Abstract
Superoxide anion radical (O •−2 ) is a toxic reactive oxygen species (ROS). Schiff-base metal complexes have been widely studied as synthetic antioxidants to scavenge ROS. However, it is toxic and shows poor water solubility. In this work, a kind of novel water-soluble biopolymer/metal complex conjugate (HO-SAM@BSA) was prepared by binding the 4-hydroxy-salicylaldehyde amino acid Schiff-base metal complexes (HO-SAM, M = Cu, Zn, Co) with water-soluble biopolymer bovine serum albumin (BSA). The conjugates were characterized using IR, UV–Vis, circular dichroism spectra (CD), and polyacrylamide gel electrophoresis (PAGE). The results show that the structure of BSA is maintained when the binding rate (nHO-SAM: nBSA) of amino acid Schiff-base metal complexes is 10. In addition, the O •−2 scavenging activities of resultant conjugates were determined via nitrobule tetrazolium assay method. After combining HO-SAM into BSA, the poor water-solubility of HO-SAM is improved, and the O •−2 scavenging activity of BSA increases dramatically. The conjugation HO-SCCu@BSA displays excellent O •−2 scavenging activity. When the EC50 value was 0.10 μmol/L, the analog quantity reached 41 % of natural SOD. Therefore, it can act as a bifunctional mimic of enzyme, which has a great application prospect in antioxidant drug.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Reactive oxygen species (ROS) is derived from the respiratory chain within the mitochondria [1]. ROS includes superoxide anion radicals (O •−2 ), hydroxyl radicals (·OH), hydrogen peroxide (H2O2) and the singled oxygen (O2), which are natural by-products of the metabolism of human body. However, when excessive, those by-products can attack biological molecules such as lipids, proteins, enzymes, DNA and RNA, leading to cell or tissue injury [2]. Superoxide anion radicals (O •−2 ) were the first oxygen radicals produced in the reduction of molecular oxygen. Superoxide dismutase (SOD) is a kind of metal enzyme in vivo [3], and can catalyze the disproportionation reaction of O •−2 to balance O •−2 in the body. Despite the excellent O •−2 scavenging activity, natural SOD has several shortcomings as well, such as complex extraction process, high cost, short half-life, lack of immunogenicity of foreign proteins and poor penetration through the cell membrane. Therefore, the preparation of SOD enzyme mimics has become a hot spot of research [4]. Schiff-base metal complexes have been widely studied as SOD enzyme mimics [5, 6]. However, most of Schiff-base metal complexes with biological activity are toxic and show poor solubility in water, which limits their applications [7]. Therefore, it is very necessary to develop a way to reduce the toxicity of complexes, maintain or improve drug efficacy, and improve water solubility. At present, the main methods are as below. The amino acid Schiff-base in the parent compound is sulfonated by introducing the hydrophilic group. Besides, the second ligand is introduced to improve the water solubility and biological activity [8].
Amino acid is a kind of important biological ligand and contains the special structure of many N and O atoms, which are necessary for cell growth. In addition, cancer cells demand much more amino acid than normal cells [9]. Therefore, much attention has been paid to the researches on amino acid Schiff-base and its metal complexes [10]. Salicylic acid and its derivatives are a class of common anti-rheumatic, antipyretic, and analgesic drugs. Long-term clinical application proves that it has no obvious toxicity. The copper complex consisting of 3,5-diisopropyl salicylic acid ligands has been studied deeply [11]. It possesses the effects of the anti-ulcer [12], cancer treatment [13], anti-mutation [14], diabetes treatment [15] and anti-reperfusion injury. Michael [16] and Lumme [17] reported that salicylaldehyde amino acid Schiff-base metal complexes exhibit higher inhibitory effects on murine L1210 lymphoid leukemia cell. Our group found that the amino acid Schiff-base complexes could be easily prepared with amino acid, salicylaldehyde and metal salts in alcohol, and it could effectively activate O2, which being applied to oxidize olefins [18]. The BSA is a versatile protein, which can easily bind with a wide range of insoluble endogenous and exogenous compounds [19]. Since BSA is considered to be non-antigenic, biodegradable, and readily available, it has been used as a bio-material, such as drug delivery and novel hydrophilic carriers [20]. We found that the solubility and hydroxyl free radical (–OH) scavenging activity of amino acid Schiff base complexes could be improved after being incorporated in BSA [21].
