with gadolinium-based small molecule contrast agents is bolstered by labeling dendrimers with a high payload of gadolinium to enhance the overall longitudinal relaxivities [112]. Cheng et al. demonstrated this, engineering gadolinium-labeled, folic acid-modified PAMAM dendrimer into nanoclusters for in vivo imaging. The relaxivities per gadolinium are higher in the dendrimer nanoclusters than in a molecular diethylenetriaminopentaacetic acid chelator. The presence of folic acid endows the nanoclusters with the capability to target folate receptor, enabling magnetic resonance imaging of folate-positive tumor in mice with subcutaneous KB cell xenografts.
2.5 Cancer Phototherapy
Cancer remains a leading cause of death globally, killing 8.8 million people in 2015 [113]. Scientists are interested in reducing this burden, aiming to introduce new non-invasive treatment modalities that complement established modalities such as surgery, radiotherapy, and chemotherapy. Phototherapy is emerging as a non-invasive modality to treat cancer with the research groups of Kono [114,115,116] and Kataoka [117,118,119] being at the forefront of this pursuit developing dendrimer-based photothermal and photodynamic therapies to treat superficial tumors. Kono et al., for instance, constructed a dendrimer-based photothermal technology that features surface plasmon resonance in the near infrared (NIR) region [114]. They accessed these dendrimers by conjugating a cystamine-cored PEG-modified PAMAM dendrimer to gold nanorod (GNR) to form a dendrimer–GNR hybrids, which generate heat on NIR irradiation, increasing the temperature of tumor in mice, a phenomenon that decrease the tumor volume. As a different strategy, Kataoka and coworkers employed the nanocavities of PAMAM and PPI dendrimers to encapsulate photosensitizers, rose bengal and protoporphyrin IX, which on irradiation, generate singlet oxygen that are cytotoxic to cancer cells [116]. Their contributions further expand the scope of biomedical application of dendrimers.
3 Conclusion
Since the seminal reports of Newkome and Tomalia, significant progress has occurred in the field, pushing the frontier into diverse landscapes including biomedicine (Fig. 2). Today, dendrimer-enabled biomedical technologies are commercially available and many are in clinical trials. The motivation stems from the ability to functionalize dendrimers as well as the close to uniform dispersity, and the solubility properties of dendrimers. No doubt, a dendrimer remains a promising scaffold to develop biomedical technology but its tedious iterative synthesis poses an obstacle to large-scale commercialization. Besides, most of the investigations explore the PAMAM dendrimer, leaving other dendrimers such as metallo-dendrimers under-exploited. We recommend a shift to other families of dendrimers, as well as the development of accelerated synthetic routes that are less tedious and more amenable to scale-up. The issues of cyto- and eco-toxicity must be addressed if these macromolecules are to be translated into marketable products to assist patients and other consumers. It is, therefore, important that dendrimers be optimized for biocompatibility as well as eco-compatibility.
