Abstract
Trinidad and Tobago (TT) is the country with the highest breast cancer mortality in the Caribbean. It is unknown whether biological, behavioral, environmental, or clinical factors play a significant role in such outcome. A total of 2,614 incident cases, histologically confirmed and recorded in the TT cancer registries between 1995 and 2005, with follow-up through 2009 were analyzed. Half of the cases were diagnosed between the ages of 40–59 years, 12.5 % before the age of 40 years; 45 % of women were diagnosed at localized stage and 43.7 % were hormone receptor positive. Women diagnosed with distant staging were more likely to undergo chemotherapy compared to those with localized staging (OR 1.39; 95 % CI 1.01–1.89). Hormone receptor negative cases were significantly less likely to undergo radiation or surgery therapy (OR 0.66; 95 % CI 0.56–0.79 and OR 0.67; 95 % CI 0.51–0.88 respectively) compared to those who were hormone receptor positive, but more than 1.5 times as likely to undergo chemotherapy. In multivariate analyses, advanced stage disease and negative hormone receptor status were independently significantly associated with poorer survival outcome. No racial/ethnic differences were observed with respect to treatment or survival. Although access to breast cancer screening and treatment is free in Trinidad and Tobago, breast cancer diagnosis occurs at advanced stages; use of multimodality therapy as a first course of treatment is low.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common cancer among women, with significant differences in the incidence observed between developed and developing countries. In 2008, a World Health Organization (WHO) International Agency on Cancer review of breast cancer reported the age-standardized mortality rate in the Caribbean was 39.1 per 100,000 women, which was similar to the worldwide rate, and significantly less than the rate in the United States (US) (76.7 per 100,000 women) [1]. Although the overall incidence of cancer is lower in the Caribbean, the mortality burden is greater, mainly due to presentation at more advanced stages, which is partly related to decreased access to cancer care [2]. However, the relative contribution of socio-economic factors versus biological characteristics to the high mortality rates observed in Latin America and the Caribbean is difficult to quantify because of a paucity of epidemiologic studies, when compared to the US and Europe.
With a population of approximately 1.3 million, of which nearly 630,000 are women, Trinidad and Tobago (TT) is the second largest English-speaking country in the Caribbean. The 2000 population census described the ethnic composition of the population as being equally split between subjects of African descent and of Indian sub-continent descent, with an additional 20 % of mixed ancestry which included White, Asian, and others [3]. A 2002 Pan American Health Organization (PAHO) report documented that the age-standardized breast cancer mortality rate for women aged 25–74 years in TT was the highest among the Caribbean countries [4]. Between 2000 and 2002, The Cancer Registry of TT estimated that breast cancer was the most common site for new cancer among women, and a 2010 study indicated that age-standardized mortality rates for breast cancer in TT have been steadily increasing over the past 35 years (from 14.9 per 100,000 women in 1970 to 24.4 per 100,000 women in 2004) [5, 6].
To address this major public health challenge, governmental efforts, such as the National Oncology Programme (NOP) and non-profit organizations, such as the Trinidad and Tobago Cancer Society (TTCS), have developed initiatives to increase screening for early diagnosis, awareness, education, and treatment [7]. Within TT, all citizens are entitled to cancer care and treatment free of charge; however a doctor must refer patients before they can access treatment. This procedure may somehow contribute to delays in screening and early treatment. A recent study of women who underwent breast cancer screening at TTCS found that screening in TT is still more of a diagnostic tool rather than preventive measure [8, 9]. A 2010 study showed that women who reside in TT were more likely to be diagnosed with breast cancer at a later stage and had poorer survival outcomes from the disease compared to Caribbean women living in the US [10]. The scanty information makes it difficult to understand what factors (biological, behavioral, environmental, or clinical) play a significant role in breast cancer outcomes in women of Afro Caribbean descent.
The present study was designed with several purposes: (1) to describe the distribution of socio-demographic factors, disease characteristics, and treatment outcomes among newly diagnosed breast cancer cases, (2) to identify factors predicting the likelihood of receiving individual and various combinations of therapy modalities and (3) to assess the impact of personal factors and treatment outcomes on overall survival.
