Abstract
To determine if pharmacy-initiated interventions improved the rate of influenza and pneumococcal vaccinations in adult patients with asthma and/or chronic obstructive pulmonary disease (COPD). Adult patients who filled prescriptions at one of three community pharmacies, who had a dispensing history indicative of an asthma and/or COPD diagnosis were randomized to receive a personal phone call or standardized mailed letter recommending influenza and pneumococcal vaccinations, or control with no vaccination information. The rate of influenza and pneumococcal vaccinations was measured for each group and measured using Chi square. Of 831 eligible participants, 210 patients completed the study, and self-reported a diagnosis of asthma and/or COPD. The influenza vaccine was administered to 56 (72.7%), 55 (87.3%), and 62 (88.6%) patients (p = 0.019); pneumococcal vaccine was administered to 46 (59.7%), 39 (61.9%), and 39 (55.7%) patients in the phone call, letter, and control groups, respectively. While the control group had significantly more influenza vaccinations, between the interventions the letter showed a higher rate of influenza vaccination over the phone call. Reviewing patients under age 65, the letter had a significantly higher rate of influenza vaccination than the phone call (p = 0.021). No significant improvement was found for the pneumococcal vaccination. Patients under age 65 who received a mailed letter had a significantly higher rate of influenza vaccination than those who received a phone call, and had a higher rate of pneumococcal vaccination. A standardized, mailed letter may help community pharmacists improve vaccination rates in patients with asthma and/or COPD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Asthma and chronic obstructive pulmonary disease (COPD) affect an estimated 18.4 and 12.7 million adults in the United States, respectively [1, 2]. Symptoms of asthma and COPD can reduce patients’ quality of life and place an increased burden on the health care system [3, 4]. In 2008, 53% of adults with asthma suffered an exacerbation that led to an average of 5 missed work days. Asthma also led to an estimated 3.2 million emergency department or urgent care visits that year [3, 4]. Further, COPD is the third leading cause of death in America and cost the healthcare system $49.9 billion in 2010, including 715,000 hospital admissions [2]. In addition to health complications, the economic cost of COPD, asthma, and pneumonia was $106 billion in 2009, with $81 billion in direct healthcare expenditures,[5] or 6.4% of all community health expenditures in the year [6].
Both influenza and invasive pneumococcal infections can cause complications in patients with asthma and COPD, including acute exacerbations [7]. Exacerbations are largely caused by an increase in irritation and inflammation to already inflamed airways, and result in reduced airflow and difficulty breathing [8]. Further, patients with asthma are also more likely to colonize Streptococcus pneumoniae in the upper airways, which can result in more asthma symptoms [7]. For these reasons, the Centers for Disease Control and Prevention and Advisory Committee on Immunization Practices (ACIP) recommend that patients with asthma and/or COPD receive both the yearly influenza vaccine and two doses of the pneumococcal 23-polyvalent vaccine (Pneumovax–Merck) appropriately spaced based on age [9, 10].
Influenza and pneumococcal vaccinations can reduce complications in patients with chronic lung diseases [11]. One study found that adults over age 65 with chronic lung diseases were hospitalized 52% more frequently when they did not receive the influenza or pneumococcal vaccinations. Additionally, among patients who received both influenza and pneumococcal vaccines, there was a 70% reduction in risk of death due to respiratory infection and fewer outpatient physician visits [11].
Across the United States, influenza vaccination rate is low, including patients with asthma [12]. According to the CDC, during the 2010–2011 flu season, only 34.6% of adults aged 18–49 years with asthma received an influenza vaccine. This is only moderately higher than the 25.4% of people receiving the vaccination in the non-asthma population [12]. The rate of pneumococcal vaccinations is even lower, with a Veteran’s Health Administration study finding that a mere 16.8% of COPD patients received the vaccine [13]. These low rates demonstrate the need for increased vaccination of patients with asthma or COPD.
In most states, pharmacists are able to administer both influenza and pneumococcal vaccines [14]. Previous studies have demonstrated that pharmacist participation in vaccine administration leads to increased vaccination rates [15, 16]. One study demonstrated that a pharmacist-led intervention resulted in a 12.1% herpes zoster vaccination rate compared to a control pharmacy of 1.5% (p < 0.01) [17]. Although studies such as Bryan et al. demonstrate a positive impact of pharmacists on vaccination rate, evidence is needed to assess the impact of pharmacist interventions on pneumococcal and influenza vaccination rates among patients with asthma or COPD.