References
G.R. Newkome, Z. Yao, G.R. Baker, V.K. Gupta, J. Org. Chem. 50, 2003, (1985)
D.A. Tomalia, H. Baker, J. Dewald, M. Hall, G. Kallos, S. Martin, J. Roeck, J. Ryder, P. Smith, Polym. J. 17, 117, (1985)
A. Caminade, D. Yan, D.K. Smith, Chem. Soc. Rev. 44, 3870 (2015)
G.R. Newkome, C.N. Moorefield, S. Chakraborty, J. Inorg. Organomet. Polym. (2017). https://doi.org/10.1007/s10904-017-0676-8
C.N. Moorefield, S. Perera, G.R. Newkome, in Dendrimer-Based Drug Delivery Systems: From Theory to Practice, ed. by Y. Cheng (Wiley, Hoboken, 2012), pp. 1–54
P. Wang, C.N. Moorefield, K. Jeong, S. Hwang, S. Li, S.Z.D. Cheng, G.R. Newkome, Adv. Mater. 20, 1381 (2008)
G.R. Newkome, C. Shreiner, Chem. Rev. 110, 6338 (2010)
S. Hwang, C.N. Moorefield, G.R. Newkome, Chem. Soc. Rev. 37, 2543 (2008)
H. Thrien-Aubin, X.X. Zhu, C.N. Moorefield, K. Kotta, G.R. Newkome, Macromolecules 40, 3644 (2007)
G.R. Newkome, C.N. Moorefield, Chem. Soc. Rev. 44, 3954 (2015)
C.N. Moorefield, A. Schultz, G.R. Newkome, Braz. J. Pharm. Sci. 49, 67 (2013)
S. Hwang, C.D. Shreiner, C.N. Moorefield, G.R. Newkome, New J. Chem. 31, 119 (2007)
G.R. Newkome, C.D. Shreiner, Polymer 49, 1 (2008)
R. Sarkar, K. Guo, C.N. Moorefield, M.J. Saunders, C. Wesdemiotis, G.R. Newkome, Angew. Chem. Int. Ed. 53, 12182 (2014)
C. Agatemor, N. Etkin, A.S. Abd-El-Aziz, J. Inor. Organomet. Polym. 25, 47 (2015)
A.S. Abd-El-Aziz, P.O. Shipman, P.R. Shipley, Macromol. Rapid Commun. 31, 459 (2010)
J. Yang, Q. Zhang, H. Chang, Y. Cheng, Chem. Rev. 115, 5274 (2015)
A. Caminade, A. Ouali, R. Laurent, C. Turrin, J. Majoral, Chem. Soc. Rev. 44, 3890 (2015)
S. Zhang, H. Sun, A.D. Hughes, R. Moussodia, A. Bertin, Y. Chen, D.J. Pochan, P.A. Heiney, M.L. Klein, V. Percec, Proc. Natl. Acad. Sci. USA 111, 9058 (2014)
W. Tian, Y. Ma, Chem. Soc. Rev. 42, 705 (2013)
E. Ficici, I. Andricioaei, S. Howorka, Nano Lett. 15, 4822 (2015)
N. Kottari, Y.M. Chabre, T.C. Shiao, R. Rej, R. Roy, Chem. Commun. 50, 1983 (2014)
S. Mignani, S. El Kazzouli, M.M. Bousmina, J. Majoral, Chem. Rev. 114, 1327 (2014)
S.M. Mignani, N. El Brahmi, S. El Kazzouli, R. Laurent, S. Ladeira, A. Caminade, E. Pedziwiatr-Werbicka, E.M. Szewcyk, M. Bryszewska, M.M. Bousmina, T. Cresteil, J. Majoral, Mol. Pharm. 14, 4087 (2017)
A.W. Bosman, H.M. Janssen, E.W. Meijer, Chem. Rev. 99, 1665 (1999)
C.C. Lee, J.A. MacKay, J.M. Fréchet, F.C. Szoka, Nat. Biotechnol. 23, 1517 (2005)
M. Fischer, F. Vögtle, Angew. Chem. Int. Ed. 38, 884 (1999)
E. Abbasi, S.F. Aval, A. Akbarzadeh, M. Milani, H.T. Nasrabadi, S.W. Joo, Y. Hanifehpour, K. Nejati-Koshki, R. Pashaei-Asl, Nanoscale Res. Lett. 9, 247 (2014)
D. Astruc, Nat. Chem. 4, 255 (2012)
T. Pentek, E. Newenhouse, B. O’Brien, A.S. Chauhan, Molecules 22, 137 (2017)
