Abstract
A secondary amino group modified MCM-41 (mobile crystalline material number 41) was synthesized and used as a support for the immobilization of a salen oxovanadium complex via a multi-grafting method. The immobilized complex was characterized by UV–Vis spectroscopy, X-ray diffraction (XRD), N2 adsorption and ICP analysis techniques. The immobilized complex was found to be an effective catalyst for oxidation of cyclohexane using H2O2 as an oxidant under mild conditions. A conversion of 45.5% of cyclohexane was obtained with a selectivity of 100% of the cyclohexanone/cyclohexanol mixture when the reaction was run at 60 °C for 12 h in acetonitrile. Decomposition of the complex, which leads to the deactivation of the catalyst, is observed and a decomposition mechanism is discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The selective partial oxidation of alkanes to afford oxygenated petrochemicals such as alcohols and carbonyl compounds is very important in utilizing petroleum and natural gas-based resources [1–3]. This process can be carried out by employing various oxidizing reagents such as oxygen [4–6], H2O2 [7–10], tert-butyl hydroperoxide [11–13] and catalyzed by many kinds of metal complexes. Among all the complexes vanadium complexes play a very important role in the catalytic partial oxidation of alkanes as a consequence of their low radius/charge ratio, which makes them suitable for the activation of peroxidic reagents [14, 15]. Soluble vanadium complexes are known to catalyze hydrocarbon oxidations with molecular oxygen, hydrogen peroxide and other oxygen donors [16–19]. However, under homogeneous conditions vanadium complexes also face the problem of separation from reaction mixtures; and, they often decompose or polymerize during catalytic reaction. Therefore, immobilization of vanadium complexes by anchoring or encapsulation on or into various supports [20–22] has received much attention as these supported complexes possess the advantageous features of heterogeneous as well as homogeneous catalysts.
MCM-41 (Mobile Crystalline Material Number 41), an ordered mesoporous compound with high surface area (typically 1000 m2/g) with a uniform large pore size (20 Å) and one dimensional, hexagonally ordered pore structure, has been extensively used as a support in the heterogenization of homogeneous catalysts [23]. The large surface area and uniform large pore size of MCM-41 have allowed bulky organic molecules to diffuse in and out of the mesopores with minimum steric hindrance. Compared to other commonly used supports, such as organic polymers, MCM-41 also has the advantage of high thermal stability in heterogeneous catalysis. Due to its large surface area and a uniform large pore size, these materials can act as excellent supports for the immobilization of soluble metal complexes [23]. Generally functionalized MCM-41 can be easily obtained either by covalently grafting various organic species onto the interior surface by post-synthesis grafting or by organosiloxane/siloxane co-condensation method [24]. Relative to the former method, a more homogeneous distribution, a higher loading of functional groups and an easier one-pot process make the latter method preferred.
Herein we used the second approach to prepare a secondary amino-modified MCM-41. The material was used as a support through a multi-step grafting method to immobilize a salen vanadium complex (abbreviated as salen VO complex). The heterogenized complex is used for the catalytic oxidation of cyclohexane under mild conditions with hydrogen peroxide as an oxidant.
2 Experimental
2.1 Materials
Cetyltrimethyl ammonium bromide (CTAB), salicylaldehyde, acetonitrile, hydrogen peroxide (H2O2, 30%), acetic acid and sodium hydroxide were purchased from Tianjin Kermel Chemical Reagents Development Centre. Tetraethoxysilane (TEOS) was provided by Tianjin No. 1 Chemical Regent Factory. Bis(3-(triethoxysilyl)propyl)-amine (BTEA) was obtained from Beijing Shenda Fine Chemical Company and distilled prior to use. VOSO4 was produced by Shenyang Haizhongtian Fine Chemical Factory. Cyclohexane was obtained from Acros Organics. 5-Chloromethylsalicylaldehyde was synthesized according to the procedure described in Ref. [25]. All organic solvents were dried and distilled prior to use.