In this paper, in order to exploit the effective, low toxic and water-soluble SOD enzyme mimics in biomedical field, the 4-hydroxy salicylaldehyde amino acid Schiff-base metal complexes (HO-SAM, M = Cu2+, Co2+, Zn2+) were conjugated to biopolymer BSA, which afforded water-soluble conjugates (HO-SAM@BSA). Their structures, molecular weight and the superoxide anion radicals (O •−2 ) scavenging activity of resultant conjugates (HO-SAM@BSA) were investigated.
2 Experimental Section
2.1 Reagents and Instrumentation
The BSA was purchased from Shanghai Sangon Biological Engineering Technology & Services Co. Ltd. 4-hydroxy salicylaldehyde was purchased from Shanghai Gaoqiao Chemical Reagent Factory. Glycine (Gly) and histidine (His) were obtained from Shanghai Institute of Biological Products. L-Cysteine (Cys) was purchased from Shanghai Kangda Amino Acid Plant, biochemical reagents. Zn(OAc)2·2H2O was obtained from Xi’an Chemical Reagent Factory. Cu(OAc)2·H2O and Co(OAc)2·4H2O were purchased from Tianjin Kaitong Chemical Reagent Co. Ltd. Other chemicals were of analytical reagent grade and used without further purification. The double-distilled water was used throughout.
Infrared spectra were recorded from KBr pellet (4000–400 cm−1) on a Digilab FTS 3000 FT-IR spectrophotometer. The UV–Vis absorption spectra were obtained using an Agilent 8453 UV–Vis spectrophotometer with an Agilent temperature control unit. CD spectra analysis was recorded using a JASCO-820 spectropolarimeter. Native polyacrylamide gel electrophoresis (PAGE) analysis was performed using DYY-12C Electrophoresis apparatus.
2.2 Preparation of Amino Acid Schiff-Base Ligands and Their Complexes
Salicylaldehyde amino acid Schiff-base ligands and their complexes were synthesized using published methods [16, 18, 21]. The salicylaldehyde amino acid Schiff-base ligands (HO-SAH2) were dried in vacuum, and the yields of salicylaldehyde l-Cysteine Schiff-base ligand (HO-SCH2), Glycine Schiff-base ligand (HO-SGH2) and l-Histidine Schiff-base ligand (HO-SHH2) were all above 80 %.
Salicylaldehyde amino acid Schiff-base complex (HO-SCM) was prepared as following method: Firstly, Cu(OAc)2·H2O was dissolved in anhydrous ethanol. Then the solution of Cu2+ (1 mmol) was added slowly to 20 mL of the anhydrous ethanol solution of ligand (HO-SCH2, 1 mmol). After reaction for 3 h with stirring at 55 °C, the products were evaporated, filtered and washed with 95 % ethanol, then recrystallized with ethanol, and dried in vacuum. Finally, the yellow powder, salicylaldehyde amino acid Schiff-base complex (HO-SCCu) was obtained. Other metal complexes, such as HO-SCZn, HO-SCCo, HO-SGM and HO-SHM, were also synthesized and the process was the same as that of the HO-SCCu. The structrue of ligands and their complexes were cheracterized by FT-IR spectra. The strong and broad absorption peaks of hydroxyl groups (–O–H) in ligand and complexes appears at 3000–3450 cm−1. The characteristic peaks of carboxyl groups (–COO−), such as νasCOO, νsCOO appears at 1400–1590 cm−1. Schiff-base (–C=N–) stretching vibrations appears at 1600–1640 cm−1, and νPh–O appears near 1250 cm−1. After forming complexes, the characteristic peaks of –OH and Ph–O were kept. However, the absorption peak of –C=N– bond and –COO− bond of metal complexes (HO-SCM) shift clearly, which indicates that N, and O atoms are involved in coordination reaction.