N. Dwivedi, J. Shah, V. Mishra, M.C.I.M. Amin, A.K. Iyer, R.K. Tekade, P. Kesharwani, J. Biomater. Sci. Polym. Ed. 27, 557–580 (2016)
A. Caminade, Chem. Soc. Rev. 45, 5174 (2016)
A. Caminade, Chem. Commun. 53, 9830 (2017)
D. Shcharbin, N. Shcharbina, V. Dzmitruk, E. Pedziwiatr-Werbicka, M. Ionov, S. Mignani, F.J. de la Mata, R. Gómez, M.A. Muñoz-Fernndez, J. Majoral, M. Bryszewska, Colloids Surf. B 152, 414 (2017)
V. Leiro, S.D. Santos, C.D. Lopes, A.P. Pêgo, Adv. Funct. Mater. (2017). https://doi.org/10.1002/adfm.201700313
A. Azzouz, R. Roy, Can. J. Chem. 95, v (2017)
D.A. Tomalia, A.M. Naylor, W.A. Goddard, Angew. Chem. Int. Ed. Engl. 29, 138 (1990)
R.M. Kannan, E. Nance, S. Kannan, D.A. Tomalia, J. Intern. Med. 276, 579 (2014)
C.J. Hawker, K.L. Wooley, J.M.J. Frechet, J. Chem. Soc. Perkin Trans. 1287 (1993)
G.R. Newkome, C.N. Moorefield, G.R. Baker, M.J. Saunders, S.H. Grossman, Angew. Chem. Int. Engl. 30, 1178 (1991)
M. Liu, K. Kono, J.M.J. Frechet, J. Controlled Release 65, 121 (2000)
Y. Wang, G. Qi, J. He, ACS Macro Lett. 5, 547 (2016)
E.M.M. de Brabander-van den, E.W. Berg, Meijer, Angew. Chem. Int. Ed. 32, 1308 (1993)
J.M.J. Fréchet, Science 263, 1710 (1994)
J.M.J. Fréchet, C.J. Hawker, I. Gitsov, J.W. Leon, J. Macromol. Sci. Pure Appl. Chem. 33, 1399 (1996)
T.H. Mourey, S.R. Turner, M. Rubinstein, J.M.J. Fréchet, C.J. Hawker, K.L. Wooley, Macromolecules 25, 2401 (1992)
C. Agatemor, N. Etkin, A.S. Abd-El-Aziz, J. Inorg. Organomet. Polym. 25, 47 (2015)
D. Astruc, E. Boisselier, C. Ornelas, Chem. Rev. 110, 1857 (2010)
D. Astruc, F. Chardac, Chem. Rev. 101, 2991 (2001)
D. Astruc, C. Ornelas, J. Ruiz, Acc. Chem. Res. 41, 841 (2008)
L. Pu, in Chemosensors: Principles, Strategies, and Applications, ed. by B. Wang, E.V. Anslyn (Wiley, Hoboken, 2011) pp. 121–162
V. Balzani, P. Ceroni, S. Gestermann, C. Kauffmann, M. Gorka, F. Vögtle, Chem. Commun. 853 (2000)
S. Svenson, D.A. Tomalia, Adv. Drug Deliv. Rev. 64, 102 (2012)
A.J.L. Villaraza, A. Bumb, M.W. Brechbiel, Chem. Rev. 110, 2921 (2010)
R.T. Hayes, D.J. Owen, A.S. Chauhan, V.R. Pulgam, H. Vardhan, PEHAM Dendrimers for Use in Agriculture, US Patent, US 9585387 B1, (2017)
S.P. Chaplot, I.D. Rupenthal, J. Pharm. Pharmacol. 66, 542 (2014)
P. Kesharwani, K. Jain, N.K. Jain, Prog. Polym. Sci. 39, 268 (2014)
P. Kesharwani, A.K. Iyer, Drug Discov. Today 20, 536 (2015)
D.A. Tomalia, J.B. Christensen, U. Boas, Dendrimers, Dendrons, and Dendritic Polymers: Discovery, Applications, and the Future (Cambridge University Press, Cambridge, 2012)
M. Wang, H. Liu, L. Li, Y. Cheng, Nat. Commun. 5, 3053 (2014)
M.W. Tibbitt, J.E. Dahlman, R. Langer, J. Am. Chem. Soc. 138, 704 (2016)
J.L. Shamshina, S.P. Kelley, G. Gurau, R.D. Rogers, Nature 528, 188 (2015)
T. Lv, T. Yu, Y. Fang, S. Zhang, M. Jiang, H. Zhang, Y. Zhang, Z. Li, H. Chen, Y. Gao, Mater. Sci. Eng. C 75, 182 (2017)
C. Zhang, D. Pan, K. Luo, W. She, C. Guo, Y. Yang, Z. Gu, Adv. Healthc. Mater. 3, 1299 (2014)
C. Zhang, D. Pan, K. Luo, N. Li, C. Guo, X. Zheng, Z. Gu, Polym. Chem. 5, 5227 (2014)