Objective
This study sought to investigate if pharmacy-initiated interventions (outbound phone call or mailed letter) improved influenza and/or pneumococcal vaccination rate among adult patients with asthma or COPD compared to control.
Methods
This study was a randomized, controlled trial. Study sites included three pharmacies within a grocery store chain located within the Kansas City metropolitan area. All three study pharmacies operated under a collaborative practice agreement for vaccine administration.
Patients included in the study were 18 years of age and older with a possible diagnosis of asthma and/or COPD based on a dispensing history that included more than one fill for leukotriene receptor antagonists, short-acting beta2 agonist inhalers, long-acting beta2 agonist inhalers, corticosteroid inhalers, or anticholinergic inhalers between August 29, 2012 and August 29, 2014. Patients were excluded if the dispensing history reflected only one fill of an albuterol rescue inhaler or leukotriene inhibitor within the specified timeframe.
Patients who met the inclusion criteria were randomized into one of three study arms: phone call intervention, mailed letter intervention, or no intervention (control). A phone call script was utilized for the phone call intervention; patient specific questions were fielded on an individual basis. The letter intervention group received a standardized letter addressed to each specific patient. Both the phone call script and letter referenced the 2014 CDC immunization schedule and guidelines [9]. The interventions began in October 2014 and were completed by November 2014. All subjects were exposed to in-store advertising for the seasonal influenza vaccine and received flyers advertising on-site immunizations when picking up prescriptions during the study period. Influenza and pneumococcal consent forms were used to establish appropriateness and need for vaccination per CDC recommendations. The consent form was also used to obtain demographic information, disease state information, and record of prior vaccination. In February and March 2015, a review of electronic pharmacy vaccination records and consent forms was performed to determine vaccination rates within the study groups. If no documentation of vaccination was found via electronic pharmacy dispensing record or consent form, one follow-up phone call was made to determine if the patient received an influenza or pneumococcal vaccination at a non-study pharmacy, clinic, or other location. Diagnosis of asthma and/or COPD was also verified at this time. A brief voicemail with a direct contact phone number was left for phone calls that were unanswered.
This study was granted exempt status from the University of Kansas Medical Center Human Subjects Committee. Sequential numbers were assigned to each patient and groups were created through a random number generator to create three approximately equal sized groups. After randomization, patient demographics were compared to ensure groups were equal prior to intervention implementation. After randomization and during completion of the intervention, patients were excluded if there was invalid contact information, including incorrect phone numbers or returned letters. Patients were considered lost to follow-up if, during the follow-up phone call, a message was unreturned or the patient had invalid contact information. All patients who were excluded or lost to follow-up were removed from the final analysis. For the purposes of the statistical analysis, all patients identified as having both asthma and COPD were counted as a single patient. Patient demographics were assessed using descriptive statistics. The primary objective of vaccination rate among the intervention arms was assessed using Chi square. Further differences between interventions and age comparisons (subjects over versus under age 65) were also assessed through Chi square. The sub-analysis for age was performed due to an additional indication for the pneumococcal vaccine in patients over age 65. Demographics between those included in the final analysis and those lost to follow-up were compared using ANOVA and Chi square to assess for response bias. SPSS v. 22.0 (Armonk, NY) was used for the statistical analysis. The statistical significance level was set a priori at α = 0.05.
Results
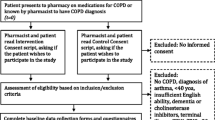
A total of 831 patients were eligible for the study and randomized into one of the three intervention arms; 276 patients in the phone call group, 277 patients in the letter group, and 278 patients in the control group. After randomization, 95 patients were excluded from the study: 61 patients in the phone call group, 25 in the letter group, and 9 in the control group. Further, 425 patients were lost to follow-up due to inability to contact patients (96 in the phone call, 149 in the letter, and 180 in the control groups, respectively). Inability to contact patients during the follow-up period resulted most often due to an invalid phone number or an unreturned voicemail (Fig. 1). Patients were included in the statistical analysis if all information was complete (disease state and vaccination status); within the three study arms, this resulted in 120 patients from the phone call group, 102 patients from the letter group, and 89 patients from the control group. Of these patients included in the statistical analysis, 77 patients, 63 patients, and 70 patients self-reported a positive diagnosis of asthma and/or COPD in the phone call, letter, and control groups, respectively.
Demographics were reviewed for all patients who completed the study (self-reported having asthma and/or COPD and vaccination status known) and were not significantly different between groups (Table 1). Mean age across groups was in the mid-50s, and approximately one-third of the patients were male. Prevalence of insurance coverage was also found to be similar between the three groups. Chi square showed a significant difference for the influenza vaccine (Table 2); with the control group having a significantly higher vaccination rate. The highest rate of pneumococcal vaccination fell within the letter group (Table 2), although no significant difference was found between the groups.