2.2 Synthesis of the Second Amino Group Modified MCM-41 (MCM-41-NH)
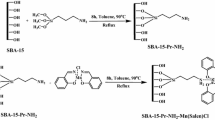
The secondary amino group modified MCM-41 was synthesized by the following procedure. The reaction mixture contained CTAB/TEOS/BTEA/NaOH/H2O (1.0:8.16:0.37:2.55:4857) based on a molar ratio. The mixture of 9.0 g (24.7 mmol) of CTAB, 31.5 mL (63.0 mmol) of NaOH (2.0 M, aqueous), and 2161.6 g (120.1 mol) of H2O were heated at 80 °C for 30 min to reach pH of 12.3. To this clear solution, 41.9 g (201.6 mmol) TEOS and 3.9 g (9.2 mmol) BTEA were added sequentially and rapidly via injection. Following the injection, a white precipitate was observed after 3 min of stirring. The reaction temperature was maintained at 80 °C for 3 h. The mixture was transferred into a Teflon-lined autoclave at 80 °C for 3 days to crystallize the product. The crystallized product was isolated by filtration, washed with distilled water, and dried at 100 °C for 10 h. The dried solid (12.4 g) was extracted with a mixture of ethanol (100 mL) and concentrated hydrochloric acid (1.0 mL) at 80 °C for 6 h. Finally the Si-MCM-41-NH (1) was obtained after filtering and washing with ethanol.
2.3 Immobilization of Salen VO Complex on MCM-41-NH
To 50 mL of THF were added 4 g of MCM-41-NH and 0.53 g (3.1 mmol) of 5-chloromethylsalicylaldehyde. The mixture was refluxed under a nitrogen atmosphere for 10 h. The mixture was filtered and the resulting powder (2) was washed and extracted with THF in a soxhlet extractor for 10 h. The resulting powder was added to 50 mL of absolute ethanol followed by addition of 0.36 g (3.1 mmol) of 1,2-diaminocyclohexane. The mixture was refluxed for 14 h, then filtered. The resulting powder (3) was extracted with ethanol in a Soxhlet extractor for 10 h. Then the power was suspended in 50 mL of ethanol and to this suspension was added 0.77 g (6.2 mmol) of salicylaldehyde. The suspension was refluxed for 18 h and filtered to afford a powder (4). The powder was washed with ethanol and then extracted with ethanol in a Soxhlet extractor for 10 h.
To a suspension of 2.5 g of 4 and 50 mL of absolute ethanol was added a solution of 1.56 g (6.2 mmol) of VOSO4 in 50 mL of absolute ethanol. The powder immediately turned green indicating the formation of a vanadium complex. The suspension was stirred at reflux for 3 h. The suspension was filtered and a green powder (5) was separated. The powder was washed and extracted with ethanol in a Soxhlet extractor for 10 h [26].
2.4 Characterization Procedures
The X-ray diffraction (XRD) patterns were carried out on a Riguku D/max-2500 X-ray diffractometer using Cu-KD radiation at 40 kV and 100 mA, in the 2θ range 1.5–9°. Diffuse reflectance UV–Vis spectra of the solid samples were carried out on a Varian Cary 300 UV–Vis spectrophotometer. Adsorption and desorption isotherms of nitrogen were measured at 77 K on a Micromeritics ASAP 2010 volumetric. The vanadium content of the catalysts was analyzed by an ICP-9000 (N + M) spectrophotometer, Thermo Jarrell-Ash Corp., USA, after the samples were dissolved in HF. Adsorption and desorption isotherms of nitrogen were measured at 77 K on a Micromeritics ASAP 2010. The pore volume and pore radius were obtained from BJH equation (below) [27]
in which, σ is the surface tension of liquid nitrogen, V is the liquid molar volume of nitrogen, r k is the radius of the capillary in cm. (later converted to Angström units for practical purposes), T is the absolute temperature (K) and 8.316 × l07 is the gas constant in ergs K−1 mol−1.
2.5 Oxidation of Cyclohexane
The oxidation of cyclohexane was carried out according to the method reported in the literature [28, 29]. In a typical reaction, cyclohexane (5 mmol), 30% H2O2 (10 mmol), and the catalyst (0.02 mmol) were mixed in 4 mL of acetonitrile. The reaction mixture was heated at 60 °C with continuous magnetic stirring. During the reaction, the reaction progress was monitored using a Shandong Lunan Ruihong Gas Chromatograph, SP-6800A, equipped with a SE 30 capillary column, 30 m × 0.25 mm and the products were analyzed by GC-MS (Trace DSQ, USA) equipped with a CP-SIL 5 column.