Methods
Study Setting and Participants
The database included data extracted from the TT cancer registry which included 3,097 patients. All cases were incident, newly diagnosed cancers recorded in the cancer registries between 1995 and 2005, with follow-up through 2009. Cases that were entered into the registry at the time of death and/or through autopsy (prevalent cases) were excluded from analyses (n = 483), leaving 2,614 histologically confirmed patients. Each patient self-reported their race/ethnicity, place of birth, and marital status. The CUNY University Integrated Internal Review Board approved the study; a waiver to obtain informed consent was approved for this research, and HIPAA authorizations were waived for all subjects.
Measures
The TT cancer registry classified staging as “local/regional” for stages I–III and “distant” disease for stage IV cases. The dataset indicated whether or not patients received hormone therapy as part of their treatment, but hormone receptor testing results were unavailable. For the purpose of this analysis, we used this as a surrogate for either Estrogen or Progesterone Receptor status, which has been previously validated as a proxy for estrogen receptor status among breast cancer patients [11]. HER2 testing was not performed or available. Histology was classified according to WHO ICD.0-3 criteria. The following histological categories were created based on the WHO ICD.0-3 criteria:
(1) ductal (ICD: 85003, 85033, 85413, 85433, 85213), (2) lobular (ICD: 85203), (3) ductal and lobular (ICD: 85223, 85233, 85243), (4) adenocarcinoma (ICD: 81403, 81413, 82003, 82113, 82603), (5) medullary (ICD: 85103, 85133), (6) mucinous (ICD: 84803, 84813), (7) carcinoma not otherwise specified (NOS), (ICD: 80103, 80003, 80203, 85753) and (8) other. With respect to treatment, the initial course of therapy given within the first six months after diagnosis was used to characterize treatment. Given the percentage of women receiving multiple therapies (63.6 % received two or more); the type of therapy was considered as well as various combinations of therapies.
Statistical Analyses
Variables analyzed include age at diagnosis, ethnicity, place of birth, marital status, hormone receptor status, stage at diagnosis and type of therapy (radiation, surgery, or chemotherapy). For descriptive analyses, categorical variables are presented as frequencies and proportions with continuous variables presented as means and standard deviations. A series of univariate and multivariate logistic regression models were conducted to assess the relationship between demographic and clinical characteristics and the likelihood of receiving chemotherapy, surgery, or radiation.
To assess differences in overall survival across treatment outcomes, Kaplan–Meier survival curves were generated for cancer mortality with a log-rank test performed. A multivariable Cox proportional hazards model was conducted to assess the effects of treatment outcomes on five year survival, adjusted for demographic and clinical characteristics. For cases that were alive, follow-up time in months was calculated as the difference between the date of initial diagnosis and the date of last contact. For cases that were dead, date of expiration was used in place of date of last contact. All statistical tests conducted were two-sided and a p value of <0.05 was considered statistically significant; analyses were performed in Stata SE 12 (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP).
Results
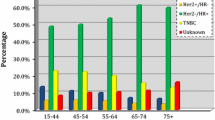
Demographic and clinical characteristics (Table 1) show that half of the cases were diagnosed between the ages of 40–59 years, with 12.5 % having been diagnosed before the age of 40 years; 45 % of women self-reported their race as African, while one-third identified themselves as Asian or Indian. More than 80 % of women were born within Trinidad and Tobago and slightly more than half of participants were married or cohabiting. Clinically, 45 % of women were diagnosed at localized stage and 43.7 % were hormone receptor positive. With respect to treatment, 95 % of women received some type of therapy, with 23 % receiving just surgery, 40 % receiving a combination of two therapies, and nearly a quarter receiving all therapies (surgery, radiotherapy, and chemotherapy).
Age at diagnosis had a significant impact on likelihood of receiving chemotherapy, but not radiation (Table 2). In adjusted models, women diagnosed later on in life were significantly less likely to undergo chemotherapy, compared to those diagnosed between 18-39 years. With respect to surgery, only women diagnosed at age 70 or older were less likely to undergo a surgical treatment (OR 0.35; 95 % CI 0.23–0.55). No significant racial/ethnic differences were observed with the exception of women who failed to self-report their race/ethnicity. In adjusted models, there were no significant differences in likelihood of receiving treatment between women born in or outside of Trinidad and Tobago. Women diagnosed with distant staging were significantly more likely to undergo chemotherapy but less likely to undergo surgery compared to those with localized/regional staging (OR 1.39; 95 % CI 1.01–1.89 and OR 0.15; 95 % CI 0.11–0.20 respectively).
