Abstract
Preparation of plate-shaped nanostructures of a new 1D polymeric lead(II) complex containing the Pb2-(μ-I)2 motif, [Pb(neo)I2] n ,(1) where neo is the abbreviation of neocuproine, using a sonochemical method is described. The new coordination polymer is characterized by scanning electron microscopy, X-ray diffraction (XRD), elemental analysis and infrared spectroscopy. The single crystalline material is obtained using a heat gradient applied to a solution of the reagents. Single-crystal XRD analysis indicates that a coordination number of six for PbII ions, (i.e., PbN2I4) with an asymmetrical coordination sphere and “stereo-chemically active” electron lone pairs. They also show that the chains interact with each other through π–π stacking interactions creating a 3D framework. PbO nanoparticles are obtained by thermolysis of 1 at 180 °C with oleic acid as a surfactant. Scanning electron microscopy confirms formation of PbO particles with a size of ~25 nm.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The aim of coordination polymers and crystal engineering is the development of new crystalline materials for their possible utilization in a variety of applications considering their wide range of possibilities [1]. This is why considerable progress has been made in theoretical prediction and network-based approaches for controlling topology and architecture of networks in order to produce useful functional materials [2–8]. Most researcher so far has mainly focused on s-, d-, or f-metal coordination polymers, meanwhile despite wide range of applications in electroluminescent devices, fluorescent sensors, photovoltaic convertor, and organic light-emitting diodes [9–12], less consideration has been given to the heavy metals of the p block as coordination center. Lead(II), with its electronic configuration [Xe] 4f14 5d10 6s2, is one of the post-transition metal elements that exhibits the so-called inert-pair effect. Its compounds are interesting and frequently discussed in considering the stereo-chemical activity of valence shell electron lone pairs [13–16].
Halo compounds of metals with d10 configuration show various coordination numbers and configurations [17]. It is observed that chloro-compounds are commonly bridged polymeric, while bromo- and iodo-derivatives are usually monomeric. Iodo-compounds often behave differently in terms of structural, chemical, photophysical and redox properties. It has been suggested that the tendency of iodine to reduce the coordination number of the metal atom is due to an increase in electron density at the metal center caused by the softer character and larger size of iodine relative to lighter halogens [18, 19]. Although I− is reluctant, in general, to generate polymeric networks with most of the metal ions, Cu(I) and Ag(I) form myriad iodine bridged dimers, cubanes, chains, strands, and so on [17, 20]. Lead(II) halide organic–inorganic hybrid compounds, and more specifically PbX2-based coordination polymers with nitrogen-containing Lewis bases, due to their diverse electrical, magnetic and optical properties, as well as their excellent film processability [21] have attracted lots of interest [22–25].
Nano-scaled materials often exhibit new, interesting, size-dependent physical and chemical properties that cannot be observed in their bulk analogues. Because of these interesting properties, nano-sized coordination polymers are interesting candidates for applications in catalysis, molecular adsorption, magnetism, nonlinear optics, luminescence, and molecular sensing. So far only a few studies about the nano-scale coordination polymers were reported [26–34].
Several different synthetic approaches have been proposed for the preparation of metal coordination compounds [35]. Some examples are (1) slow diffusion of the reactants into a polymeric matrix, (2) diffusion from the gas phase, (3) evaporation of the solvent at ambient or reduced temperatures, (4) precipitation or recrystallization from a mixture of solvents, (5) temperature-controlled cooling, and (6) hydrothermal synthesis [36]. Recently the application of ultrasound in synthetic organic chemistry has attracted attention because ultrasonic waves in liquids are known to facilitate chemical reactions in both of homogeneous or heterogeneous systems [37, 38].
We focus our attention on design and synthesis lead(II) coordination compounds [39–44], and recently we have reported a variety nano lead(II) coordination polymer [43, 44].
In this paper, we report the preparation and crystal structure of a novel lead(II) coordination polymer involving the Pb2-(μ-I)2 unit, [Pb(neo)I2] n . We also describe a simple synthetic sonochemical preparation of plate-shaped structures of this coordination polymer and their conversion into nano-structured lead oxide using calcination at moderately elevated temperatures. This is he first plate shape lead(II)–iodo coordination compound.
