Abstract
Interferon-gamma release assays have potentially been transformative to screening programs focused on latent tuberculosis infection (LTBI) in foreign-born persons. We sought to add to this assessment by presenting the impact of a well-established refugee screening and treatment program switching from skin testing to Quantiferon®-TB Gold (QFT). We completed a retrospective cohort of refugees screened for tuberculosis between November 1, 2009–April 30, 2011 (pre-QFT) and May 1, 2011–October 31, 2012 (post-QFT). Among 2244 refugees screened that met the inclusion criteria, there was a significant difference in the proportion of refugees diagnosed with LTBI between the two time periods (p = <0.0001). In multivariate analysis, refugees tested with QFT had a greater odds of treatment initiation (adjusted odds ratio 1.53; 95 % CI 1.02–2.29, p = 0.040). However, test type had no impact on treatment completion (odds ratio 0.88; 95 % CI 0.57–1.36, p = 0.560). Although we demonstrated increased efficiency in LTBI diagnosis in this group, treatment completion rates indicate other barriers to treatment that must be addressed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the United States, tuberculosis (TB) is largely a disease of the foreign-born population. In 2013, nearly 65 % of active TB cases in the US were in foreign-born persons [1]. Compared to individuals born in the US, foreign-born persons develop active TB disease at rates that mirror their countries of origin [2–4]. Further, untreated latent tuberculosis infection (LTBI) accounts for about 80 % of all new active TB cases in the United States [5–7]. Screening and potential LTBI treatment in these groups is complicated by a number of factors, including the use of the bacille Calmette Guerin (BCG) vaccine, which can interfere with using standard, inexpensive tuberculin skin tests (TST). Interferon-gamma release assays (IGRA) have been potentially transformative to screening programs focused on foreign-born persons. How much of a transformation requires on-going assessment. This paper adds to this assessment by presenting the experience of a well-established refugee screening and treatment program in Portland, OR switching from TST- to IGRA-based screening and the impact of that programmatic change.
While a relatively small fraction of all immigrants to the United States, refugees usually arrive from high TB endemicity areas. In addition to being at higher risk for TB disease than US-born persons, refugees also appear to have a higher risk of developing TB disease when compared to other foreign-born persons in the US [8, 9]. To prevent reactivation of LTBI after arrival to the United States, the Centers for Disease Control and Prevention (CDC) recommends screening and treatment for LTBI in newly arrived refugees [3, 10]. Prior to May 2011, Multnomah County’s refugee screening and LTBI treatment program relied primarily on TST testing to detect possible LTBI cases; in May 2011, we shifted to IGRA testing for refugees between 5 and 50 years of age.
While TST screening for TB screening has been used reliably for decades, it has some limitations. Cross-reactivity with BCG vaccine and non-TB mycobacteria complicates interpreting TST results for individuals from TB-endemic settings [11]. Interferon-gamma (INF-γ) release assays (IGRAs), such as the QuantiFERON-TB Gold (QFT) [12] detect MTB infection by measuring in vitro IFN-γ release following stimulation of peripheral blood lymphocytes with purified MTB-specific antigens. Thus, IGRA tests have less cross-reactivity than TST with BCG or non-TB mycobacteria [11]. While the CDC finds that TST and IGRA testing can both be used to detect MTB infection for surveillance or to identify those individuals who stand to benefit from treatment, IGRA can sometimes be the preferred option [11].
The goal of this retrospective analysis is to assess the effectiveness of identification of latent TB infection among refugees both pre and post QFT implementation, with effectiveness defined as a decrease in LTBI diagnosis. We (1) describe the demographics of refugees in Multnomah County both pre and post QFT implementation; (2) compare the difference in the proportion of refugees diagnosed with LTBI by TST and QFT; and (3) analyze the demographic factors associated with LTBI treatment initiation and completion. This information will be useful to other public health clinics that wish to assess the impact of QFT on their refugee and asylee populations, and can inform efforts to improve care for this population.
Methods
Settings and Subjects
Multnomah County Health Department (MCHD) is Oregon’s largest local public health agency and safety-net provider. As part of our local TB control strategy, Multnomah County has provided culturally and linguistically appropriate medical care for refugees with LTBI for over 20 years.