Women who were hormone receptor negative were significantly less likely to undergo radiation or surgery therapy (OR 0.66; 95 % CI 0.56–0.79 and OR 0.67; 95 % CI 0.51–0.88 respectively) compared to those who were hormone receptor positive, but more than 1.5 times as likely to undergo chemotherapy. With respect to surgery, women with adenocarcinoma or carcinoma NOS were significantly less likely to undergo surgery compared to those with ductal histology; those with carcinoma NOS were also significantly less likely to receive radiation compared to women with ductal breast cancer. Women with histology in the other category (which included mostly cribriform and phyllodes types) were significantly less likely to undergo radiation compared to those with ductal carcinoma.
At multivariate analyses, advanced stage disease and negative hormone receptor status were independently significantly associated with poorer survival outcome (Table 3). No statistically significant differences with respect to age, race/ethnicity, place of birth, or marital status were observed. With respect to clinical characteristics, compared to ductal, carcinoma NOS was associated with prolonged survival. With respect to therapy, undergoing surgery alone (adjusted hazard ratio: 0.44; 95 % CI 0.32–0.60), receiving radiotherapy or chemotherapy (adjusted hazard ratio: 0.70; 95 % CI 0.50–0.99), having a combination of two therapies (adjusted hazard ratio: 0.47; 95 % CI 0.36–0.63), or receiving all therapies (adjusted hazard ratio: 0.41; 95 % CI 0.30–0.56) was associated with prolonged survival compared to those who received no treatment.
Discussion
This study, conducted using the cancer registry in Trinidad and Tobago, provided several important findings. Although the women with breast cancer within this registry belong to a variety of racial/ethnic backgrounds, no racial/ethnic differences were observed with respect to treatment or survival. Within the US, several studies have shown that despite lower incidence rates, mortality rates due to breast cancer are higher among blacks than whites [12, 13]. To explain these differences in survival, studies exploring differences in breast cancer screening and treatment have examined the effects of a range of sociodemographic and clinical factors [14–17]. Several studies have shown that black women are more likely than white women to have late-stage breast cancer at diagnosis [16–20]. Although there are contradictory findings in the literature [21–24], there are a number of studies that report racial/ethnic differences in breast cancer treatment, with non-Hispanic black women less likely to receive treatment or more likely to experience delays in treatment after diagnosis [12, 25–32]. One of the characteristics of TT is the universal health system, therefore breast cancer screening and treatment in these islands is free for citizens; this study showed that when access to care is equal, racial differences in outcomes attenuate.
In prior studies, differences in outcomes have also been attributed to a higher likelihood of unfavorable disease characteristics, such as hormone receptor-negative status and high-grade histology, which have impacted treatment [33–38]. Many of the breast cancers cases within the TT registry are aggressive, which may contribute to poor survival outcomes; 13 percent of women were diagnosed before the age of 40 years, nearly 50 percent of cancer cases are of regional or distant staging, and 54 percent are hormone receptor negative. Within our analyses, this study confirmed that both advanced staging and negative hormone receptor status were associated with poorer survival, regardless of treatment modality.
With respect to treatment, the study confirmed that women who had the indication and were treated with multimodality therapy are those with the best survival. Within this population, the prevalence of multimodality therapy is lower than in the US, particularly the use of radiation therapy following surgery, likely because the disease is diagnosed at advanced stages when the indication is for endocrine and/or chemotherapy [10]. A small study of Caribbean breast cancer patients who had received treatment in their home nations found that only 18 % of patients had received radiotherapy after undergoing a surgical resection of the tumor, and among those who did not receive radiation, the majority denied having been offered it [39]. Limited data exist regarding determinants of therapy from the physician perspective, but a 2006 study of breast cancer experts from 12 Latin American countries found that most Caribbean oncologists considered local radiation therapy facilities inadequate [40]. A follow-up study reported that greater than 90 % of countries had no national law or guideline for mammography screening [41]. As such, it is possible that differences in treatment, follow-up care, or both play a role in overall survival, together with the late stage of disease diagnosis. A new finding in this analysis is that women age 70 years or older are less likely to undergo treatment; the reasons for this result are not known, but could possibly be attributed to the presence of co-morbidities that prevent a full scale treatment, excessive collateral effects from therapy, or lack of access to proper care among the elderly. Upon examining differences in clinical characteristics by age, women age 70 years or older were significantly more likely to have distant staging (11.6 vs. 8.6 %; p < 0.0001) but less likely to be hormone receptor negative (45 vs. 57.5 %; p < 0.0001) compared to women younger than 70 years of age. It is possible that in older ages we observe a more indolent tumor (more likely to be hormone receptor positive) that gets diagnosed at a later stage.