2 Experimental
2.1 Physical Property Measurements
All chemicals were obtained from Aldrich and used as received. Infrared spectra were recorded as KBr pellets using a Perkin-Elmer 883-IR and on a Nicolet 520 Fourier transform infrared (FTIR) spectrophotometers. Elemental analyses (C, H, N) were performed on Perkin-Elmer 2400 II elemental analyzer. X-ray powder diffraction (XRD) measurements were performed using a X’pert diffractometer (Panalytical Co.), with monochromatized Cukα radiation. The crystallite sizes of selected samples were estimated using the Scherrer formula. The samples were characterized with a scanning electron microscope (SEM, Philips XL 30), with gold coating. Melting points (uncorrected) were measured on an Electrothermal 9100 apparatus. Crystallographic data were collected at 100 K with the Oxford Xcalibur CCD area detector diffractometer, using graphite monochromatic MoKα (λ = 0.71069 Å) radiation. Data reduction and absorption correction were performed using CrysAlis RED 1.171.26 (Oxford Diffraction). The structure was solved by direct methods using SIR2004 [45] and refined by full-matrix least squares using SHELX-97 [45]. Hydrogen atoms were generated in calculated position using SHELX-97 [46]. Materials for publication were prepared using SHELXTL [46] and ORTEPIII [47].
2.2 Preparation of [Pb(neo)I2] n (1)
To prepare the plate-shape structure of [Pb(neo)I2] n (1), a solution of Pb(CH3COO)2 (25 mL of a 0.1 M) in H2O was positioned in a high-density ultrasonic probe operating at 20 kHz with a maximum power output of 600 W. Into this solution the ligands, neocuproine (25 mL of a 0.1 M) and potassium iodide (25 mL of a 0.2 M) were added dropwise. The obtained precipitates were filtered, washed with water and air dried. Product 1: m.p. = 224 °C. Anal. Calc. for C14H12I2N2Pb: C: 25.12, H: 1.81, N: 4.19 %; found: C: 25.20, H: 1.80, N: 4.20 %.
IR (cm−1) selected bands: 690m, 1470s, 1508, 1575s, 2993m, 3012w [48].
To isolate single crystals of 1, neocuproine (0.20 g, 1 mmol) was placed in one arm of a branched tube [24] and mixture of lead(II) acetate (0.38 g, mmol) and potassium iodide (0.34 g, 2 mmol) in the other. Methanol was then carefully added to fill both arms, the tube sealed and the ligand-containing arm immersed in a bath at 60 °C, while the other was left at ambient temperature. After 9 days, crystals (m.p. 227 °C) suitable for an X-ray structure determination had deposited in the arm at ambient temperature. They were then filtered off, washed with acetone and ether, and air dried. Yield: 51 %. Anal. Calc. for C14H12I2N2Pb: C: 25.12, H: 1.81, N: 4.19 %; found: C: 25.10, H: 1.70, N: 4.10 %.
IR: ν = 690m, 1470s, 1508, 1575s, 2995m, 3015w cm−1 [48].
2.3 Synthesis of PbO Nanoparticles
Compound 1 (0.1 mmol) was ground and dissolved in oleic acid (1.58 mL) to form a pale yellow solution. This solution was degassed for 20 min and heated to 180 °C for 2 h forming a black precipitate. A small amount of toluene and a large excess of EtOH were added to the reaction solution, and PbO nanoparticles were separated by centrifugation. The solid was washed by EtOH and dried under air.
3 Results and Discussion
The reaction of the “neo” ligand with a mixture of lead(II) acetate and potassium iodide using two different routes provided crystalline materials of the general formula [Pb(neo)I2] n (1). Scheme 1 gives an overview of the methods used for the synthesis of 1 using these two different routes.
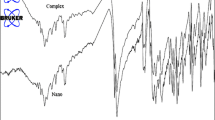
The elemental analysis and IR spectra of the sonochemically prepared nano-crystals and the single crystalline material are indistinguishable (elemental analysis of single crystal: C: 25.10, H: 1.70, N: 4.10 %, melting point of single crystal: 227 °C; elemental analysis of nano crystals: C: 25.20, H: 1.80, N: 4.20, melting point of nano crystal: 224 °C). IR spectra display the characteristic absorption bands of the “neo” ligands (Fig. 1). The relatively weak band around 3,015 cm−1 is attributed to the absorption of the aromatic CH bonds and the band around 2,995 cm−1 is attributed to the absorption of the aliphatic CH bonds [48].