We examined a retrospective cohort to evaluate LTBI services in refugees before and after widespread implementation of QFT. We compared 18 months of data prior to introduction of routine QFT testing (pre-QFT) to 18 months after implementation (post-QFT). Initial data were obtained from the MCHD’s electronic health record data warehouse from November 1, 2009 to April 30, 2011 (pre-QFT) and May 1, 2011 to October 31, 2012 (post-QFT). This information identified TB screening findings from the initial refugee screening visit conducted through the county’s Refugee Medical Assistance Program contract. Additional information for persons in this cohort was subsequently identified from two more data sources: the CDC Electronic Disease Notification (EDN) System for information on TB Class B status and country of origin, and our local TB program database (Microsoft Access 2003) for information on TB evaluation and subsequent treatment. Information from EDN is not available when a person’s jurisdiction is not in Multnomah County; there is a transfer to another jurisdiction; or a parolee or immigrant does not have Class A or B conditions. Chart review was performed as necessary when TST or QFT results were missing or in notes fields. For specific analyses, country of origin was dichotomized into lower versus higher TB prevalence according to World Health Organization 2013 data, with lower defined as prevalence <75/100,000 (Iraq, Cuba, Iran, Saudi Arabia, Kuwait, Jordan, Egypt, Israel, and Syria) and higher defined as prevalence ≥75/100,000 (Afghanistan, Myanmar, Bhutan, Burundi, Congo, Democratic Republic of Congo, China, Central African Republic, Eritrea, Ethiopia, Gabon, India, Kenya, Malaysia, Mozambique, Nepal, Pakistan, Rwanda, Somalia, Sudan, Thailand, Tanzania, Uganda, Vietnam, or Zambia) [13].
Individuals >5 and <50 years of age with an electronic encounter for “refugee screening” were selected for inclusion into this cohort. Refugees receive their initial screening appointment within 30 days of their arrival in the United States, and undergo comprehensive health screening, including testing for TB.
Exclusion criteria included being part of a pilot project wherein refugees from former Soviet Union (FSU) countries and Romania were offered QFT before the rest of the refugee population (based on likelihood of repeated BCG in country of origin [14]); being a case or suspect case of active TB disease; or failing to return for repeat QFT testing related to a QFT test recall in October 2012.
LTBI Evaluation and Treatment
Communicable Disease Services’ TB Clinic (TBC) performs evaluations on all refugees with a positive TST ≥10 mm or IGRA, symptoms of lung TB, or a history of TB or Immigration B Waiver (evidence of inactive TB on a chest x-ray at the time of immigration). LTBI diagnosis for this analysis was defined as TB infection, not clinically active, with or without evidence of past clinical activity). Individuals diagnosed with LTBI during the course of this study period were offered standard treatment, typically 6–9 months of INH or 4–6 months of Rifampin depending on age and TB risk factors. Treatment initiation here is based on patient acceptance of treatment, not on physician prescription of LTBI medication.
Statistical Analyses
For descriptive statistics of the entire cohort, categorical data were compared using Chi square tests, while differences between continuous variables were assessed with t tests. For LTBI treatment initiation and completion, the analysis only included refugees who were eligible for treatment at the Multnomah County TB Clinic (treatment ineligibility includes age ≥50 years, current pregnancy, medical contraindication, or history of prior adequate treatment). To assess factors associated with treatment initiation, multivariate logistic regression was performed. Adjusted odds ratios (AORs) were calculated for variables associated with treatment initiation. Variables in the multivariate model were included if their univariate p value was ≤0.15. The association between birth region and both age and gender was assessed by using interaction terms in the final models, using p value of ≤0.05 as significant. Smaller sample size in the number of refugees completing treatment limited our ability to use multivariate logistic regression. For this outcome, we used bivariate analysis only. All data were analyzed using SAS 9.3 (SAS Institute, Cary, NC, USA).
Ethical Review
The work presented here is an evaluation of our testing guidelines and informs our public health practice. Review by an institutional review board was not required.
Results
Evaluation Cohort Description
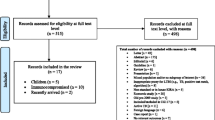
There were 2447 refugees >5 and <50 years of age screened for TB through MCHD’s Mid-County Health Center between November 1, 2009 and October 31, 2012. Two hundred and three met exclusion criteria of being from FSU countries (n = 156); being an active or suspicious case or contact to an active or suspicious case (n = 21); failing to return for repeat QFT testing due to a test kit recall (n = 24); or having a cancelled test with no re-test (n = 2). Of 2244 refugees screened with a valid TST or QFT result from their refugee screening—1215 in the pre-QFT period and 1029 in the post-QFT period—all had matching record information from outside data sources to create a full history of TB screening and LTBI treatment.