3 Results and Discussion
3.1 Preparation of the Immobilized Catalyst
The route to the synthesis of the immobilized catalysts is shown in Scheme 1. First, a secondary amino-functionalized MCM-41 was synthesized by copolymerization of TEOS and BTEA in the presence of C16TMABr as a template as described in the literature [30–32]. The secondary amino group, which is attached to the matrix, reacts smoothly with 5-chloromethyl salicylaldehyde by a nucleophilic substitution reaction to attach the salicylaldehyde moiety onto the MCM-41 surface. In the subsequent procedure the attached salicylaldehyde condenses readily with one amino group of 1,2-diaminocyclohexane, and then the remaining amino group reacts with salicylaldehyde in a next step, as it does under homogeneous conditions [33], to give the MCM-41 anchored salen ligand. Similar to the coordination reaction under homogeneous condition [34], the supported ligand coordinates with VOSO4 smoothly to give the supported complex catalyst. The heterogenized catalyst is extracted until a colorless extract is obtained. This is a generally accepted process to remove absorbed compounds or complexes on the interior surface of the supported catalysts [26].
The vanadium content (4.84% by wt.) of the heterogenized catalyst was determined by ICP. The extraction procedure removed all the compounds or complexes from the MCM-41 surface. To confirm the fact that the extraction process can remove the compounds absorbed on the MCM-41 surface in a nonspecific bonding fashion, the product was mixed with VOSO4 directly in absolute ethanol and treated as the procedure in the preparation of the heterogenized catalyst. The collected solid was subjected to ICP analysis. Only 0.14% vanadium was found, which indicates that the vanadium content of the heterogenized catalyst is mainly from the formation of the salen vanadium complex and not from nonspecific bonding to surface silanols.
3.2 Characterization of the Catalyst
Figure 1 shows the XRD patterns of MCM-41-NH and the immobilized complex, salen VO/Si-MCM-41. The XRD pattern of the MCM-41-NH was similar to the pattern of pure MCM-41 published in the literature [34], which exhibited four peaks at low angles that correspond to the d100, d110, d200, d210 reflections of a regular hexagonal array of pores. Interplanar spacing d100 of the MCM-41-NH was observed at the value of 4.12 nm. The pattern of the immobilized complex has no significant change relative to that of MCM-41-NH except for intensity decreases of the peaks, which indicate that the MCM-41 framework structure remains after modification by the multi-step grafting method.
3.3 Nitrogen Sorption
Table 1 presents the specific surface areas and the range of pore structural parameters of the MCM-41-NH and the supported complex catalyst. The MCM-41-NH has an area of 872.1 m2/g lower than that of the pure silicon MCM-41 [35]. Decreases in surface area from 872.1 to 596.7 m2/g, pore volume from 0.77 to 0.60 cm3/g and pore diameter from 35.2 to 30.0 Å were observed after the complex was supported on MCM-41-NH.
Figure 2 shows the pore radius distribution from BJH of the MCM-41-NH and the supported complex catalyst. The two samples give a maximum pore radius of about 2.53 and 2.49 nm, respectively. The decrease in maximum pore radius after complexation indicates that complexation takes place more easily in the pores of large radius. Figure 2 also shows a decrease in pore volume after the complex is supported on MCM-41-NH. A similar trend is observed by other researchers; and, the reduction in the surface area and pore size has been assigned to the lining of the walls of Si-MCM-41 with the organic moieties [26]. In other words the immobilization of the complex occurs within the mesopores of MCM-41-NH.
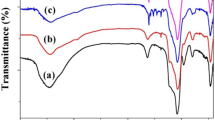
Curves a and b in Fig. 3 show the nitrogen adsorption–desorption isotherm of MCM-41-NH and the supported complex catalyst, respectively. Both curves display a typical type IV isotherm, which has three well-distinguished regions: mono- and multi-layer adsorption on the pore walls, capillary condensation, and multi-layer adsorption on the outer surface [36, 37]. This is in agreement with the typical shape for mesoporous MCM-41.
