Abstract
Reaction of Ph3SnCl and K3[Cu(CN)4] with quinoxaline (qox) at room temperature affords the novel 3D-supramolecular coordination polymer (SCP) 3∞ [Cu2(CN)4·(Ph3Sn)2·qox], I, as the first example of the organotin-CuCN SCP containing the Ph3Sn fragment. The structure of I displays two Cu(CN)2 building blocks connected by a Ph3Sn cation and qox ligand creating the rhombic [Cu2(μ-CN)2] motif. The structure of I contains three different fused rings, the minicycle [Cu2(μ-CN)2], the 18-atomic [Cu4(Ph3Sn)2(CN)4(qox)2], and the 24-atomic [C8N8Cu4(Ph3Sn)4] rings, which create a non-interpenetrating 3D-network. These rings form a box-like structure with cavities suitable to accommodate bulky phenyl groups. SCP I belongs to the rapidly growing family of supramolecular assemblies containing the quite unusual [Cu2(μ-CN)2] motif, the repeated appearance of which raises the question whether cuprophilic interactions therein are the basic cause of the existence of I.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The first super-prussian blue (SPB) compound was Ag3[Co(CN)6], which contained the linear [Co–CN···Ag···NC–Co] fragment [1]. Later, the first crystallographically established organometallic SCP derivative was [(Me3Sn)3Co(CN)6], which contained nanometer-sized 3∞ [Co{μ-CNSn(Me3)NC}3] spacers [2]. Since then numerous SPB derivatives of general composition [M(CN) n ·mR3E(CN)] x-solvent, wherein M was Fe, Co, Cu or Ru, R was an alkyl group, E an Sn moiety or Pb-atom and x = 0–3, have been reported [3–9]. In the presence of water, a slightly elongated spacer –[N → Sn(R3) ← O(H)–H···NC–]– may be formed [10]. Incorporation of an acceptor L different from a terminal cyanide ligand leads to considerably more extended spacers such as –[–CN → Sn(Me3) ← O(H)–H···L···H–O(H) → Sn(Me3) ← NC] with L = 1,4-dioxane or 4,4-bipyridine [11, 12]. On the other hand, a limited number of SPB compounds containing organotin copper cyanide adducts with charged or uncharged L ligands is known [13–15]. The structure of these SCP compounds is based on mixed-spacers system of the type Cu–L–Cu and –[–CN ← Sn(Me3) ← NC–Cu–]–, which create voluminous 3D-networks wherein one carbon atom of each [CNSn(Me3)NC] spacer interacts with a pair of copper ions.
We report here the synthesis and the crystal structure of a related mixed-ligand system containing Ph3Sn cation and qox ligand.
2 Experimental Section
All chemicals and solvents used in this study were of analytical grade.
2.1 Synthesis of 3∞ [Cu2(CN)4·(Ph3Sn)2·qox], I
A solution of K3[Cu(CN)4] (90 mg, 0.31 mmol) in H2O (10 mL) was added with gentle stirring to a mixture of solutions containing Ph3SnCl (366 mg, 0.95 mmol) in acetonitrile (10 mL) and qox (40 mg, 0.31 mmol) in acetonitrile (10 mL). After a week at room temperature, yellow prismatic crystals formed. After filtration, subsequent washing with small portions of H2O and acetonitrile and overnight drying, about 260 mg (yield, 79%, based on K3[Cu(CN)4]) of yellow prismatic crystals were obtained. Anal. Calc. for I (C48H36N6Cu2Sn2): C, 54.31; H, 3.39; N, 7.92.98; Cu, 11.98%. Found: C, 54.24; H, 3.43; N, 7.85 Cu, 11.84%.
2.2 Single Crystal Structure Determination
Structural measurements for I were performed on a Kappa CCd Enraf Nonius FR 90 four circle goniometer with graphite monochromatic MoK α radiation {[λMoK α] = 0.71073 Å} at 25 ± 2 °C. The structure was solved using direct methods and all of the non-hydrogen atoms were located from the initial solution or from subsequent electron density difference maps during the initial stages of the refinement. After locating all of the non-hydrogen atoms in each structure the models were refined against F 2, first using isotropic and finally using anisotropic thermal displacement parameters. The positions of the hydrogen atoms were then calculated and refined isotropically, and the final cycle of refinements was performed. The cyano groups of all structures are ordered unless otherwise stated. Crystallographic data for I is summarized in Table 1. Selected bond distances and bond angles are given in Table 2.