A sub-analysis was conducted for patients with asthma and/or COPD under age 65 (Table 3). In this subset of patients, the letter group had significantly higher influenza vaccination rate (p < 0.021) compared to the phone call group.
Discussion
To the authors’ knowledge, this is the first study to assess the impact of pharmacist-initiated interventions on influenza and pneumococcal vaccination rates among adult patients with asthma and/or COPD. These findings can serve as a guide for pharmacists developing tools they can utilize to improve influenza and pneumococcal vaccination rate among patients with asthma and/or COPD.
Previous research has shown that letter-based interventions improved the rates of patients receiving preventative health measures [18,19,20,21]. Szilagyi et al. found that a mailed letter was able to achieve a higher, although not significant, rate of 56% for up-to-date childhood vaccinations, compared to a rate of 53% with a telephone reminder; preventative care appointments also had a higher attendance rate of 65% in the letter group, compared to 63% of completed visits in the telephone group [18]. The observed increase in preventative care measures of the letter over a phone call intervention may have resulted when patients who received the letter intervention had a physical reminder with a recommendation that could be utilized during the patient’s next trip to the physician office or pharmacy. A mailed letter may still be readily accessible by the patient weeks or months after the intervention date, whereas a phone call could have been forgotten without a physical written reminder.
When adults feel healthy, they may be less likely to seek regular visits with their care provider and be less likely to receive indicated preventative care measures. For example, Bender et al. found that patients with a lower dose of fluticasone propionate/salmeterol inhaler, indicating less symptomatic asthma or feeling more healthy, was associated with fewer days supply of medication obtained over a 12 month period than a higher dose [22]. In addition, health care providers may be less familiar with the specific recommendations for the pneumococcal vaccine, as opposed to a patient over age 65 with dual indications for vaccination; Wisnivesky et al. found that some physicians’ nonadherence to disease guidelines for asthma was due to limited familiarity with specific recommendations [23].
Given that patients over age 65 have a second age-based indication for receiving the pneumococcal vaccination, data were analyzed secondarily among study patients under age 65 [10]. This sub-analysis was intended to demonstrate the impact that pharmacist interventions could achieve for patients with solely a disease state indication for vaccination (i.e., asthma and/or COPD). This partially supports the hypothesis that a pharmacy-initiated intervention could improve rate of influenza and pneumococcal vaccination in the asthma and/or COPD population.
Among all study participants, both influenza and pneumococcal vaccination rates were high compared to national statistics and previous studies [12, 13]. This high rate of vaccination may have stemmed from a number of variables. The study subjects had a slightly lower proportion of private insurance compared to the 2016 national average (65.3 and 69.2% respectively) [24] as well as a much lower proportion of patients who were uninsured (5.5%) compared to the 2016 national average (12.4%) [24]. Further, all three study pharmacies were located within an affluent, suburban area; all were within the same zip code that shows as the sixth highest income-earning zip code in the state of Kansas, with an average income of $67,000/year, [25] which may raise the vaccination rate for the region. Dyda et al. demonstrated that patients with a higher income are significantly more likely to receive an influenza vaccination over patients with a lower income [26]. This may be a result of placing more value on preventative health care services when excess income is available, rather than anticipating other financial obligations that may take priority and limiting the value a patient places on vaccines.
In addition, numerous studies have found higher vaccination rates correlates with marketing for vaccination, [27,28,29,30] and this study occurred during the peak months of influenza season. Although the vaccination rate was high among the three groups of patients with asthma and/or COPD, the rate of influenza vaccination in patients in the control group was found to be statistically significantly higher when compared to the phone call group; this is in contrast to one study [18] that demonstrated a letter or phone call reminder increased vaccination. The high vaccination rate within the control group observed in the current study may have resulted from a high baseline level of vaccination within the study pharmacies, largely due to a combination of the socioeconomic status of the study participants, and the increase in vaccination advertisements during the study time period. Further, during the course of the phone call intervention, many patients expressed the desire to discuss the recommendation at their next physician appointment after the conclusion of the study period; patients in the letter group may have experienced this same desire.
Further studies need to be performed to confirm the impact that community pharmacy interventions can have on improving vaccination rates. Future research may focus on the inclusion of economically and geographically diverse study locations, other patient populations with specific vaccination indications, as well as re-surveying the current study population to determine long-term impact of the interventions. Additional research is also needed to confirm the superiority of a mailed letter intervention in comparison to a phone call, to gain insight into the optimal intervention methods to improve vaccination rates in specific patient populations.