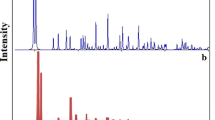
Figure 2a shows the XRD pattern calculated from the single crystal data of 1, compared to the XRD pattern of a typical nano-crystal of 1 prepared by the sonochemical process (Fig. 2b). There is a good match with very slight differences in peak positions which indicates that both materials have a single crystalline phase. The significant broadening of the peaks of the nano-structure (Fig. 2b) is an indication of nanometer dimension of synthesized particles. The average size of the particles was estimated by the Scherrer formula, D = 0.891λ/βcosӨ, where D is the average grain size, λ the X-ray wavelength (0.71069 Å), Ө the diffraction angle and β the full-width at half maximum of an observed peak. The obtained value of D is 85 nm. Figure 3 shows the nano-layers that observed by scanning electron microscopy. The morphology of compound 1 prepared by the sonochemical method (Fig. 3) is very interesting. It can be seen that it is composed of hexagonal cross-sheets with thickness of about 85 nm (Fig. 3). The mechanism of formation of this network structure needs to be further investigated, however it may be a result of the crystal structure of the compound which is a two-dimensional layered network (see below), that means the packing of the structure on a molecular level might have influenced the morphology of the nano-structure of the particles (Fig. 4).
Crystal structure analysis of [Pb(neo)I2] n (Table 1) showed that the complex crystallizes in the monoclinic system with space group I2/a, forming a one dimensional polymer alligned along the a axis. Such arrangement is observed in several other inorganic polymer structures [49–52]. Table 2 shows the selected bond lengths and angles of complex 1.
As shown in Fig. 5, the coordination number of lead atom is six, with the N,N′-bidenate neocuprine ligand necessarilybeing in cis conformation in the coordination sphere. The polymer, illustrated in Fig. 5, is generated by a succession of Pb(μ-I)2Pb rhombs, with the lead(II) atoms bridged by four iodide ions.
The lead atom is located on a crystallographic 2 axis which also passes through the midpoint of the bond(s) linking the two aromatic rings. The polymer is generated by the crystallographic inversion centres located at the centres of the symmetry-related PbI2Pb rhombs on both sides of the lead atom. The octahedral coordination of lead is completed by two iodine atoms of a symmetrically equivalent molecule, which are located at a longer distance [3.547(8) Å] with respect the two iodides in the asymmetric unit [3.144(8) Å]. The longer Pb–I bonds are trans to the Pb–N vectors, even though the angle between these two cis Pb–I vectors are the largest cis angle in the coordination sphere [173.76(3)°]. This arrangement is perhaps suggestive of some sterically active lonepair component that occupies a hole of the coordination geometry around the metal ions [16]. The observed asymmetry of the coordination sphere, due to the difference between the short Pb–N bonds on the side of the Pb(II) ion opposite to the lone pair [2.559(7) Å] and the much longer Pb–I bond [3.542(8) Å], adjacent to the lone pair, supports their presence [53]. These distances and angles are comparable with previously reported 1,10-phenanthroline and iodo adducts of lead(II) [I–Pb–Iii angle is 168.65(3)°, 2.530(9) Å compared with 3.323(1) Å adjacent to the lone pair] [21]. The Pb···Pb distances through the iodide bridges are 4.769 and 4.763 Å.
The mode of packing of the polymer strands about their mean planes, parallel to bc, exhibits a 3D supramolecular architecture arising from lone pair activity and π–π stacking interactions (Fig. 6). It is evident from Fig. 7 that there can be a partial overlap between the aromatic rings of the “neo” ligands, related by the twofold axis, with an interplanar distance of 3.59 Å [54, 55]. Thus, lone pair activity and π–π stacking, are two factors that may control the coordination sphere of this complex.
3.1 Nano Structure of PbO
Nano-powders of PbO have been generated by thermal decomposition of plates of compound 1 under an air atmosphere. The powder XRD pattern (Fig. 8), matches the standard pattern of orthorhombic PbO with a = 5.8931 Å and z = 4 (JCPDS card file No. 77-1971). The morphology and size of the prepared PbO samples were further observed using SEM. Figure 9 shows a SEM image of the PbO nano powder. Calcination of the bulk powder of 1 produces regularly shaped Pb(II) oxide nanoparticles with a diameter of about 25 nm (Fig. 9).
4 Conclusion
In this work, a novel Pb(II) nano-plate coordination polymer formed by Pb2-(μ-I)2 units and containing an aromatic amine (neocuproine) is sonochemically prepared. The complex takes the form of a one dimensional polymer streched along the a axis. The arrangement of ligands suggests a gap in coordination geometry around the metal ions possibly occupied by a stereo-active lone pair of electrons on Pb. Two factors, lone pair activity and π–π stacking, may control the coordination sphere of this complex. The morphology of 1 prepared sonochemically is a nano crystalline hexagonal plate. This is the first plate shape lead(II)–iodo coordination compound. In addition, PbO nanoparticles were prepared by thermolysis of 1 at 180 °C with oleic acid as a surfactant. The scanning electron microscopy shows that the size of the PbO particles is ~25 nm.
