Abstract
New thiophene-2,5-dicarboxylate complexes with the formula of [M(μ-tdc)(pen)2] n (M = Ni(II) (1) and Cu(II) (2), pen = propane-1,3-diamine, tdc = thiophene-2,5-dicarboxylate) have been synthesized and characterized by using thermal (TG/DTG, DTA and MS) analysis, IR and UV–Vis. spectroscopies, magnetic measurement and single crystal X-Ray diffraction techniques. The Ni(II) and Cu(II) ions are distorted octahedrally coordinated by two oxygen atoms of two bridging thiophene-2,5-dicarboxylate ligands and four nitrogen atoms of the trans-pen ligands. The polymeric chains are connected together by hydrogen bonds interactions, forming three-dimensional network.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Synthesis, structures and properties of metal–organic coordination polymers is receiving increasing attention owing to their potential applications in materials science [1, 2]. In the construction of metal–organic frameworks, complexes based on carboxylates are of special interest. Carboxylic acids play an important role in the design of various coordination polymers because of their usage as building blocks. Polycarboxylate ligands such as benzencarboxylates, pyridinecarboxylates have been well established to be useful building blocks [3–8]. Like these carboxylic acids which show diverse coordination modes, thiophene-2,5-dicarboxylate can potentially be a monodentate, bridging or chelating ligands in its metal complexes. [9–11]. Because of these rich bridging modes thiophene-2,5-dicarboxylate (tdc) complexes are useful building blocks in the construction of coordination polymers. The diverse coordination modes of tdc are shown in Scheme 1. [9–15].
In our own investigations we are interested in the synthesis of new coordination polymers based on transition metal carboxylates and multidentate N-donor ligands. During these investigations we have obtained several compounds with 2,3-pyrazine-dicarboxylic acid, orotic acid and thiophene-2,5-dicarboxylic acid [16–18]. Thiophene-2,5-dicarboxylate anion is employed as exo-bidentate ligands for the design and construction of inorganic–organic hybrid frameworks owing to its thermal stability and symmetry [9]. As well known, rigid thiophene ring possesses unique physical and chemical activities. Because of the bigger radius of the S atom than C, N and O atoms, its lone pair of electrons can be more easily delocalized within the heterocyclic, and the ligand exhibits good charge-transfer ability [19].
To the best of our knowledge, six coordinate copper(II) complexes are uncommon with tdc, the geometry around the metal ion is generally trigonal bipyramidal or square pyramidal. We see that copper(II) complexes with tdc are the octahedral coordination mode. Furthermore, in our previous work we synthesized three copper(II) complexes of thiophene-2,5-dicarboxylic acid with dissimilar ligands and in all complexes the metal ion is in distorted octahedral geometry. This study represents the continuation of our extensive synthesis, spectral, thermal and structural characterization studies on the mixed-ligand complexes of tdc with metal atoms [16]. We report here the synthesis, crystal structures, spectroscopic and thermal properties of two new coordination polymers, [Ni(μ-tdc)(pen)2] n (1) and [Cu(μ-tdc)(pen)2] n (2).
2 Experimental
2.1 Materials and Physical Measurements
All chemicals and solvents purchased used for the synthesis were of reagent grade and used without further purification. C, H, N elemental analyses were performed on an Elementar Micro Vario CHNS. IR spectra of the complexes were determined as KBr discs using Perkin Elmer 100 FT-IR spectrometer within the 4000–400 cm−1 frequency range. Magnetic susceptibility measurements were performed using a Sherwood Scientific MXI model Gouy magnetic balance at room temperature. The UV–Visible spectra were obtained for the aqueous solution of the complex (10−3 M) with a Shimadzu UV-3150 spectrometer in the range of 900–190 nm. Thermal analyses were performed with a Diamond TG/MS thermal analyzer in static air from 30 to 700 °C at heating rate of 10°C/min. using platinum crucibles.