3 Results and Discussion
3.1 Crystal Structure of 3∞ [{Cu(CN)2}2 μ-(Ph3Sn)2 μ-(qox)], I
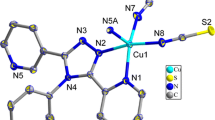
The ORTEP drawing of the asymmetric unit of I shows two crystallographically different copper sites forming two Cu(CN)2 building blocks, which are connected by the qox ligand while each of the Cu(CN)2 building block is attached by a Ph3Sn group (Fig. 1). These Cu(CN)2 building blocks form elongated corrugated chains, which are bridged by the Ph3Sn units. These chains are connected by the qox molecules creating a 3D-network structure (Fig. 2). The expanded structure of I indicates the formation of a unique rhombic [Cu2(μ3-CN)2] motif in which the Cu(I) atoms are four-coordinated to form a pseudo-tetrahedral (T-4) CuC3N conformation, in which two CN ligands bridge two copper ions most probably through their carbon atoms (Fig. 2; Table 2). The cyanide ligands of I represent the no longer elusive (vide infra) type of bifurcated μ3-CN bridges. The bond distances for Cu(3)\–C(8) and Cu(4)\–C(18) are 2.207(2) and 2.225(2) Å and that of the C(8)–Cu(3) and Cu(4)–C(18) are 2.029(2) and 2.018(2) Å, respectively (Table 2). In this case two of the cyanide ligands assume a linear Cu–CN geometry; ca. Cu(3)–C(6)–N(14) = 178.4°(2) and Cu(4)–C(12)–N(22) = 177.9°(2), while the other cyanide ligands are non-linear; ca. Cu(3)–C(8)–N(10) = 134.76°(14) and Cu(3)\–C(8)–N(10) = 150.91°(15) and Cu(4)–C(18)–N(9) = 134.3°(2) and Cu(4)\–C(18)–N(9) = 152.1°(2). Thus, each Cu(I) atom in the rhombic [Cu2(μ3-CN)2] motifs is coordinated to two bifurcated cyanide ligands, one linear cyanide ligand and the qox molecule.
It is a matter of fact that the cyanide ligand CN− is well known to bridge two metal atoms in a quasi-linear M-C ≡ N→M\ fashion, whereas the connection of two metal centers exclusively by its carbon atom, which is most common for the isoelectronic CO ligand, is extremely rare. A quite exceptional case of almost carbon monoxide-like bridging of CN− is, however, realized in the rhombic [Cu 12 (μ3-CN)2] motif that may be considered as basic building blocks of an increasing number of 2D or 3D supramolecular assemblies [14–20]. White et al. [21] have shown that almost anhydrous CuCN crystallizes in layers owing to the presence of [Cu2(μ-CN)2(CN)2] units. Bifurcated cyanide ligands may even help interconnecting layered coordination polymers to generate 3D-frameworks [22, 23]; and, a closer inspection of already published structures is likely to detect additional examples [24–26]. It is observed that the Cu(I) atom usually coordinates to the carbon end of the cyanide group, however, the experimental conformation of the anticipated carbon-to-copper coordinate of the μ3-CN ligand is not free of ambiguity [27]. Also, it should be kept in mind that at present there is no unambiguous method to identify [Cu2(μ-CN)2] fragments with direct carbon or nitrogen bridges. The previously described 3D-framework of the coordination polymer [Cu2(CN)2-pip] (pip = piperazine) seems to involve μ4-CN units while, in the other case, both the carbon and nitrogen atoms connect a pair of copper ions [25]. Yet, in the case of I, one end of the cyanide group should bond to a copper(I) atom while the other end should connect to the tin atom. Thus, we could argue that (a) the actual Sn–N bond distances compare well with the earlier reported data (Table 3), (b) the TBPY-5 configured {R3SnXX′} units, even with only one carbon atom in the axial position, are still extremely rare [28, 30] (to the best of our knowledge we are only aware of two compounds involving Me n Sn (n = 3, 2) units and Sn–C (cyanide) bonds with Sn–C distances as long as 2.49 and even 2.68 Å, respectively [31–34]), (c) there are some gold(I) compounds that have been synthesized with unusual coordination numbers for small nonmetals [35–37]. For example, the [C(AuPR3)6]2+ cation has carbon with the unusual covalency of six. While carbon has no low energy d-orbitals, there is nothing to prevent it from forming a1g (2S) and t1u (2P) MOs and forming three-center bonds, and (d) in the limit of a symmetric arrangement of the [Cu2(μ3-CN)2] motif with both Cu–C distances and the Cu–C≡N, Cu–C–Cu angles, the bonding would be regarded as two three-center Cu–C–Cu bonds, which is reminiscent of the bonding in diborane [25]. Also, these bond distances and bond angles are comparable to those in (CuCN)2(butda) [25], (CuCN)2(2,5-dmp) [24] and (CuCN)(phpip) [25], (CuCN)2(bpe) [24]. Thus, the above arrangements favor the formation of the unique [Cu2(μ3-CN)2] motif with a pentacoordinate carbon atom and bent cyanide group. On the other hand, in view of the current discussion of cuprophilic interactions [14, 15, 38–46], one might also suggest that the formation of a [Cu2(μ3-CN)2] rhomb is triggered by a weak, but non-negligible, Cu···Cu attraction. According to a detailed quantum chemical study [47], which focused on pairs of Cu1XY systems, a faint Cu···Cu attraction, which is weaker than aurophilic forces, seems to be realistic. The Cu···Cu distances in favor of cuprophilic attraction are usually longer than 2.70 Å [14, 15, 38–46]. It might be argued that the shorter Cu···Cu distances; e.g., Cu(3)···Cu(3) = 2.5021 Å and Cu(4)···Cu(4) = 2.5303 Å (Table 2), in the [Cu2(μ3-CN)2] rhombs are obviously due to efficient cyanide bridging in spite of the fact that they fall within the range of cuprophilic interactions. Interestingly, the Cu···Cu contacts in I are only slightly shorter than in metallic copper (2.55 Å) [48]. Actually, the bifurcated cyanide bridge is hardly observed outside assemblies that involve [Cu2(μ3-CN)2] fragments. Furthermore, it might be speculated that the asymmetric CN bridge would be more a consequence of cuprophilicity than the primary cause of comparatively short Cu···Cu distances.
The tin atom is coordinated to three phenyl groups and two cyanide ligands in axial positions via coordinate bonds to form a TBPY-5 structure. The phenyl groups are arranged in a TP-3 conformation with C–Sn–C angles ≈ 120° (Table 2) while the cyanide ligands occupy axial positions, which are perpendicular to the plane defined by the phenyl groups. The Sn–C and Sn–N distances are in the range of 2.125–2.133 and 2.3094–2.3439 Å, respectively, which are in accordance to the same bond distances reported for other SCPs (Table 3).
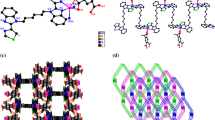
The network structure of I can be considered as consisting of repeating units of the unique minicycle [Cu2(μ3-CN)2] motif connected by the CN–Sn–NC spacer on one side and the qox spacer on the other side to form a 3D-network (Fig. 3). In this case, three different types of rings are formed. Ring 1 has the minicycle [Cu2(μ3-CN)2] motif and ring 2 is the 18-atomic [Cu4 (Ph3Sn)2 (CN)4 (qox)2] ring with four rhomb [Cu2(μ3-CN)2] motifs at the corner. This ring exhibits wide spacing; 12.033 Å × 14.657 Å, which is enough to accommodate the phenyl groups. Four phenyl groups interweave the two qox molecules of ring 2 along the c axis in a quasi-parallel fashion where the angles between the plane of the phenyl ring and the qox molecule are 6.78° and 11.97° (Fig. 4). In contrast, each two neighbor rings 2 accommodate also four interwoven phenyl rings in the middle of the space of ring. The four phenyl rings spread to form wing-like shapes (Fig. 5). Ring 3 is a 24-atom [C8N8Cu4 (Ph3Sn)4] ring, which is almost planar that is shifted from coplanarity by 7.25°. Rings 3 are fused, form ribbons along the a axis, and contain wide, roughly ellipsoidal pores; 14.912 Å × 14.977 Å. Along the a axis, ring 3 exhibits four minicycle [Cu2(μ3-CN)2] motifs, which tilt out of the plane of the ring by 51.79° and 53.22°. However, along the b axis each of the two facing minicycles are parallel while the plane of one of the two facing minicycles is tilted out of the plane of the other two facing minicycles by 75.48°. Thus, the network structure of I can be considered as constructed of fused rings that form an unprecedented 3D-network structure via the rhomb minicycle [Cu2(μ3-CN)2] motif, which contains the pseudo T-4 Cu(I) sites. The 3D-network structure of I acquires non-interpenetrating rings creating a box-like structure with cavities suitable to accommodate the bulky phenyl groups. Figures 4 and 5 show how the 3D-network structure is assembled in a unique way where the rhomb [Cu2(μ3-CN)2] motifs connect the different rings in 3D-fashion. This unique 3D-network structure of I exhibits four diamondoid minicycle [Cu2(μ3-CN)2] motifs and phenyl moieties of the qox molecules, which are directed to opposite positions and look like wings. The numerous bulky phenyl groups are assembled in a neat manner to form puckered infinite chains that look like waves along the a axis. These chains can be stabilized by π–π stacking (3.074–3.646 Å) and H-bonds (2.510–2.984 Å).