C. Zhang, D. Pan, J. Li, J. Hu, A. Bains, N. Guys, H. Zhu, X. Li, K. Luo, Q. Gong, Z. Gu, Acta Biomater. 55, 153 (2017)
Z. Maeno, T. Mitsudome, T. Mizugaki, K. Jitsukawa, K. Kaneda, Chem. Lett. 41, 801 (2012)
Y. Tsai, C. Hu, C. Chu, T. Imae, Biomacromolecules 12, 4283 (2011)
D. Kannaiyan, T. Imae, Langmuir 25, 5282 (2009)
T. Kibata, T. Mitsudome, T. Mizugaki, K. Jitsukawa, K. Kaneda, Chem. Commun. 49, 167 (2013)
N.Q. Tran, C.K. Nguyen, T.P. Nguyen, Adv. Nat. Sci.: Nanosci. Nanotechnol. 4, 045013 (2013)
D.S. Conti, D. Brewer, J. Grashik, S. Avasarala, S.R. da Rocha, Mol. Pharm. 11, 1808 (2014)
B.T. Luk, L. Zhang, ACS Appl. Mater. Interfaces 6, 21859 (2014)
E. Luque-Michel, E. Imbuluzqueta, V. Sebastián, M.J. Blanco-Prieto, Expert Opin. Drug Deliv. 14, 75 (2017)
H. Zhang, Y. Ma, Y. Xie, Y. An, Y. Huang, Z. Zhu, C.J. Yang, Sci. Rep. 5, 10099 (2015)
World Health Organization, World Health Statistics 2017: Monitoring Health for the SDGs, Sustainable Development Goals (World Health Organization, Geneva, 2017)
World Health Organization, Global Action Plan on Antimicrobial Resistance (World Health Organization, Geneva, 2017)
A.S. Abd-El-Aziz, C. Agatemor, N. Etkin, D.P. Overy, M. Lanteigne, K. McQuillan, R.G. Kerr, Biomacromolecules 16, 3694 (2015)
A.S. Abd-El-Aziz, C. Agatemor, N. Etkin, R. Bissessur, D. Overy, M. Lanteigne, K. McQuillan, R.G. Kerr, Macromol. Biosci. 17, 1700020 (2017)
A.S. Abd-El-Aziz, A.A. Abdelghani, S.K. El-Sadany, D.P. Overy, R.G. Kerr, Eur. Polym. J. 82, 307 (2016)
A.S. Abd-El-Aziz, A.A. Abdelghani, J.K. Pearson, M.K. Awad, D.P. Overy, R.G. Kerr, Macromol. Chem. Phys. 217, 987 (2016)
C. Yang, X. Ding, R.J. Ono, H. Lee, L.Y. Hsu, Y.W. Tong, J. Hedrick, Y.Y. Yang, Adv. Mater. 26, 7346 (2014)
A. Pascual, J.P.K. Tan, A. Yuen, J.M.W. Chan, D.J. Coady, D. Mecerreyes, J.L. Hedrick, Y.Y. Yang, H. Sardon, Biomacromolecules 16, 1169 (2015)
Y. Li, K. Fukushima, D.J. Coady, A.C. Engler, S. Liu, Y. Huang, J.S. Cho, Y. Guo, L.S. Miller, J.P.K. Tan, P.L.R. Ee, W. Fan, Y.Y. Yang, J.L. Hedrick, Angew. Chem. Int. Ed. 52, 674 (2013)
J. Hoque, P. Akkapeddi, V. Yadav, G.B. Manjunath, D.S.S. Uppu, M.M. Konai, V. Yarlagadda, K. Sanyal, J. Haldar, ACS Appl. Mater. Interfaces 7, 1804 (2015)
W. Cheng, C. Yang, X. Ding, A.C. Engler, J.L. Hedrick, Y.Y. Yang, Biomacromolecules 16, 1967 (2015)
H.W. VanKoten, W.M. Dlakic, R. Engel, M.J. Cloninger, Mol. Pharm. 13, 3827 (2016)
R. Ruiz-González, F. Setaro, s Gulías, M. Agut, U. Hahn, T. Torres, S. Nonell, Org. Biomol. Chem. 15, 9008 (2017)
S.F. Badylak, R.M. Nerem, Proc. Natl. Acad. Sci. USA 107, 3285 (2010)
Y. Vida, D. Collado, F. Najera, S. Claros, J. Becerra, J.A. Andrades, E. Perez-Inestrosa, RSC Adv. 6, 49839 (2016)
E. Murugan, S. Arumugam, RSC Adv. 4, 35428 (2014)
Y. Wang, Q. Zhao, H. Zhang, S. Yang, X. Jia, Adv. Mater. 26, 4163 (2014)
X. Bi, J. Amie Luckanagul, A. Allen, M. Ramaboli, E. Campbell, D. West, P. Maturavongsadit, K. Brummett, Q. Wang, J. Biomater. Sci. Polym. Ed. 26, 669 (2015)
P.N. Desai, Q. Yuan, H. Yang, Biomacromolecules 11, 666 (2010)