5 Supplementary Material
Crystallographic data for the structures reported in this paper has been deposited with the Cambridge Crystallographic Data Centre as supplementary publication CCDC 884117 for [Pb(neo)I2]n (1). Copies of the data can be obtained on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-3360-33; e-mail: de-posit@ccdc.cam.ac.uk.
References
C. Janiak, J. Chem. Soc. Dalton Trans. 2003, 2781 (2003)
X.-H. Bu, M.-L. Tong, H.-C. Chang, S. Kitagawa, S.R. Batten, Angew. Chem. Int. Ed. 43, 192 (2004)
C.N.R. Rao, S. Natarajan, R. Vaidhyanathan, Angew. Chem. Int. Ed. 43, 1466 (2004)
O.R. Evans, W.-B. Lin, Acc. Chem. Res. 35, 511 (2002)
B. Zhao, P. Cheng, Y. Dai, C. Cheng, D.-Z. Liao, S.-P. Yan, Z.-H. Jiang, G.-L. Wang, Angew. Chem. Int. Ed. 42, 934 (2003)
G. Férey, C. Serre, C. Mellot-Draznieks, F. Millange, S. Surble, J. Dutour, I. Margiolaki, Angew. Chem. Int. Ed. 43, 6296 (2004)
P.J. Hagrman, D. Hagrman, J. Zubieta, Angew. Chem. Int. Ed. 38, 2638 (1999)
R.J. Hill, D.-L. Long, N.R. Champness, P. Hubberstey, M. Schröder, Acc. Chem. Res. 38, 337 (2005)
A.A. Bol, A. Meijerink, Phys. Chem. Chem. Phys. 3, 2105 (2001)
H. Nikol, A. Vogler, J. Am. Chem. Soc. 113, 8988 (1991)
S.K. Dutta, M.W. Perkovic, Inorg. Chem. 41, 6938 (2002)
P. Singh, M.M. Richter, Inorg. Chim. Acta 357, 1589 (2004)
P. Pyykkö, Chem. Rev. 88, 563 (1988)
P. Schwerdtheger, G.A. Heath, M. Dolg, M.A. Bennett, J. Am. Chem. Soc. 114, 751 (1992)
A. Andres, A. Bencini, A. Garachalios, A. Bianchi, P. Dapporto, E. Garcia-Espna, P. Paoletti, P. Paoli, J. Chem. Soc. Dalton Trans. 1993, 3507 (1993)
L. Shimoni-Livny, J.P. Glusker, C.W. Bock, Inorg. Chem. 37, 1853 (1998)
F.A. Cotton, G. Wilkinson, C.A. Murillo, M. Bochmann, Advanced inorganic chemistry, 6th edn. (John Wiley, New York, 1999)
H. Strasdeit, W. Saak, S. Pohl, W.L. Driessen, J. Reedijk, Inorg. Chem. 27, 1557 (1988)
Y.Y. Niu, H.G. Zheng, H.K. Fun, I.A. Razak, S. Chantrapromma, X.Q. Xin, Synth. React. Inorg. Met. Org. Chem. 33, 1 (2003)
C. Janiak, L. Uehlin, H.-P. Wu, P. Klufers, H. Piotrowski, T.G. Scharmann, J. Chem. Soc. Dalton Trans. 1999, 3121 (1999)
G.A. Bowwmaker, J.M. Harrowfield, H. Miyamae, T.M. Shand, B.W. Skelton, A.A. Soudi, A.H. White, Aust. J. Chem. 49, 1089 (1996)
L.M. Engelhardt, J.M. Patrick, C.R. Whitaker, A.H. White, Aust. J. Chem. 40, 2107 (1987)
H. Miyamae, Y. Numahata, M. Nagata, Chem. Lett. 663 (1980)
J.M. Harrowfield, H. Miyamae, B.W. Skelton, A.A. Soudi, A.H. White, Aust. J. Chem. 49, 1121 (1996)
V.N. Kokozay, A.V. Sienkiewicz, Polyhedron 14, 1547 (1995)
Z.R. Ranjbar, A. Morsali, Ultrason. Sonochem. 18, 644 (2011)
H. Sadeghzadeh, A. Morsali, CrystEngComm 12, 370 (2010)
A.M. Spokoyny, D. Kim, A. Sumrein, C.A. Mirkin, Chem. Soc. Rev. 38, 1218 (2009)
L. Chen, Y. Shen, B.J. Yongming, C. Wang, J. Solid State Chem. 182, 2298 (2009)
A. Pramanik, G. Das, CrystEngComm 12, 401 (2010)