2.2 Synthesis of the Complexes
A solution of Ni(CH3COO)2·4H2O (0.58 g, 2.33 mmol) in water (10 mL) was added to a solution of thiophene-2,5-dicarboxylic acid (0.5 g, 2.90 mmol) in water (20 mL) after 10 min light green precipitate was formed and the mixture was stirred about 3 h at 50 °C. After that, aqueous solution of the pen (0.43 g, 5.80 mmol) was added dropwise with stirring. Clear solution was observed, stirred for 3 h at 60 °C and then cooling to room temperature. After several days light purple blocks were formed, collected and washed with ethanol. Anal. Calcd. for C12H22N4O4SNi: C, 38.22; H, 5.88; N, 14.86. Found: C, 38.37; H, 5.92; N, 14.71.
The preparative method used for 2 was the same as that for 1, using a solution of Cu(CH3COO)2·H2O (0.58 g, 2.90 mmol). Glossy dark blue crystals were obtained and washed with ethanol (yield >60% based on Cu). Anal. Calcd. for C12H22N4O4SCu: C, 37.74; H, 5.81; N, 14.67. Found: C, 37.72; H, 5.73; N, 14.71.
2.3 X-Ray Crystallography
For the crystal structure determination was used for data collection on a four-circle Rigaku R-AXIS RAPID-S diffractometer equipped with a two-dimensional area IP detector. The graphite-monochromatized Mo Kα radiation (λ = 0.71073 Å) and oscillation scans technique with Δω = 5° for one image were used for data collection. Images for 1 and 2 were taken successfully by varying ω with three sets of different χ and φ values. For each compounds the 108 images for six different runs covering about 99.8% of the Ewald spheres were performed. The lattice parameters were determined by the least-squares methods on the basis of all reflections with F2>2σ(F2). Integration of the intensities, correction for Lorentz and polarization effects and cell refinement was performed using CrystalClear software [20]. The structures were solved by direct methods (SHELXS-97) [21] and non-H atoms were refined by full-matrix least-squares method with anisotropic temperature factors (SHELXL-97) [21].
3 Results and Discussion
3.1 UV–Vis Spectra and Magnetic Properties
The electronic spectra of water solutions of the complexes 1 and 2 exhibit broad d-d absorption band with a maximum at 543 nm (ε = 38 L mol−1 cm−1) and 571 (ε = 93.6 L mol−1 cm−1), which are assigned to the 3A2g → 3T2g and 2Eg → 2T2g transition, respectively. 3A2g → 3T1g and 3A2g → 3T2g (P) transitions for Ni(II) complex were not observed which are shift to the UV region. The strong absorption bands below 300 nm are due to intraligand transitions.
Magnetic behavior of the complexes have been investigated at room temperature. 1 has the magnetic moment value of 2.62 which corresponds to two unpaired electron, 2 exhibits magnetic moment value of 1.83 which corresponds to one unpaired electron.
3.2 IR Spectra
The main IR group frequencies of the complexes are presented in Table 1. In the IR spectrum of complex 1 and 2 characteristic bands of the NH2 stretching bands of propane-1,3-diamine ligands were observed at 3290 and 3128 cm−1 for 1, 3296 and 3278 cm−1 for 2.
The asymmetric stretching band of the carboxylate function, ν as(COO) is seen at 1575 and 1567 cm−1, respectively. The symmetric stretching band is clearly seen at 1340 and 1363 cm−1 in 1 and 2. These Δν values [Δν = ν as(COO)−ν s(COO)] can be attributed respectively to the bridging bidentate coordination mode ((к1)−(к1)−μ2−tdc2−) (Δν = 235 and 204 cm−1) of the carboxylate functions of tdc2− [22].