Despite the fact that this study gives a comprehensive picture of breast cancer treatment and outcome in TT, it has some limitations: the data set lacks information on environmental, behavioral, and dietary factors which may be responsible for a more aggressive form of breast cancer. For example, prior studies have reported the extensive use of pesticides and other estrogen disruptors in Caribbean countries [42]. Due to the retrospective nature of the study, there are missing and/or incomplete information for some of the patients; such data cannot be retrieved or recovered because the data set is anonymous. There are several strengths to the study including the diversity of the population, its large sample size, and the inclusion of various breast cancer clinical characteristics, such as histology, hormone receptor status, and stage.
Conclusions
In conclusion, although access to breast cancer screening and treatment is free in Trinidad and Tobago, screening is still more diagnostic than preventive; use of multimodality therapy is low, perhaps because of the advanced stage of disease at diagnosis. Governmental agencies and community-based organizations should consider implementing policies and educational initiatives to increase screening, particularly among high risk-groups. Future research should also examine the role of physician and institutional level factors in the access, timing, and quality of breast cancer care among women screened within this population.
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–917.
Goss PE, Lee BL, Badovinac-Crnjevic T, Strasser-Weippl K, Chavarri-Guerra Y, StLouis J, Villarreal-Garza C, Unger-Saldaña K, Ferreyra M, Debiasi M, Liedke PE, Touya D, Werutsky G, Higgins M, Fan L, Vasconcelos C, Cazap E, Vallejos C, Mohar A, Knaul F, Arreola H, Batura R, Luciani S, Sullivan R, Finkelstein D, Simon S, Barrios C, Kightlinger R, Gelrud A, Bychkovsky V, Lopes G, Stefani S, Blaya M, Souza FH, Santos FS, Kaemmerer A, deAzambuja E, Zorilla AF, Murillo R, Jeronimo J, Tsu V, Carvalho A, Gil CF, Sternberg C, Dueñas-Gonzalez A, Sgroi D, Cuello M, Fresco R, Reis RM, Masera G, Gabús R, Ribeiro R, Knust R, Ismael G, Rosenblatt E, Roth B, Villa L, Solares AL, Leon MX, Torres-Vigil I, Covarrubias-Gomez A, Hernández A, Bertolino M, Schwartsmann G, Santillana S, Esteva F, Fein L, Mano M, Gomez H, Hurlbert M, Durstine A, Azenha G: Planning cancer control in Latin America and the Caribbean. Lancet Oncol 2013; 14(5):391–436.
Central_Statisitical_Office. Mid year population estimates 2000–2010, based on 2000 census. CSO Population Division. 2000. http://www.cso.gov.tt/statistics/statistics/-in-statistics/statistics/population-statistics. Accessed 4 Apr 2013.
Robles SC, Galanis E. Breast cancer in Latin America and the Caribbean. Rev Panam Salud Publica. 2002;11(3):178–85.
Trinidad & Tobago Cancer Registry. Trinidad and Tobago Cancer Registry: annual statistical report, 2000–2002, 2004. http://www.health.gov.tt/sitepages/default.aspx?id=122. Accessed 16 Jan 2013.
Naraynsingh V, Hariharan S, Dan D, Bhola S, Nagee K. Trends in breast cancer mortality in Trinidad and Tobago-a 35-year study. Cancer Epidemiol. 2010;34(1):20–3.
Trinidad and Tobago Ministry of Health Cancer care overview. 2012. http://www.health.gov.tt/sitepages/default.aspx?id=135. Accessed 1 Feb 2013.
Joseph MD, Thorpe L, Annadsingh C, Laquis G, Young JC, Kwasniewski J, Lee R, Taioli E. Breast cancer diagnosis from screening in Trinidad and Tobago: Opportunities for cancer prevention. J Immigr Minor Health Jan 2013.
McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns s markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1159–69.