2.3 Preparation of BSA Conjugating Amino Acid Schiff-Base Complexes
Firstly, BSA was dissolved in 10 mL of PBS solution (pH 7.4) and HO-SCCu was dissolved in 5 mL of DMSO. Then 0.5 mL of BSA solution (1 mmol/L) and 1.14 mL of HO-SCCu (4.4 mmol/L) were added to 2.5 mL of PBS. The mixture was incubated for 12 h with rotation in dark at room temperature. The complex was dialyzed in the PBS to remove the unreacted HO-SCCu and DMSO at 5–10 °C, which afforded BSA conjugating amino acid Schiff-base complexes (HO-SCCu@BSA) (n(HO-SCCu): n(BSA) = 10:1). The preparation process of HO-SCZn@BSA and HO-SCCo@BSA were similar to that of HO-SCCu@BSA. After dialyzing, the concentrations of HO-SCM@BSA complexes were calculated based on the final concentration of the BSA. ([HO-SCCu@BSA] = 6.45 × 10−5 mol/L, [HO-SCCo@BSA] = 8.3 × 10−5 mol/L, [HO-SCZn@BSA] = 8.3 × 10−5 mol/L).
BSA conjugating l-Histidine Schiff-base complexes (HO-SHM@BSA) and BSA conjugating glycine Schiff-base complexes (HO-SGM@BSA) were prepared with the same way.
2.4 Superoxide Anion Radical (O •−2 ) Scavenging Ability
Superoxide anion (O •−2 ), the one-electron reduction product of dioxygen (O2), is a toxic ROS [22]. Excessive accumulation of superoxide anion oxygen in the cells results in an adverse effect on the body, which is also one of the most important factors in inflammation and aging. Scavenging activity of superoxide anion radicals (O •−2 ) was assayed by the inhibition of nitrobule tetrazolium (NBT) reduction. Riboflavin luminescence method was used as the source of O •−2 . The formation of blue formazane was monitored at 560 nm (phosphate buffer), and the inhibition rate (F %) of O •−2 was calculated according to the Eq. 1.
Where Δ is the absorbance in the presence of the tested compound; Δ0 is the absorbance in the absence of the tested compound.
The action mixture was a mixed solution of riboflavin (3.4 × 10−6 mol L−1), methionine (0.01 mol L−1) and NBT (4.66 × 10−5 mol L−1). All of action components were connected with PBS (pH 7.80, 0.05 mol L−1). The action mixture was saturated at 25°C for 0.5 h. During the test, 3 mL of action mixture and different quantities of antioxidant were illuminated with light intensity of 4000 (±100) Lux, and then the absorbance of the action mixture in 560 nm was measured (time interval was 30 s.). The SOD-like activity of studied complexes was compared with native Cu,Zn-SOD.
3 Results and Discussion
3.1 Preparation and Characterization of HO-SAM@BSA
The N,O-Schiff-base ligand with a strong coordination ability was easily obtained by condensing salicylaldehyde (Sal) with amino acid (AA). The ligand can coordinate with different metal ions to form complexes [23]. Here, firstly, using cysteine (Cys), glycine (Gly), and histidine (His) as amino acid resources, three Schiff-base ligands (HO-SAH2: HO-SCH2, HO-SGH2 and HO-SHH2) were synthesized by salicylaldehyde and amino acid (Cys, Gly, and His) (Scheme 1). Then, amino acid Schiff-base metal complexes (HO-SAM), such as HO-SCM, HO-SGM and HO-SHM, were prepared by coordinating with metal ions (Cu, Co, Zn).
Secondly, bovine serum albumin (BSA), a typical soluble biopolymer, was applied to conjugate with amino acid Schiff-base metal complexes (HO-SAM). BSA can modulate the activity of conjugated substances, so it is regarded as one of the most important raw materials in medical and biotechnological products [24]. The result shows that the obtained BSA conjugating amino acid Schiff-base metal complexes (Scheme 2) possess good water solubility, and overcome the shortcoming of poor water solubility of small molecules.