3.4 UV–Vis Spectroscopy
Generally metal salen complexes and ligands exhibit characteristic bands in their optical spectra [38]. The anchoring of complexes to solid surfaces can be conveniently followed by diffuse reflectance UV–Vis spectroscopy [39, 40]. Figure 4 shows the diffuse reflectance UV–Vis spectra of MCM-41-NH, the intermediates in the synthesis of the catalyst and the supported catalyst. It can be seen there is no obvious absorbance band in the spectrum of 1. In spectrum of 2 the bands maximum at 214, 254 nm are derived from the π → π* transitions of phenyl and C=O groups and the band around 325 nm is from n → π* of C=O. The band maximum around 405 nm in spectra of 3 and 4 is attributed to the π → π* transition of C=N. The absorbance band around 700 nm in spectrum of 5 is possibly due to the phenolate-V(IV) ligand-metal charge-transfer [41], which indicates the successful anchoring of salen-VO complex onto the MCM-41-NH.
3.5 Catalytic Oxidation of Cyclohexane
The immobilized salen VO complex was used as a catalyst for the oxidation of cyclohexane (Scheme 2). The immobilized complex showed good catalytic activity under mild conditions. Several parameters influencing the catalytic properties were investigated.
The effect of the immobilized catalyst on reaction time was studied in the presence of H2O2 at 60 °C in acetonitrile. The data are summarized in Table 2. The conversion of cyclohexane was 23.9 mol% after 6 h and increased rapidly to 45.5 mol% after 12 h. Thereafter the conversion increased slowly and reached a maximum of 46.7 mol% after 24 h. In addition, the yield of cyclohexanone increased with the prolongation of reaction time, but the yield of cyclohexanol decreased accordingly due to the further oxidation of cyclohexanol to cyclohexanone under the catalytic reaction conditions.
The effect of H2O2 loading on the oxidation is given in Table 3. The conversion increased from 17.4 to 75.8 mol% when the H2O2 loading increased from 5 to 20 mmol. However, the yield of cyclohexanone-cyclohexanol mixture decreased sharply possibly due to the further oxidation of cyclohexanol and cyclohexanone by excess H2O2. It is noteworthy that the selectivity of cyclohexanone/cyclohexanol mixture was 100% if the H2O2 was kept below 10 mmol under the reactions.
The effect of reaction temperature from 40 to 70 °C on the catalytic oxidation of cyclohexane with H2O2 and the distribution of products is summarized in Table 4. The conversion of cyclohexane increased quickly to a maximum of 45.5 mol% at 60 °C. At the same time the selectivity of cyclohexanone/cyclohexanol mixture was still 100%. When the reaction is run at 70 °C, the conversion decreased to 42.9% and the selectivity of cyclohexanone/cyclohexanol mixture also decreased. This may be a result of the decomposition of H2O2 and further oxidation of cyclohexanone and (or) cyclohexanol at high reaction temperature.
The oxidation of cyclohexane with other oxidants and catalysts were previously published [29, 42, 43]. Jin et al. [42] reported the synthesis and characterization of zeolite Y supported transition metal tetrahydro-schiff base complexes. When using Cu-[H4]salen/Y as a catalyst, the largest cyclohexane conversion was 9.5%. A hetero-binuclear macrocyclic Co-V complex bonded to a chemically modified alumina support was also applied to the oxidation of cyclohexane using oxygen as an oxidant. Although high selectivity for cyclohexanone/cyclohexanol mixture was observed, the conversion is still low [42]. Compared with the catalysts reported in literature [29, 42, 43], 5 shows high activity and high selectivity for the cyclohexanone/cyclohexanol mixture within a reasonable reaction time and temperature.
In theory, the activity of the catalyst used here can be derived from 1, the vanadium compounds that are nonspecifically bonded to surface silanols of MCM-41-NH, and 5. 1 and 1 that is doped with VOSO4 as described in above were also subjected to the oxidation of cyclohexane under the same conditions as 5. However, no reaction products were observed. Therefore, we conclude that the catalytic activity of the catalyst originated from 5.