Taioli E, Attong-Rogers A, Layne P, Roach V, Ragin C. Breast cancer survival in women of African descent living in the US and in the Caribbean: effect of place of birth. Breast Cancer Res Treat. 2010;122(2):515–20.
Srasuebkul P, Dobbins TA, Elements of Cancer Care (EoCC) Investigators, Pearson SA. Validation of a proxy for estrogen receptor status in breast cancer patients using dispensing data. Asia Pac J Clin Oncol. 2012;. doi:10.1111/ajco.12015.
Bassett MT, Krieger N. Social class and black-white differences in breast cancer survival. Am J Public Health. 1986;76:1400–3.
Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Arch Intern Med. 2003;163:49–56.
McCarthy EP, Burns RB, Coughlin SS, Freund KM, Rice J, Marwill SL, Ash A, Schwartz M, Moskowitz MA. Mammography use helps to explain differences in breast cancer stage at diagnosis between older black and white women. Ann Intern Med. 1998;128(9):729–36.
Li CI. Racial and ethnic disparities in breast cancer stage, treatment, and survival in the United States. Ethn Dis. 2005;15:S5–9.
Centers for Disease Control and Prevention. Vital signs: racial disparities in breast cancer severity—United States, 2005–2009. MMWR Morb Mortal Wkly Rep. 2012;61(45):922–6.
Lantz PM, Mujahid M, Schwartz K, Janz NK, Fagerlin A, Salem B, Liu L, Deapen D, Katz SJ. The influence of race, ethnicity, and individual socioeconomic factors on breast cancer stage at diagnosis. Am J Public Health. 2006;96:2173–8.
Hunter CP, Redmond CK, Chen VW, Austin DF, Greenberg RS, Correa P, Muss HB, Forman MR, Wesley MN, Blacklow RS, et al. Breast cancer: factors associated with stage at diagnosis in black and white women. Black/White Cancer Survival Study Group. J Natl Cancer Inst. 1993;85(14):1129–37.
Eley JW, Hill HA, Chen VW, Austin DF, Wesley MN, Muss HB, Greenberg RS, Coates RJ, Correa P, Redmond CK, et al. Racial differences in survival from breast cancer. Results of the National Cancer Institute Black/White Cancer Survival Study. JAMA. 1994;272:947–54.
Warner ET, Gomez SL. Impact of neighborhood racial composition and metropolitan residential segregation on disparities in breast cancer stage at diagnosis and survival between black and white women in California. J Community Health. 2010;35(4):398–408.
Griggs JJ, Hawley ST, Graff JJ, Hamilton AS, Jagsi R, Janz NK, Mujahid MS, Friese CR, Salem B, Abrahamse PH, Katz SJ. Factors associated with receipt of breast cancer adjuvant chemotherapy in a diverse population-based sample. J Clin Oncol. 2012;30(25):3058–64.
Nelson C, Bai H, Neboori H, Takita C, Motwani S, Wright JL, Hobeika G, Haffty BG, Jones T, Goyal S, Moran MS. Multi-institutional experience of ductal carcinoma in situ in black vs white patients treated with breast-conserving surgery and whole breast radiation therapy. Int J Radiat Oncol Biol Phys. 2012;84(3):e279–83.
Wu X, Lund MJ, Kimmick GG, Richardson LC, Sabatino SA, Chen VW, Fleming ST, Morris CR, Huang B, Trentham-Dietz A, Lipscomb J. Influence of race, socioeconomic status, insurance, and hospital type on receipt of guideline concordant adjuvant systemic therapy for locoregional breast cancers. J Clin Oncol. 2012;30:142–50.
Lipscomb J, Gillespie TW, Goodman M, Richardson LC, Pollack LA, Ryerson AB, Ward KC. Black-white differences in receipt and completion of adjuvant therapy among breast cancer patients in a rural region of the US. Breast Cancer Res Treat. 2012;133(1):285–96.
Pierce L, Fowble B, Solin LJ, Schultz DJ, Rosser C, Goodman RL. Conservative surgery and radiation therapy in black women with early stage breast cancer. Cancer. 1992;69:893–904.
Bickell NA, Wang JJ, Oluwole S, Schrag D, Godfrey H, Hiotis K, Mendez J, Guth AA. Missed opportunities: racial disparities in adjuvant breast cancer treatment. Cancer. 2006;24:1357–62.