3.2 UV–Vis Spectra of HO-SAM@BSA
The conjugations of BSA and metal complexes were characterized by UV–Vis spectra (Fig. 1). Before conjugation, the characteristic absorption peak of BSA appears at 278 nm, and the characteristic peak of the HO-SCCu approximately appears at 260 nm. After conjugation, the characteristic absorption peak of the HO-SCM@BSA moves redshift. A new absorption peak appears at 300–500 nm in the HO-SCM@BSA. It indicates that the HO-SCM is conjugated with the BSA, and the HO-SCM@BSA was successfully prepared.
3.3 Circular Dichroism Spectra of HO-SAM@BSA
The circular dichroism (CD) spectroscopy was used to monitor the change of the secondary structure in protein. As being showed in Fig. 2, BSA in PBS shows negative absorption bands with maxima at around 208 and 222 nm. The Schiff-base complexes do not show any CD signals in this region. So the far-UV region of CD spectroscopy was used to investigate the interaction of Schiff-base complexes with BSA. The absorption peaks of BSA in HO-SCM@BSA (BSA: HO-SCM = 1:10) are all weakened, which indicates that the conformation of BSA in HO-SCM@BSA has changed. The conformation of BSA is affected by HO-SCCu obviously, followed by HO-SCZn and HO-SCCo. Therefore, the mol ratio of the amino acid Schiff-base complexes to the BSA is 10, α-helical content of BSA changes, but the total band of complex is basically the same as that of BSA. This indicates BSA is affected by Schiff-base complexes to some extent, but the change is slight.
3.4 Native-PAGE
Native-PAGE results of HO-SCM@BSA were exhibited in Fig. 3. Migration speed of metal complexes is the same as that of BSA, which indicates the non-covalent interactions have occurred between the metal complexes and BSA. Because the denaturing agent (mercaptoethanol) is added into the Native-PAGE, the non-covalent interaction disappears when the proteins undergo high-temperature boiling process. The more proteins are conjugated with covalent bond, the larger the molecular weight is for complexes. At the same time, electrophoretic speed of this type of proteins is slower than that of proteins unmodified. On the contrary, if proteins bind metal complexes with non-covalent bond, metal complexes will remove from it under the effect of denaturant. Further, both the molecular weight and electrophoretic speed are the same as those of the pure proteins.
3.5 Superoxide Anion Radical (O •−2 ) Scavenging Ability
The conjugations were prepared by the same metal ion and different amino acid groups integrating with BSA. Median extinction concentration (EC50) is an important detection index of the antioxidant activity. For antioxidant scavenger, the lower EC50 value the conjugates have, the better scavenging activity they own. As is showed in Table 1, the EC50 of HO-SCCu@BSA is 0.10 μmol/L, which is the lowest of all.
The O •−2 scavenging activity of natural SOD was measured via NBT assay, and the EC50 of natural SOD was 0.041 μmol/L. HO-SCCu@BSA possesses lower EC50 than the standard compound M-40403 [25] and its analog quantity reaches 41.00 % compared with natural Cu, Zn-SOD. Thus, in the three groups of conjugations, HO-SCM@BSA exhibits the strongest antioxidant activity. It indicates that different amino acids have a great influence on the antioxidant activity of conjugation. The sulfhydryl of cysteine residue plays an important role in O •−2 scavenging activity. This result may be caused by micro-environmental change of the binding site between HO-SCM and amino acid residue in the presence of sulfhydryl, further leading to the difference of the O •−2 scavenging activity.