Table 2 also shows that conversion increased only 1.2 mol% from 12 to 24 h, which indicates that the catalyst almost completely loses its activity after 12 h. It is observed that the color of the reaction solution changed from colorless to blue with prolonged reaction time. Concomitantly, the catalyst loses its characteristic color gradually. ICP analysis shows that the V content is only 0.38% at this stage. If the blue filtrate is used as the solvent to run the reaction without adding additional fresh catalyst, no reaction occurs. The results indicate that the supported complex decomposes to non-active components entering into solution during catalysis. Mishra and Pombeiro reported previously a similar immobilized vanadium complex in the oxidation of cyclohexane by molecular oxygen [44]. They found that the catalyst can be recycled without activity reduction. On comparing the structures and the recycling test results of the two supported complexes, it can be concluded that the decomposition of the supported complex in this study is possibly induced by water from the aqueous H2O2 and accelerated by the carboxylic acids derived from further oxidation of cyclohexane. The results also indicate that aqueous H2O2 is not the most suitable oxidant for the catalyst due to the presence of water. It is expected water-free oxidants such as molecular oxygen and tert-butyl hydroperoxide may resolve the problem of catalyst decomposition.
4 Conclusions
Secondary amino modified MCM-41 was successfully synthesized by copolymerization and used as a support to immobilize a salen VO complex via a multi-grafting method. The immobilized complex showed good activity and selectivity in the oxidation of cyclohexane using H2O2 as an oxidant within a relative short time. Long reaction times and high temperatures lead to the decomposition of the supported complex induced by water from aqueous H2O2 and a decrease in selectivity of cyclohexanone/cyclohexanol mixture.
References
D.H.R. Barton, A.E. Martell, D.T. Sawyer, The Activation of Dioxygen and Homogeneous Catalytic Oxidation (Plenum, New York, 1993)
R.A. Sheldon, R.A. van Santen, Catalytic Oxidation: Principles and Applications (World Scientific, Singapore, 1995)
T. Punniyamurthy, L. Rout, Coord. Chem. Rev. 252, 134 (2008)
A. Kumar, G.S. Mishra, A. Kumar, J. Mol. A Catal. Chem. 201, 179 (2003)
H. Tang, C. Shen, M. Lin, A. Sen, Inorg. Chim. Acta 300–302, 1109 (2000)
K.S. Anisia, A. Kumar, J. Mol. A Catal. Chem. 219, 319 (2004)
H.R. Mardani, H. Golchoubian, J. Mol. A Catal. Chem. 259, 197 (2006)
G.B. Shul’pin, A.R. Kudinov, L.S. Shul’pina, E.A. Petrovskaya, J. Organomet. Chem. 691, 837 (2006)
G.B. Shul’pin, G.V. Nizova, Y.N. Kozlov, V.S. Arutyunov, A.C.M. dos Santos, A.C.T. Ferreira, D. Andelli, J. Organomet. Chem. 690, 4498 (2005)
S. Velusamy, T. Punniyamurthy, Tetrahedron Lett. 44, 8955 (2003)
V.B. Valodkara, G.L. Tembeb, M. Ravindranathanb, H.S. Ramaa, J. Mol. Catal. A Chem. 223, 31 (2004)
T. Kojima, H. Matsuo, Y. Matsuda, Inorg. Chim. Acta 300–302, 661 (2000)