The structure of I is quite different than that of [Cu2(CN)2(qox)] [24], which adopts the common network structure constructed of {CuCN}∞ chains linked through quinoxaline ligands into a 2D-sheet. I exhibits more puckered [Cu(CN)2] chains than [Cu2(CN)2(qox)] owing to the presence of the Ph3Sn units, which connect the [Cu(CN)2] building blocks. Also, I contains the unique rhombic [Cu2(CN)2] motifs, which is not the case for [Cu2(CN)2(qox)].
4 Conclusion
The molecular formula of I contains six phenyl groups and the qox molecule. Such bulky groups need unique arrangements of the network structure to avoid crowding and steric hindrance. In such case, the presence of repeating units of the rhombic [Cu2(μ3-CN)2] motif connected by the CN–Sn–CN spacer on one side and the qox spacer on the other side creates a 3D-network containing wide spacing, which is enough to accommodate the phenyl groups. The formation of a box-like structure is a general feature of the prototype SCP. Thus, the rhombic [Cu2(μ3(CN)2] motifs can be considered as the basic building blocks in the structures of the SCP containing such bulky groups.
5 Supplementary Data
CCDC 737374 contains the supplementary crystallographic data for SCP I. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or e-mail: deposit@ccdc.cam.ac.uk. Supplementary data associated with this.
References
L. Pauling, P. Pauling, Proc. Natl. Acad. Sci. USA 60, 362 (1968)
K. Yünlü, N. Höck, R.D. Fischer, Angew. Chem. Int. Ed. Engl. 24, 879 (1985)
D.C. Appreley, N.A. Davies, R.K. Harris, A.K. Brimah, S. Eller, R.D. Fisher, Organometallics 9, 2672 (1990)
D.C. Appreley, N.A. Davies, R.K. Harris, S. Eller, P. Schwarz, R.D. Fisher, J. Chem. Soc. Chem. Commun. 740 (1992)
T. Niu, J. Liu, X. Wang, J.D. Krop, A.J. Jacobson, Inorg. Chem. 37, 5324 (1998)
U. Behrins, A.K. Brimah, T.M. Soliman, R.D. Fischer, D.C. Apperly, N.A. Davies, R.K. Harris, Organometallics 11, 1718 (1992)
M.Sh. Ibrahim, A.F. Morgan, A.B.K. Mecky, S.E.H. Etaiw, Pol. J. Chem. 78, 173 (2004)
A.M.A. Ibrahim, J. Organomet. Chem. 556, 1 (1998)
E.M. Poll, J.U. Schütze, R.D. Fischer, N.A. Davies, D.C. Apperley, R.K. Harris, J. Organomet. Chem. 621, 254 (2001)
J. Liu, W.T. Harrison, A.J. Jacobson, Inorg. Chem. 35, 4271 (1996)
M. Adam, A.K. Brimah, R.D. Fischer, X.F. Li, Inorg. Chem. 29, 1595 (1990)
R. Eckhardt, H. Hanika-Heidl, R.D. Fischer, Chem. Eur. J. 9, 1795 (2003)
A.M.A. Ibrahim, E. Siebel, R.D. Fischer, Inorg. Chem. 37, 3521 (1998)
E. Siebel, A.M.A. Ibrahim, R.D. Fischer, Inorg. Chem. 38, 2530 (1999)
H. Hanika-Heidl, S.E.H. Etaiw, M.Sh. Ibrahim, A.S. Badr El-din, R.D. Fischer, J. Organomet. Chem. 684, 329 (2003)
J.H. Yu, J.Q. Xu, Q.X. Yang, L.Y. Pan, T.G. Wang, C.H. Lü, T.H. Ma, J. Mol. Struct. 658, 1 (2003)
D.J. Chesnut, J. Zubieta, Chem. Commun. 1707 (1998)
O. Teichert, W.S. Sheldrick, Z. Anorg. Allg. Chem. 625, 860 (1999)