The majority of persons screened were born in sub-Saharan Africa (29 %) (Table 1); the most common birth countries individually, however, were Myanmar/Burma (n = 316), followed by Iraq (n = 299) and Somalia (n = 237) (individual country data not shown). More than two-thirds of refugees screened were between 18 and 49 years of age, with the majority reporting Asian descent (36 %). Most individuals screened had a refugee visa, although an additional 120 (7 %) had special immigrant visa, parolee with refugee benefits, or parolee status. Over the entire evaluation period 1537 persons had a negative LTBI screening test (69 %) and most did not have a Class B condition (n = 1537, 69 %).
Persons in the pre-QFT period did not differ from those in the post-QFT period on gender or presence of a Class B condition, but did differ in mean age, race/ethnicity, test results, type of visa, and region of birth (Table 1).
LTBI Diagnosis, Treatment Initiation, and Treatment Completion
There were 1215 refugees screened for LTBI in the pre-QFT period, and 1029 screened in the post-QFT period (Fig. 1), with 420/1215 (35 %) evaluated in pre-QFT and 346/1029 (34 %) evaluated in post-QFT. The proportion of clients diagnosed with LTBI was significantly different between the pre QFT period (393 diagnosed/420 evaluated, 94 %) and the post QFT period (287 diagnosed/346 evaluated, 83 %) (p < 0.0001) (Fig. 1). There were significant differences in the proportion of patients who were candidates for treatment (333/393, 85 % in pre-QFT, 215/287, 75 % in post-QFT, p = 0.001 and those who started treatment (221/333, 66 % in pre-QFT, 160/215, 74 % in post-QFT, p = 0.046). There was no significant difference in the proportion of refugees completing treatment between periods (154/221, 70 % in pre-QFT, 107/160, 67 % in post-QFT, p = 0.560). Reasons for treatment non-completion were similar in the pre/post QFT periods; loss to follow up (67 overall, 18 % of all refugees initiating treatment) and patient stopping treatment (31, 8 %) were the most common explanations.
Flow of patients from refugee screening into Multnomah County TB Clinic, November 2009–October 2012 (n = 2244). aSignificant difference in proportion of patients diagnosed with LTBI between study periods (p < 0.0001). bSignificant difference in proportion of patients who were candidates for treatment (p = 0.001) between study periods. cSignificant difference in proportions of patients who were started LTBI treatment (p = 0.046) between study periods. dNo significant difference in proportions of patients who completed LTBI treatment (p = 0.560) between study periods. TBC TB Clinic, LTBI latent tuberculosis infection
When country of origin was dichotomized into higher versus lower TB prevalence, there was a significant association with diagnosis of LTBI (38.5 vs. 18.5 %, p < 0.0001). When stratified by pre- versus post-QFT time period, however, no association was seen (higher prevalence countries: p = 0.336; lower prevalence countries: p = 0.909) (data not shown).
There were 381 refugees who initiated treatment for LTBI during the study period out of 548 eligible for treatment (70 %) (Fig. 1; Table 2). After controlling for age and immigration class, refugees who were tested with QFT were significantly more likely to initiate treatment than those tested before implementation of QFT (AOR 1.53; 1.02–2.29; p = 0.040). Further, refugees from East Asia and Pacific birth countries were significantly more likely to initiate treatment (AOR 1.86; 1.11–3.12; p = 0.018) than refugees from sub-Saharan African birth countries. Refugees from Middle East and North African birth countries were significantly less likely to initiate treatment (AOR 0.39; 0.21–0.73; p = 0.003) than refugees from sub-Saharan African birth countries. Interaction terms for region of birth by age and sex were not statistically significant.
There were 381 refugees who initiated treatment for LTBI during the evaluation period, with 261 completing treatment (68 %) (Fig. 1; Table 3). In bivariate analysis, medication type was a significant predictor of LTBI treatment completion (OR for Rifampin vs. INH 2.57; 1.21–5.45; p = 0.014). Age group was not found to be a significant predictor of treatment completion; (OR for 18–49 year olds, 0.63; 0.36–1.08; p = 0.094). Refugees from South Asian birth countries had a higher odds of treatment completion than refugees from sub-Saharan African birth countries (OR 2.32; 1.19–4.51; p = 0.013).