3.3 Crystal Structures
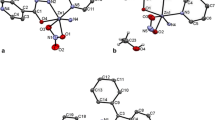
The compounds 1 and 2 are isomorphous and crystallize in the orthorhombic crystal system space group Pna21. Details of crystal structures are given in Table 2. The selected bond distances and angles together with the hydrogen bonding geometry for 1 and 2 are listed in Table 3. Crystallographic analysis reveals that the crystal structure of 1 and 2 are one dimensional wave-like chain polymer along the b axis (Fig. 1). M(II) atoms (M = Ni(II) and Cu(II)) have a distorted octahedral geometry with the basal plane comprised of four nitrogen atoms of pen ligands. The axial position is occupied by the oxygen atoms (O1 and O2) of the tdc ligands. In 1, Ni1–O bonds distances [Ni1–O1 = 2.108(6) Å and Ni1–O2 = 2.087(5) Å] and the equatorial Ni1–N bond distances [average Ni1–N = 2.097 Å] are is nearly equal, whereas these bond distances in 2 are significantly longer than those the equatorial Cu1–N bonds [average Cu1–N = 2.031 Å] due to the Jahn–Teller effect. Furthermore, Cu1–O1/O2 bond distances are also significantly longer than the corresponding bonds in [Cu(tdc)(bipy)(H2O)]·(bipy) [1.946(1) Å] [11], [Cu(tdc)(phen)(H2O)2]·3H2O [1.928(3) Å] [9], [Cu(tdc)(im)4] [2.372(2) Å], [Cu(tdc)(py)2] n [1.938(2) and 1.949(2) Å] [10] and [Cu(tdc)(dpya)(H2O)]·DMF·H2O} n [1.958(2) Å] [23]. The M···Mi, M···Mii and M···Miii distances within the intra- and interchain are 9.120, 6.905 and 6.907 Å, respectively for 1, 9.351, 7.747 and 6.739 Å, respectively for 2 [(i) = 2−x, −y, −1/2 + z; (ii) 1.5−x, −1/2 + y, z and (iii) 1.5−x, 1/2 + y, z for 1 and (i) = −x, −y, 1/2 + z; (ii) −1 + x, y, z and (iii) 1/2 + x, −1/2−y, z for 2].
The crystal packing is stabilized by intra- and intermolecular hydrogen bonds interaction. The strong N–H···O hydrogen bonds between nitrogen atoms of pen ligands (N1, N2, N3 and N4) and oxygen atoms of uncoordinated oxygen atom of carboxylate groups (O3 and O4) (average N···O = 3.022 Å for 1 and N···O = 3.094 Å for 2) were found within the adjacent chains (Fig. 2; Table 3.). All of these intermolecular interactions give a three-dimensional framework results.
3.4 Thermal Analyses
Thermal analyses (TA, DTA and DTG) were carried out to examine the thermal stability of complexes. The thermal behavior of complex 2 was investigated using differential thermoanalysis and thermogravimetry coupled with mass spectroscopy. Complexes are very stable in air at ambient temperature and almost soluble in common solvents such as water, methanol and ethanol, but insoluble in acetonitrile, N,N′-dimethylformamide and acetone. The TA curves of the complexes show that 1 and 2 stable up to 196 and 216 °C, respectively. Complexes indicate two obvious weight losses as shown in (Figs. 3, 4). The TA curves exhibit a continuous mass loss. Therefore, it is almost impossible to calculate mass loss value for each step. For complex 1 the first stage is related to exothermic removal of pen ligands and tdc moiety in the 216–445 °C temperature range. The solid product of the thermal decomposition was identified by IR spectroscopy as NiO. The overall weight loss of 80.60% (calcd. 84.44%) agrees with the propose structure well. For complex 2 stages of the temperature range of 195–244 °C, complex is related to the successively decomposition of pen ligands by giving exothermic effect (DTAmax = 244–244 °C) and the emission of CO2. The following stage involves the highly exothermic decomposition of tdc ligands (DTAmax = 433 °C). The solid product of the thermal decomposition was identified by IR spectroscopy as CuO with total mass loss of was (the overall weight loss, found 79.90, calcd. 83.37%).
4 Conclusion
In summary, we have structurally characterized two new coordination polymers, namely [Ni(μ-tdc)(pen)2]n (1) and [Cu(μ-tdc)(pen)2]n (2), which were prepared from Ni(OAc)2·4H2O, Cu(OAc)2·H2O, pen and H2tdc by a different method from the literature. Although in literature there are coordination polymers with tdc but lots of them are prepared by hydrothermal reaction. In this work we have been synthesized the [M(μ-tdc)(pen)2] n coordination polymers where M = Cu(II) and Ni(II) constructed from syn,syn-bridging bidentate thiophene-2,5-dicarboxylate ion which bridges the [M(pen)2] units to form a one dimensional wave-like chain polymer. The polymeric chains are connected via hydrogen bonds into a one dimensional network structure.