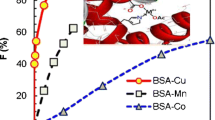
It was found that metal ion shows O •−2 scavenging capacity [26], but the ability is weak. After the prepared metal complexes are incorporated in BSA, the O •−2 scavenging activity increases dramatically. The scavenging activities of HO-SCM@BSA conjugates with different metal ions were showed in Fig. 4. The scavenging activities are as follows: HO-SCCu@BSA > HO-SCCo@BSA > HO-SCZn@BSA. Obviously, in this system, HO-SCCu@BSA shows the strongest scavenging activity. Because it is easy for Cu2+ to coordinate with amino acid residues, the proportion of the combined Cu2+ is high, and the O •−2 scavenging activity of HO-SCCu@BSA increases therein.
Compared with BSA, the O •−2 scavenging activity of HO-SCCu@BSA is improved dramatically. Therefore, it is a kind of excellent oxygen radical scavenger. In summary, not only different amino acid groups but also the different metal ions affect the O •−2 scavenging activity of conjugation.
3.6 Mechanism of O •−2 scavenging
BSA has weak ability to scavenge free radicals, while BSA-Schiff-base complexes have strong antioxidant activity. The results show that the more BSA Schiff-base complexes combine with, the higher antioxidant activity BSA-Schiff-base complexes have. So the metal ions in conjugates play an important role in ROS scavenging.
It was found that Mn was the metalloenzyme in the reduced state and Mn+1 was the enzyme in the oxidized state [27, 28]. Zhou [29] found that arginine was indeed essential for high SOD activity and could steer the O •−2 substrate to the metal ion. Based on reported catalytic mechanism, a possible mechanism of the BSA conjugating amino acid Schiff-base complexes for scavenging O •−2 was proposed (Fig. 5) as following: Firstly, a super oxide anion gathers in the activity center composed of small molecules Schiff-base; Secondly, the super oxide anion binding directly to the metal ion can rapidly exchange between the axial and the planar position of the distorted square pyramid, which induces it to give up its electron and the active center to transform Mn+ into M(n−1)+. Thirdly, the electrically neutral oxygen molecule leaves. Fourthly, a second super oxide anion gathers in the activity center composed of small molecules Schiff-base. Fifthly, the super oxide anion binds again to the M(n−1)+ ion to accept an electron and a proton from the buffer. Since the proton exchange between the substrates and buffer is a rapid process, the superoxide anion further combines another proton from the solution to form a H2O2 molecule. Finally, electrically neutral H2O2 leaves, completing a catalytic cycle.
4 Conclusions
Three kinds of BSA conjugating amino acid Schiff-base metal complexes with good antioxidant activity have been prepared. BSA acts as scaffold while the Schiff-base metal complexes acts as the catalytic center. The O •−2 scavenging activity of conjugation is measured via NBT assay, which demonstrates the poor water-solubility of the Schiff-base metal complexes can be dramatically improved and that the oxidative radical scavenging activity increases obviously when Schiff-base metal complexes binding with BSA. HO-SCCu@BSA shows remarkable ability in scavenging O •−2 , therefore, it is a kind of alternatives for scavenge free radicals.