To test whether the decrease in diagnosis of LTBI was explained by the decrease in patients from high prevalence countries, we performed a stratified analysis. Among refugees from high prevalence countries, there continued to be a decrease in the proportion of positive tests from the pre and post-QFT period (38 % positive in pre-QFT period vs. 33 % in post-QFT period, p = 0.028). A decrease in the proportion of positive tests also remained among refugees from lower prevalence birth countries (18 % in pre-QFT vs. 11 % in post-QFT, p = 0.04).
Discussion
Programs to screen foreign-born individuals for LTBI are an integral part of TB control in the United States. Multnomah County’s refugee screening program tests this high-risk group for LTBI and offers culturally competent patient education and treatment. Continuing efforts to test and diagnose newly-arrived refugees for LTBI is imperative, especially since the highest rate of TB disease occurs in persons who have resided in the US for 1 year or less [4]. This evaluation found that implementation of routine Quantiferon testing had a significant programmatic impact on diagnosis of LTBI in Multnomah County, with concomitant reductions in LTBI diagnosis and treatment.
We found that the proportion of refugees starting treatment for LTBI was significantly different between the time periods examined in this study. However, the proportion completing treatment was not significantly different. This finding may imply that the type of test chosen influenced patient behavior in terms of treatment initiation, but not enough to influence treatment completion. A study of QFT impact on a public health clinic in another urban setting found that test type did not affect treatment completion rates [15]. Overall, the LTBI treatment initiation rates reported in this evaluation are higher than in other studies in Minnesota (49 %) and Canada (49 %), lower than Baltimore (91 %) and similar to Australia (77 %) and San Diego (76 %) [3, 9, 16–18]. Similarly, treatment completion rates can vary widely, with studies of treatment completion in foreign-born persons ranging from 22 to 90 % depending on country of origin [19]. Treatment initiation and completion are complex issues, especially among refugees, who often arrive with low health literacy. These difficulties in health education, along with language and/or cultural issues, have been shown to affect adherence to INH treatment for LTBI [19, 20]. However, results from the current evaluation demonstrate some local success, with South Asians showing a treatment completion rate double that of sub-Saharan Africans. However, the finding that 18 % of all refugees eligible for treatment were lost to follow-up indicates that the mobility of refugees coming into Multnomah County is quite high; efforts to improve follow-up among this group could improve future analyses of treatment completion in our area.
A smaller proportion of refugees were diagnosed with LTBI in the post-QFT period compared to the pre-QFT period (Fig. 1). This finding may represent increased efficiency in identifying true cases. A stratified analysis found that this decrease in LTBI diagnosis persisted both among refugees from high or low prevalence countries, suggesting that differences in place of birth during the pre and post-QFT periods do not account for the findings.
In multivariate analysis, we found that birth country was a significant predictor of treatment initiation; refugees from East Asia and Pacific countries were almost twice as likely to initiate treatment as refugees from sub-Saharan African counties. Given the recent finding that the TB case rate among Africans living in the United States is three times higher than other foreign born persons [21], this gap in treatment initiation represents an important upstream prevention opportunity. We also found that compared to sub-Saharan Africans, persons of Middle East and North African descent were significantly less likely to initiate treatment. Further research into cultural and other barriers (e.g., communication, access to our downtown TBC) is warranted.
Treatment regimen was a significant predictor of treatment completion among refugees diagnosed with LTBI, with those taking Rifampin more likely to complete treatment than those taking INH. This finding differs from that of Nuzzo et al. [18] who saw no association between treatment completion and type of treatment regimen, but is similar to other authors, who showed a higher completion rate for LTBI treatment for individuals taking rifamycin versus INH [22–25]. However, there was a national INH shortage during the post-QFT period [26]. Refugees who had already begun treatment with INH were switched to Rifampin if clinically indicated. It is possible that the switch in medication type made it more likely that these patients would complete treatment. Given that there was no association in the pre-QFT period between treatment completion and therapy type, however, this is likely not an issue in the current analysis (p = 1.0) (data not shown).