O. Teichert, W.S. Sheldrick, Z. Anorg. Allg. Chem. 626, 1509 (2000)
C.P. Cui, P. Lin, W.-X. Du, L.-M. Wu, Z.-Y. Fu, J.-C. Dai, S.M. Hu, X.-T. Wu, Inorg. Chem. Commun. 4, 444 (2001)
J.D. Kildea, B.W. Skelton, A.H. White, Aust. J. Chem. 38, 1329 (1985)
J. Cernák, K. Györyová, S. Sabolová, M. Dunaj-Jurco, Inorg. Chim. Acta 185, 119 (1991)
M. Schwarten, J. Chomic, J. Cernák, D. Babel, Z. Anorg. Allg. Chem. 622, 1449 (1996)
D.J. Chesnut, D. Plewak, J. Zubieta, J. Chem. Soc. Dalton Trans. 2567 (2001)
F.B. Stocker, T.P. Staeva, C.M. Rienstra, D. Britton, Inorg. Chem. 38, 984 (1999)
R.T. Williams, D.T. Cromer, A.C. Larson, Acta Crystallogr. B 27, 1701 (1971)
D.T. Cromer, A.C. Larson, R.B. Roof Jr., Acta Crystallogr. 19, 192 (1965)
A.K. Brimah, E. Siebel, R.D. Fischer, N.A. Davies, D.C. Apperley, R.K. Harris, J. Organomet. Chem. 475, 85 (1994)
T. Niu, A.J. Jacobson, Inorg. Chem. 38, 5346 (1999)
U. Behrens, A.K. Brimah, R.D. Fischer, J. Organomet. Chem. 411, 325 (1991)
P. Avalle, R.K. Harris, H. Hanika-Heidl, R.D. Fischer, Solid State Sci. 6, 1069 (2004)
Me3SnCN: E.O. Schlemper, D. Britton, Inorg. Chem. 5, 507 (1966)
Me2Sn(CN)2: J. Konnert, D. Britton, Y.M. Chow, Acta Crystallogr. B 28, 180 (1972)
P.G. Harrison, T.J. King, K.C. Molley, J. Organomet. Chem. 185, 199 (1980)
F. Scherbaum, A. Grohmann, B. Huber, C. Krüger, H. Schmidbour, Angew. Chem. Int. Ed. Engl. 27, 1544 (1988)
F. Scherbaum, A. Grohmann, G. Müller, H. Schmidbour, Angew. Chem. Int. Ed. Engl. 28, 463 (1989)
O. Steigelmann, P. Bissinger, H. Schmidbaur, Angew. Chem. Int. Ed. Engl. 29, 1399 (1990)
T.A. Tronic, K.E. deKrafft, M.J. Lim, A.N. Ley, R.D. Pike, Inorg. Chem. 46, 8897 (2007)
R.D. Köhn, G. Seifert, Z. Pan, M.F. Mahon, G. Koziok-Köhn, Angew. Chem. 115, 818 (2003)
R.D. Köhn, G. Seifert, Z. Pan, M.F. Mahon, G. Koziok-Köhn, Angew. Chem. Int. Ed. Engl. 42, 793 (2003)
G. Boche, F. Bosold, M. Marsch, K. Harms, Angew. Chem. 110, 1778 (1998)
G. Boche, F. Bosold, M. Marsch, K. Harms, Angew. Chem. Int. Ed. Engl. 37, 1684 (1998)
C.M. Che, Z. Mao, V.M. Miskowski, M.-C. Tse, C.-K. Chan, K.-K. Cheung, D.L. Phillips, K.-H. Leung, Angew. Chem. 112, 4250 (2000)
C.M. Che, Z. Mao, V.M. Miskowski, M.-C. Tse, C.-K. Chan, K.-K. Cheung, D.L. Phillips, K.-H. Leung, Angew. Chem. Int. Ed. Engl. 39, 4084 (2000)
A. Sundararaman, L.N. Zakharov, A.L. Rheingold, F. Jäkle, Chem. Commun. 1708 (2005)
G. Margraf, J.W. Bats, M. Bolte, H.-W. Lernera, M. Wagner, Chem. Commun. 956 (2003)
H.L. Hermann, G. Boche, P. Schwerdtfeger, Chem. Eur. J. 7, 5333 (2001)
A.F. Wells, Z. Kristallogr. 94, 447 (1936)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Etaiw, S.Ed.H., Abdou, S.N.A. A Novel 3D-Supramolecular Coordination Polymer Based on CuCN, Ph3Sn Cation and Quinoxaline. J Inorg Organomet Polym 20, 622–627 (2010). https://doi.org/10.1007/s10904-010-9383-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-010-9383-4