References
R.C. Robert, Biological Inorganic Chemistry (Elsevier, Amsterdam, 2008)
G. Ceyhan, C. Çelik, S. Uruş, İ. Demirtaş, M. Elmastaş, M. Tümer, Spectrochim Acta A 81(1), 184–198 (2011)
S.I. Liochev, Free Radic. Biol. Med 60, 1–4 (2013)
S. Cuzzocrea, E. Mazzon, R.D. Paola, T. Genovese, C. Muià, A.P. Caputi, D. Salvemini, Arthritis Rheum. 52(6), 1929–1940 (2005)
S.R. Doctrow, K. Huffman, C.B. Marcus, G. Tocco, E. Malfroy, C.A. Adinolfi, H. Kruk, K. Baker, N. Lazarowych, J. Mascarenhas, B. Malfroy, J. Med. Chem. 45(20), 4549–4558 (2002)
W. Munroe, C. Kingsley, A. Durazo, E.B. Gralla, J.A. Imlay, C. Srinivasan, J.S. Valentine, Inorg. Biochem. 101(11–12), 1872–1875 (2007)
K.G. Vladimirova, A.Y. Freidzon, O.V. Kotova, A.A. Vaschenko, L.S. Lepnev, A.A. Bagatur’yants, A.G. Vitukhnovskiy, N.F. Stepanov, M.V. Alfimov, Inorg. Chem. 48(23), 11123–11130 (2009)
S.P. Rath, S. Mondal, T. Ghosh, Transit. Metal Chem. 21(4), 309–311 (1996)
D.Z. Li, Z.M. Chen, Q. Jin, J. Beijing Norm. Univ. (Sci.) 32, 251 (1996)
P.R. Reddy, A. Shilpa, N. Raju, P. Raghavaiah, J. Inorg. Biochem. 105(12), 1603–1612 (2011)
Y.Z. Fang, R.L. Zheng, The Theory and Application of Free Radical Biology (Sciences Press, Beijing, 2002)
J.R.J. Sorenson, J. Med. Chem. 19(1), 135–148 (1976)
H.H.A. Dollwet, J.B. McNicholas, A. Pezeshk, J.R.J. Sorenson, Trace Elem. Med. 4, 13–20 (1987)
J.R.J. Sorenson, L.W. Oberley, R.K. Crouch, T.W. Kensler, V. Kishore, S.W.C. Leuthauser, T.D. Oberley, A. Pezeshk, Biol. Trace Elem. Res. 5, 257–273 (1983)
S.E. Gandy, M.G. Buse, J.R.J. Sorenson, R.K. Crouch, Diabetologia 24(6), 437–440 (1983)
M.R. Wagner, F.A. Walker, Inorg. Chem. 22, 3021–3028 (1983)
H. Elo, P. Lumme, Inorg. Chim. Acta 136(1), 149–153 (1987)
R.M. Wang, C.J. Hao, Y.P. Wang, S.B. Li, J. Mol. Catal. A 147(1–2), 173–178 (1999)
G. Li, H.F. Zhang, R.M. Wang, Y.F. He, Y.B. Xiong, Chin. Sci. Bull. 58(24), 2956–2963 (2013)
W.P. Sheffield, L.J. Eltringham-Smith, S. Gataiance, V. Bhakta, BMC Biotechnol. 9, 15 (2009)
R.M. Wang, J.J. Mao, J.F. Song, C.X. Huo, Y.F. He, Chin. Chem. Lett. 18(11), 1416–1418 (2007)
A.E.M. Ramadan, J. Mol. Struct. 1015(1), 56–66 (2012)
P.K. Sharma, S.N. Dubey, Indian J. Chem. 33A(12), 1113–1115 (1994)
A. Ravindran, A. Singh, A.M. Raichur, N. Chandrasekaran, A. Mukherjee, Colloid Surf. B 76(1), 32–37 (2010)
H. Lee, W. Park, D. Lim, Bioorg. Med. Chem. Lett. 20(8), 2421–2424 (2010)
X.X. Li, Studies on Antioxidant Functions of Feather Keratin Metal Complexes (Northwest Normal University, Lanzhou, 2012)
P.J. Hart, M.M. Balbirnie, N.L. Ogihara, A.M. Nersissian, M.S. Weiss, J.S. Valentine, D. Eisenberg, Biochemistry 38, 2167 (1999)
X.C. Yin, X.X. Li, R.M. Wang, G. Li, Y.F. He, Pure Appl. Chem. 84(12), 2641–2651 (2012)
Y.H. Zhou, H. Fu, W.X. Zhao, W.L. Chen, C.Y. Su, H. Sun, L.N. Ji, Z.W. Mao, Inorg. Chem. 46(3), 734–739 (2007)
Acknowledgments
The authors are grateful to the NSFC (21364012, 21263024) and IRTGP (1210RJIA004) for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, Z., Pan, S., Li, G. et al. Albumin Conjugating Amino Acid Schiff-Base Metal Complexes for Scavenging Superoxide Anion Radical. J Inorg Organomet Polym 25, 1313–1319 (2015). https://doi.org/10.1007/s10904-015-0242-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-015-0242-1