G.L. Tembe, P.A. Ganeshpure, S. Satish, J. Mol. Catal. A Chem. 121, 17 (1997)
V. Conte, F. Di Furia, S. Moro, J. Phys. Org. Chem. 9, 329 (1996)
A.G.J. Ligtenbarg, R. Hage, B.L. Feringa, Coord. Chem. Rev. 237, 89 (2003)
A. Butler, M.J. Clague, G.E. Meister, Chem. Rev. 94, 625 (1994)
A.E. Shilov, G.B. Shul’pin, Chem. Rev. 97, 2879 (1997)
G. Süss-Fink, L.G. Cuervo, B. Therrien, H. Stoeckli-Evans, G.B. Shul’pin, Inorgan. Chim. Acta 357, 475 (2004)
L.G. Cuervo, Y.N. Kozlov, G. Süss-Fink, G.B. Shul’pin, J. Mol. A Catal. Chem. 218, 171 (2004)
G.B. Shul’pin, G.S. Mishra, L.S. Shul’pina, T.V. Strelkova, A.J.L. Pombeiro, Catal. Commun. 8, 1516 (2007)
A. Kozlov, A. Kozlova, K. Asakura, Y. Iwasawa, J. Mol. A Catal. Chem. 137, 223 (1999)
G.S. Mishra, A.J.L. Pombeiro, J. Mol. A Catal. Chem. 239, 96 (2005)
U. Ciesla, F. Schüth, Microporous Mesoporous Mater. 27, 131 (1999)
D. Brunel, N. Bellocq, P. Sutra, A. Cauvel, M. Laspéras, P. Moreau, F. Di Renzo, A. Galarneau, F. Fajula, Coord. Chem. Rev. 178–180, 1085 (1998)
S.J. Angyal, P.J. Morris, J.R. Tetaz, J.G. Wilson, J. Chem. Soc. 3, 2141 (1950)
T. Joseph, M. Hartmann, S. Ernst, S.B. Halligudi, J. Mol. A Catal. Chem. 207, 131 (2004)
E.P. Barrett, L.G. Joyner, P.P. Halenda, J. Am. Chem. Soc. 73, 373 (1951)
M.R. Mauryaa, A.K. Chandrakar, S. Chand, J. Mol. A Catal. Chem. 270, 225 (2007)
G.C. Salomão, M.H.N. Olsen, V. Drago, C. Fernandes, L.C. Filho, O.A.C. Antunes, Catal. Commun. 8, 69 (2007)
S. Huh, J.W. Wiench, J.-C. Yoo, M. Pruski, V.S.-Y. Lin, Synth. Meth. Chem. Mater. 15, 4247 (2003)
W. Zeng, X.-F. Qian, J. Yin, Z.-K. Zhu, Mater. Chem. Phys. 97, 437 (2006)
M.H. Lim, A. Stein, Chem. Mater. 11, 3285 (1999)
L. He, J. Zhao, Y. Zhang, Chin. J. Appl. Chem. 23, 688 (2006)
J.R. Zamian, E.R. Dockal, Polyhedron 14, 2411 (1995).
V. Caps, S.C. Tsang, Appl. Catal. A Gen. 248, 19 (2003)
M. Park, S. Komarneni, Microporous Mesoporous Mater. 25, 75 (1998)
R. Schmidt, E.W. Hansen, M. Stoecker, D. Akporiaye, O.H. Ellestad, J. Am. Chem. Soc. 117, 4049 (1995)
M.J. Sabater, M. Álvaro, H. García, E. Palomares, J.C. Scaiano, J. Am. Chem. Soc. 123, 7074 (2001)
G.-J. Kim, D.-W. Park, Catal. Today 63, 537 (2000)
C. Baleizão, B. Gigante, M.J. Sabater, H. Garcia, A. Corma, Appl. Catal. A Gen. 228, 279 (2002)
A. Horn Jr., C.A.L. Filgueiras, J.L. Wardell, M.H. Herbst, N.V. Vugman, P.S. Santos, J.G.S. Lopes, R.A. Howie, Inorg. Chim. Acta 357, 4240 (2004)
C. Jin, W. Fan, Y. Jia, B. Fan, J. Ma, R. Li, J. Mol. A Catal. Chem. 249, 23 (2006)
M.J.L. Kishore, G.S. Mishra, A. Kumar, J. Mol. A Catal. Chem. 230, 35 (2005)
G.S. Mishra, A.J.L. Pombeiro, J. Mol. A Catal. Chem. 239, 96 (2005)
Acknowledgement
This work was supported by NSFC of China (grant no. 20376017).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, J., Wang, W. & Zhang, Y. Preparation of MCM-41 Supported Salen Vanadium Complex and its Catalysis for the Oxidation of Cyclohexane with H2O2 as an Oxidant. J Inorg Organomet Polym 18, 441–447 (2008). https://doi.org/10.1007/s10904-008-9221-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-008-9221-0