Markossian TW, Hines RB. Disparities in late stage diagnosis, treatment, and breast cancer-related death by race, age, and rural residence among women in Georgia. Women Health. 2012;52(4):317–35.
Sail K, Franzini L, Lairson D, Du X. Differences in treatment and survival among African-American and Caucasian women with early stage operable breast cancer. Ethn Health. 2012;17(3):309–23.
Royak-Schaler R, Pelser C, Langenberg P, Hayes J, Gardner L, Nesbitt K, Citron W, Drogula CL, Dwyer D. Characteristics associated with the initiation of radiation therapy after breast-conserving surgery among African American and white women diagnosed with early-stage breast cancer in Maryland, 2000–2006. Ann Epidemiol. 2012;22(1):28–36.
Nurgalieva ZZ, Franzini L, Morgan RO, Vernon SW, Liu CC, Du XL. Impact of timing of adjuvant chemotherapy initiation and completion after surgery on racial disparities among women with breast cancer. Med Oncol 2013; 30(1):419.
Parise CA, Bauer KR, Caggiano V. Disparities in the receipt of adjuvant radiation therapy after breast-conserving surgery among the cancer-reporting regions of California. Cancer. 2012;118(9):2516–24.
Balasubramanian BA, Demissie K, Crabtree BF, Strickland PA, Pawlish K, Rhoads GG. Black Medicaid beneficiaries experience breast cancer treatment delays more frequently than whites. Ethn Dis. 2012;22(3):288–94.
Ma H, Lu Y, Malone KE, Marchbanks PA, Deapen DM, Spirtas R, Burkman RT, Strom BL, McDonald JA, Folger SG, Simon MS, Sullivan-Halley J, Press MF, Bernstein L. Mortality risk of black women and white women with invasive breast cancer by hormone receptors, HER2, and p53 status. BMC Cancer. 2013;13:225.
Henson DE, Chu KC, Levine PH. Histologic grade, stage, and survival in breast cancer carcinoma: comparison of African American and Caucasian women. Cancer. 2003;98:908–17.
Mohla S, Sampson CC, Khan R, et al. Estrogen and progesterone receptors in breast cancer in black Americans: correlation of receptor data with tumor differentiation. Cancer. 1982;50:552–9.
Miller BA, Hankey BF, Thomas TL. Impact of sociodemographic factors, hormone receptor status, and tumor grade on ethnic differences in tumor stage and size for breast cancer in US women. Am J Epidemiol. 2002;155:534–45.
Wright JL, Reis IM, Zhao W, Panoff JE, Takita C, Sujoy V, Gomez CR, Jorda M, Franceschi D, Hurley J. Racial disparity in estrogen receptor positive breast cancer patients receiving trimodality therapy. Breast. 2012;21(3):276–83.
Chagpar AB, Crutcher CR, Cornwell LB, McMasters KM. Primary tumor size, not race, determines outcomes in women with hormone-responsive breast cancer. Surgery. 2011;150(4):796–801.
Sidhu G, Bristol D, Palanisamy N, et al. Rising incidence of tumorectomies without breast radiation in patients treated for invasive breast cancer in Caribbean nations. South Med J. 2010;103(4):307–10.
Cazap EL, Buzaid A, Chacon R, et al. Breast cancer care in Latin American and the Caribbean. Proc Am Soc Clin Oncol 2007; 18S. Abstract 21140.
Cazap EL, Buzaid AC, Garbino C, et al. Breast cancer in Latin America: results of the Latin American and Caribbean Society of Medical Oncology/Breast Cancer Research Foundation expert survey. Cancer 2008; 113(8S):2359–65.
Landau-Ossondo M, Rabia N, Jos-Pelage J, Marquet LM, Isidore Y. Saint-Aime′ C, Martin M, Irigaray P, Belpomme D, ARTAC International Research Group on Pesticides. Why pesticides could be a common cause of prostate and breast cancers in the French Caribbean Island, Martinique. An overview on key mechanisms of pesticide-induced cancer. Biomed Pharmacother. 2009;63(6):383–95.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Camacho-Rivera, M., Ragin, C., Roach, V. et al. Breast Cancer Clinical Characteristics and Outcomes in Trinidad and Tobago. J Immigrant Minority Health 17, 765–772 (2015). https://doi.org/10.1007/s10903-013-9930-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10903-013-9930-5