5 Supplementary Data
CCDC numbers 771105 and 771138 contains the supplementary crystallographic data for 1 and 2 respectively. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or e-mail: deposit@ccdc.cam.ac.uk.
References
G.F. Swiegers, T.J. Malefetse, Chem. Rev. 100, 3483–3538 (2000)
P.J. Hagrman, D. Hagrman, J. Zubieta, Angew. Chem. Int. Ed. 38, 2638–2684 (1999)
J. Lv, E.H. Shen, Y.G. Li, D.R. Xiao, E.B. Wang, L. Xu, Cryst. Growth Des. 5, 65–67 (2005)
X.L. Wang, C. Qin, E.B. Wang, Y.G. Li, C.W. Hu, L. Xu, Chem. Commun. 4, 378–379 (2004)
M. Eddaoudi, J. Kim, N. Rosi, D. Vodak, D.V. Wachter, M. O’Keeffe, O.M. Yaghi, Science 295, 469–472 (2002)
X.M. Zhang, M.L. Tong, M.L. Gong, X.M. Chen, Eur. J. Inorg. Chem. 1, 138–142 (2003)
N.L. Rosi, M. Eddaoudi, J. Kim, M. O’Keeffe, O.M. Yaghi, Angew. Chem. Int. Ed. 41, 284–287 (2002)
K. Barthelet, J. Marrot, D. Riou, G. Fe’rey, Angew. Chem. Int. Ed. 41, 281–284 (2002)
B.L. Chen, K.F. Mok, S.C. Ng, Y.L. Feng, S.X. Liu, Polyhedron 17, 4237–4247 (1998)
B.L. Chen, K.F. Mok, S.C. Ng, M.G.B. Drew, Polyhedron 18, 1211–1220 (1999)
X.Z. Sun, Y.F. Sun, B.H. Ye, X.M. Chen, Inorg. Chem. Commun. 6, 1412–1414 (2003)
X.Z. Sun, Z.L. Huang, H.Z. Wang, B.H. Ye, X.M. Chen, Z. Anorg. Allg. Chem. 631, 919–923 (2005)
J.H. Yu, C.J. Ding, K.F. Han, S.W. Zhang, H.Y. Guo, Chin. J. Inorg. Chem. 22, 607–611 (2006)
B.L. Chen, K.F. Mok, S.C. Ng, M.G.B. Drew, New J. Chem. 23, 877–883 (1999)
L. An, J. Zhou, L. Zhao, Y. Lv, Struct. Chem. 21, 159–164 (2010)
O.Z. Yeşilel, İ. İlker, O. Büyükgüngör, Polyhedron 28, 3010–3016 (2009)
O.Z. Yeşilel, A. Mutlu, O. Büyükgüngör, Polyhedron 28, 437–444 (2009)
H. Erer, O.Z. Yeşilel, O. Büyükgüngör, Polyhedron 29, 1163–1167 (2010)
K.X. Xu, Handbook of Fine Organic Chemical Engineering Raw Material and Intermediate (Chemical Industry, Beijing, 1986)
Rigaku,CrystalClear, Version 1.3.6. Rigaku American Corporation, 9009 New Trails Drive, The Woodlands, TX 77381-5209, USA (2005)
G.M. Sheldrick, SHELXS-97 and SHELXL-97. Program for Refinement of Crystal Structures (University of Göttingen, Germany, 1997)
K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, 5th edn. (Wiley Interscience, New York, 1997), pp. 59–62
Hong-Ping Xiao, Acta Cryst. E61, m2592–m2594 (2005)
Acknowledgment
This work was supported by the Eskişehir Osmangazi University by Project No. 200819042.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yeşilel, O.Z., İlker, İ. & Şahin, E. One-Dimensional Ni(II) and Cu(II) Coordination Polymers Containing Syn-Syn Thiophene-2,5-dicarboxylate and Propane-1,3-diamine. J Inorg Organomet Polym 21, 103–109 (2011). https://doi.org/10.1007/s10904-010-9414-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-010-9414-1