In Baltimore, as in the current evaluation, implementation of QFT for LTBI evaluation in a public health clinic significantly reduced the proportion of individuals who were referred and for whom LTBI was diagnosed [15]. However, during the post-QFT time period of our study, there was a voluntary market withdrawal of specific lots of antigen tubes for QFT due to potential false-positive tests. Re-testing of these refugees revealed 33 potential false positive results. Although only the valid test result was used in the current analysis, it is possible that the QFT recall might have affected our finding of a significant difference in LTBI diagnosis, since more clients were referred to TBC than would have been otherwise. However, even when refugees with a negative repeat test were excluded from analysis, the proportion diagnosed with LTBI was still lower in the post-QFT period (243/297, 82 %, p < 0.0001 (data not shown).
This study has several limitations. Our analysis was limited to demographic variables associated with treatment initiation and completion. Other studies have shown the importance of other factors, such as co-morbid health conditions or socio-economic status, in successful treatment completion [3, 27] as well as clinical risk factors increasing risk of progression to active TB [3]. Refugees from the countries of the FSU and Romania were excluded from the current evaluation because they were offered QFT beginning in 2007. This programmatic decision was made based on their likelihood of receiving multiple series of BCG. Although they do not account for a large proportion of refugees coming into Oregon (around 13 % in fiscal year 2012, and 7 % in FY 2013), they have historically had lower treatment initiation at MCHD than other refugee groups (personal communication, Dr. Jennifer Vines, July 2014). Additional work needs to be done to increase the proportion of these refugees who initiate treatment.
Strengths of this study include the use of multiple data systems to track refugees from their arrival in Multnomah County through their treatment status in the Communicable Disease Services TBC, as well as the reporting of factors associated with treatment completion, which may be of use to other large public TB prevention program. In addition, the challenges faced during the time period of this analysis (e.g., QFT recall, INH shortage) reflect real-world problems that occur in TB prevention programs around the United States.
Overall, we found that a lower proportion of incoming refugees were diagnosed with LTBI during the post-QFT period than when TST was the only TB screening test utilized, indicating increased efficiency. However, treatment completion did not vary, implying that refugees must have other barriers to treatment that need to be further studied. One possible method to increase treatment initiation and completion is to offer a 12 week combination treatment of INH and rifapentine, directly observed [28]. The shorter treatment time may help address barriers to treatment initiation and completion in our refugee population. Additionally, the direct observation appointments may provide an opportunity to provide more culturally competent outreach and education regarding TB infection [3].
References
Alami NN, Yuen CM, Miramontes R, Pratt R, Price SF, Navin TR, et al. Trends in tuberculosis—United States, 2013. MMWR Morb Mortal Wkly Rep. 2014;63(11):229–33.
American Thoracic Society. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med. 2000;161(4 Pt 2):S221–47.
Bennett RJ, Brodine S, Waalen J, Moser K, Rodwell TC. Prevalence and treatment of latent tuberculosis infection among newly arrived refugees in San Diego County, January 2010–October 2012. Am J Public Health. 2014;104(4):e95–102.
Cain KP, Haley CA, Armstrong LR, Garman KN, Wells CD, Iademarco MF, et al. Tuberculosis among foreign-born persons in the United States: achieving tuberculosis elimination. Am J Respir Crit Care Med. 2007;175(1):75–9.
Horsburgh CR, Rubin EJ. Clinical practice. Latent tuberculosis infection in the United States. N Engl J Med. 2011;364(15):1441–8.
Geng E, Kreiswirth B, Driver C, Li J, Burzynski J, DellaLatta P, et al. Changes in the transmission of tuberculosis in New York City from 1990 to 1999. N Engl J Med. 2002;346(19):1453–8.
Ricks PM, Cain KP, Oeltmann JE, Kammerer JS, Moonan PK. Estimating the burden of tuberculosis among foreign-born persons acquired prior to entering the US, 2005–2009. PLoS One. 2011;6(11):e27405.
Hadzibegovic DS, Maloney SA, Cookson ST, Oladele A. Determining TB rates and TB case burden for refugees. Int J Tuberc Lung Dis. 2005;9(4):409–14.
Varkey P, Jerath AU, Bagniewski SM, Lesnick TG. The epidemiology of tuberculosis among primary refugee arrivals in Minnesota between 1997 and 2001. J Travel Med. 2007;14(1):1–8.
Taylor Z, Nolan CM, Blumberg HM, American Thoracic Society, Centers for Disease Control and Prevention, et al. Controlling tuberculosis in the United States Recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. MMWR Recomm Rep Morb Mortal Wkly Rep Recomm Rep Cent Dis Control. 2005;54(RR-12):1–81.
Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recomm Rep Morb Mortal Wkly Rep Recomm Rep Cent Dis Control. 2010;59(Rr-5):1–25.
Cellestis. Quantiferon-TB Gold (in-tube method) package insert. 2006. http://www.cellestis.com/IRM/Company/ShowPage.aspx?CPID=1171. Accessed 01 Dec 2014.
World Health Organization. Global tuberculosis report 2013. Geneva. 2013. http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf?ua=1. Accessed 28 Aug 2014.
Drobniewski F, Balabanova Y, Zakamova E, Nikolayevskyy V, Fedorin I. Rates of latent tuberculosis in health care staff in Russia. PLoS Med. 2007;4(2):e55.
Shah M, DiPietro D, Greenbaum A, Ketemepi S, Martins-Evora M, Marsiglia V, et al. Programmatic impact of QuantiFERON-TB gold in-tube implementation on latent tuberculosis diagnosis and treatment in a public health clinic. PLoS One. 2012;7(5):e36551.
Levesque JF, Dongier P, Brassard P, Allard R. Acceptance of screening and completion of treatment for latent tuberculosis infection among refugee claimants in Canada. Int J Tuberc Lung Dis. 2004;8(6):711–7.
Trauer JM, Krause VL. Assessment and management of latent tuberculosis infection in a refugee population in the Northern Territory. Med J Aust. 2011;194(11):579–82.
Nuzzo JB, Golub JE, Chaulk P, Shah M. Analysis of latent tuberculosis infection treatment adherence among refugees and other patient groups referred to the Baltimore City Health Department TB Clinic, February 2009–March 2011. J Immigr Minor Health. 2015;17(1):56–65.
Hirsch-Moverman Y, Daftary A, Franks J, Colson PW. Adherence to treatment for latent tuberculosis infection: systematic review of studies in the US and Canada. Int J Tuberc Lung Dis. 2008;12(11):1235–54.
Butcher K, Biggs BA, Leder K, Lemoh C, O’Brien D, Marshall C. Understanding of latent tuberculosis, its treatment and treatment side effects in immigrant and refugee patients. BMC Res Notes. 2013;6:342.
Abraham BK, Winston CA, Magee E, Miramontes R. Tuberculosis among Africans living in the United States, 2000–2009. J Immigr Minor Health. 2013;15(2):381–9.
Lardizabal A, Passannante M, Kojakali F, Hayden C, Reichman LB. Enhancement of treatment completion for latent tuberculosis infection with 4 months of rifampin. Chest. 2006;130(6):1712–7.
Li J, Munsiff SS, Tarantino T, Dorsinville M. Adherence to treatment of latent tuberculosis infection in a clinical population in New York City. Int J Infect Dis. 2010;14(4):e292–7.
Menzies D, Dion MJ, Rabinovitch B, Mannix S, Brassard P, Schwartzman K. Treatment completion and costs of a randomized trial of rifampin for 4 months versus isoniazid for 9 months. Am J Respir Crit Care Med. 2004;170(4):445–9.
Page KR, Sifakis F, Montes de Oca R, Cronin WA, Doherty MC, Federline L, et al. Improved adherence and less toxicity with rifampin vs isoniazid for treatment of latent tuberculosis: a retrospective study. Arch Intern Med. 2006;166(17):1863–70.
Centers for Disease Control, Prevention. Impact of a shortage of first-line antituberculosis medication on tuberculosis control—United States, 2012–2013. MMWR Morb Mortal Wkly Rep. 2013;62(20):398–400.
Trajman A, Long R, Zylberberg D, Dion MJ, Al-Otaibi B, Menzies D. Factors associated with treatment adherence in a randomised trial of latent tuberculosis infection treatment. Int J Tuberc Lung Dis. 2010;14(5):551–9.
Centers for Disease and Prevention. Recommendations for use of an isoniazid-rifapentine regimen with direct observation to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep. 2011;60(48):1650–3.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Walters, J.K., Sullivan, A.D. Impact of Routine Quantiferon Testing on Latent Tuberculosis Diagnosis and Treatment in Refugees in Multnomah County, Oregon, November 2009–October 2012. J Immigrant Minority Health 18, 292–300 (2016). https://doi.org/10.1007/s10903-015-0187-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10903-015-0187-z