Z. Rashidi, A. Morsali, J. Mol. Struct. 936, 206 (2009)
H. Ahmadzadi, F. Marandi, A. Morsali, J. Organomet. Chem. 694, 3565 (2009)
J.K.-H. Hui, M.J. MacLachlan, Coord. Chem. Rev. 254, 2363 (2010)
C.J. Hoeller, K.M. Buschbaum, Eur. J. Inorg. Chem. 2010, 45 (2010)
S. Kawaguchi, Coord. Chem. Rev. 70, 51 (1986)
H. Sadeghzadeh, A. Morsali, Ultrason. Sonochem. 18, 80 (2011)
K.S. Suslick, in Ultrasound its chemical, Physical and Biological Effect, ed. by K.S. Suslick (VCH Publishers, Weinheim, 1989)
S.J. Tabatabaei Rezaei, M.R. Nabid, A. Yari, S. Weng Ng, Ultrason. Sonochem. 18, 49 (2011)
F. Marandi, B. Mirtamizdoust, A.A. Soudi, H.-K. Fun, Inorg. Chem. Commun. 10, 174 (2007)
F. Marandi, B. Mirtamizdoust, A.A. Soudi, S. Chantrapromma, H.-K. Fun, Z. Anorg. Allg. Chem. 633, 1329 (2007)
F. Marandi, B. Mirtamizdoust, A.A. Soudi, V.T. Yilmaz, C. Kazak, Z. Anorg. Allg. Chem. 632, 2380 (2006)
B. Shaabani, B. Mirtamizdoust, M. Shadman, H.-K. Fun, Z. Anorg. Allg. Chem. 635, 2642 (2009)
B. Shaabani, B. Mirtamizdoust, D. Viterbo, G. Croce, H. Hammud, P. Hojati-Lalemi, A. Khandar, Z. Anorg. Allg. Chem. 637, 713 (2011)
B. Mirtamizdoust, B. Shaabani, A. Khandar, H.-K. Fun, S. Huang, M. Shadman, P. Hojati-Talemi, Z. Anorg. Allg. Chem. 638, 844 (2012)
A. Altomare, M.C. Burla, M. Camalli, G.L. Cascarano, C. Giacovazzo, A. Guagliardi, A.G. Moliterni, G. Polidori, R. Spagna, J. Appl. Crystallogr. 32, 115 (1999)
G.M. Sheldrick, SHELXL-97 (University of Göttingen, Germany, 1997)
L.J. Farrugia, J. Appl. Crystallogr. 30, 565 (1997)
K. Nakamoto, Infrared and Raman spectra of inorganic and coordination compounds, Part B, 5th edn. (Wiley, New York, 1997), p. 124
K. Abu-Shandi, H. Winkler, H. Paulsen, R. Glaum, B. Wu, C. Janiak, Z. Anorg. Allg. Chem. 631, 2705 (2005)
B. Wisser, Y. Lu, C. Janiak, Z. Anorg. Allg. Chem. 633, 1189 (2007)
C.J. Holler, K. Muller-Buschbaum, Z. Anorg. Allg. Chem. 633, 2614 (2007)
A. Roth, A. Buchholz, W. Plass, Z. Anorg. Allg. Chem. 633, 383 (2007)
R.D. Hancock, M.S. Shaikjee, S.M. Dobson, J.C.A. Bceyens, Inorg. Chim. Acta 154, 229 (1998)
G. Wilkinson, R.D. Gillard, J. McCleverty (eds.), Comprehensive coordination chemistry, vol. 1–7 (Pergamon Press, Oxford, 1987)
N.N. Greenwood, A. Earnshaw, Chemistry of the elements (Pergamon Press, Oxford, 1986), p. 235
Acknowledgments
The authors acknowledge financial support by University of Tabriz Research Council (Project number sad/27/1391-3) and Università del Piemonte Orientale is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mirtamizdoust, B., Shaabani, B., Khandar, A. et al. Sonochemical Synthesis and Characterization of the New Plate-Shaped Lead(II)–Iodo Coordination Polymer: A Precursor to Produce a Pure Phase Nano-Sized Lead(II) Oxide. J Inorg Organomet Polym 22, 1293–1299 (2012). https://doi.org/10.1007/s10904-012-9754-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-012-9754-0