A.S. Abd-El-Aziz, C. Agatemor, N. Etkin, R. Bissessur, Macromol. Chem. Phys. 216, 369 (2015)
A.S. Abd-El-Aziz, A.A. Abdelghani, B.D. Wagner, E.M. Abdelrehim, Polym. Chem. 7, 3277 (2016)
A.S. Abd-El-Aziz, C. Agatemor, N. Etkin, B. Wagner, Macromol. Rapid Commun. 37, 1235 (2016)
L. Hou, Y. Cui, M. Xu, Z. Gao, J. Huang, D. Tang, Biosens. Bioelectron. 47, 149 (2013)
C. Ocaña, A. Hayat, R. Mishra, A. Vasilescu, M. del Valle, J. Marty, Analyst 140, 4148 (2015)
M. Wang, G. Zhang, D. Zhang, in Aggregation-Induced Emission: Fundamentals and Applications, Volumes 1 and 2, ed. by A. Qin, B.Z. Tang (Wiley, Chichester, 2013) pp. 165–188
X. Lou, C.W.T. Leung, C. Dong, Y. Hong, S. Chen, E. Zhao, J.W.Y. Lam, B.Z. Tang, RSC Adv. 4, 33307 (2014)
X. Han, Q. Chen, H. Lu, J. Ma, H. Gao, ACS Appl. Mater. Interfaces 7, 28494 (2015)
M.M. Barsan, M.E. Ghica, C.M.A. Brett, Anal. Chim. Acta 881, 1 (2015)
R. Gracia, D. Mecerreyes, Polym. Chem. 4, 2206 (2013)
A.E.G. Cass, G. Davis, G.D. Francis, H.A.O. Hill, W.J. Aston, I.J. Higgins, E.V. Plotkin, L.D.L. Scott, A.P.F. Turner, Anal. Chem. 56, 667 (1984)
H. Li, F. Zhao, L. Yue, S. Li, F. Xiao, Electroanalysis 28, 1003 (2016)
M. Şenel, C. Nergiz, E. Çevik, Sens. Actuators B 176, 299 (2013)
Y. Zeng, Y. Huang, J. Jiang, X. Zhang, C. Tang, G. Shen, R. Yu, Electrochem. Commun. 9, 185 (2007)
L. Xu, Y. Zhu, L. Tang, X. Yang, C. Li, Electroanalysis 19, 717 (2007)
E. Çevik, Ö Bahar, M. Şenel, M.F. Abasıyanık, Biosens. Bioelectron. 86, 1074 (2016)
M. Dervisevic, M. Şenel, T. Sagir, S. Isik, Biosens. Bioelectron. 91, 680 (2017)
Z. Cheng, D.L. Thorek, A. Tsourkas, Angew. Chem. Int. Ed. 49, 346 (2010)
World Health Organization Media Center, Cancer Fact Sheet February (2017), http://www.who.int/mediacentre/factsheets/fs297/en/. Accessed 5 December 2017
X. Li, K. Takeda, E. Yuba, A. Harada, K. Kono, J. Mater. Chem. B 2, 4167 (2014)
X. Li, M. Takashima, E. Yuba, A. Harada, K. Kono, Biomaterials 35, 6576 (2014)
C. Kojima, Y. Toi, A. Harada, K. Kono, Bioconjug. Chem. 18, 663 (2007)
N. Nishiyama, H.R. Stapert, G. Zhang, D. Takasu, D. Jiang, T. Nagano, T. Aida, K. Kataoka, Bioconjug. Chem. 14, 58 (2003)
R. Ideta, F. Tasaka, W. Jang, N. Nishiyama, G. Zhang, A. Harada, Y. Yanagi, Y. Tamaki, T. Aida, K. Kataoka, Nano Lett. 5, 2426 (2005)
Y. Li, W. Jang, N. Nishiyama, A. Kishimura, S. Kawauchi, Y. Morimoto, S. Miake, T. Yamashita, M. Kikuchi, T. Aida, K. Kataoka, Chem. Mater. 19, 5557 (2007)
Acknowledgements
We thank the Natural Sciences and Engineering Research Council of Canada for support.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper is dedicated to Professor George Newkome in recognition of his outstanding contribution to the field of dendrimers.
Rights and permissions
About this article
Cite this article
Abd-El-Aziz, A.S., Agatemor, C. Emerging Opportunities in the Biomedical Applications of Dendrimers. J Inorg Organomet Polym 28, 369–382 (2018). https://doi.org/10.1007/s10904-017-0768-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-017-0768-5