Limitations
The study had limitations. A large number of patients (520, 62.6%) were lost to follow up due to invalid contact information or unreturned phone messages. No attempt was made to acquire valid contact information beyond pharmacy software records. Also, due to time constraints and patient volume, only a single round of intervention phone calls was attempted in the phone call group and one follow-up phone call was completed for patients with incomplete data in the letter and control groups. Additionally, patients self-reported disease states as opposed to validating information from the patients’ physicians or diagnosis codes from health plans. Patients may have been unfamiliar with the indication for their inhalers and expressed that they did not have asthma and/or COPD. Further, patients were not surveyed to determine if the intervention prompted their decision to be vaccinated. Given the high vaccination rate in the control group, it is possible that other advertising or events affected their vaccination decisions. Patients may have been personally invited by pharmacy staff to receive either the influenza or pneumococcal vaccine; however, this would be expected to occur at a similar rate in all 3 study groups. In each of the three study pharmacies, printed advertisements promoting in-store immunizations were given with each prescription for the duration of the study period. Also likely impacting pneumococcal vaccination rates in patients over age 65, the CDC released a new recommendation for the pneumococcal 13-polyvalent vaccine (Prevnar–Pfizer) during the study period, which was highly publicized in print and television media, including printed advertisements at each of the study pharmacies. Due to the high level of advertisements, both physicians and patients may have become more familiar with specific vaccination recommendations. Additionally, there were some differences between those patients included in the analysis and those lost to follow-up. The patients lost to follow-up were younger and had a slightly higher proportion of cash payers which may have impacted the results. Lastly, the study population lacked economic and geographic diversity, so the results may not be generalizable to all patients with asthma and/or COPD. Selecting pharmacies in a different geographical area may have allowed for increased diversity or produced different results.
Conclusion
A mailed letter intervention was associated with a statistically significantly higher rate of influenza vaccination in adult patients under age 65 with asthma and/or COPD, and a higher rate of pneumococcal vaccination compared with a phone call. The results of this study may help community pharmacists determine which interventions can be effective for increasing immunization rates in patients with specific immunization needs. Additional research is needed to further explore effective intervention techniques in specific patient populations.
References
Centers for Disease Control and Prevention (CDC). FastStats. Retrieved August 3, 2017, from http://www.cdc.gov/nchs/fastats/asthma.htm.
American Lung Association (ALA). Trends in COPD (Chronic Bronchitis and Emphysema): Morbidity and mortality. Retrieved August 3, 2017, from http://www.lung.org/assets/documents/research/copd-trend-report.pdf.
Centers for Disease Control and Prevention (CDC). Vital signs: Asthma prevalence, disease characteristics, and self-management education—United States, 2001–2009. MMWR. Retrieved August 3, 2017, from http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6017a4.htm?s_cid=mm6017a4_w.
American Academy of Allergy Asthma and Immunology. Asthma statistics. Retrieved August 3, 2017, from http://www.aaaai.org/about-the-aaaai/newsroom/asthma-statistics.aspx.
National Heart, Lung, and Blood Institute. Disease statistics. Retrieved August 3, 2017, from http://www.nhlbi.nih.gov/about/documents/factbook/2012/chapter4.
Cohen, S. The concentration of health care expenditures and related expenses for costly medical conditions, 2009. Retrieved June 26, 2015, from http://meps.ahrq.gov/mepsweb/data_files/publications/st359/stat359.pdf.
Pesek, R., & Lockey, R. (2011). Vaccination of adults with asthma and COPD. Allergy, 66(1), 25–31.
Rennard, S. I., & Farmer, S. G. (2014). Exacerbations and progression of disease in asthma and chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society, 1, 88–92.
Centers for Disease Control and Prevention (CDC). Lung disease including asthma and adult vaccination. Retrieved August 3, 2017, from http://www.cdc.gov/vaccines/adults/rec-vac/health-conditions/lung-disease.html.
Immunization Action Coalition (IAC). Advisory committee on immunization practices. Retrieved April 28, 2015, from http://www.immunize.org/acip/.
Nichol, K. L., Baken, L., & Nelson, A. (1999). Relation between influenza vaccination and outpatient visits, hospitalization, and mortality in elderly persons with chronic lung disease. Annals of internal medicine, 130(5), 397–403.
Centers for Disease Control and Prevention (CDC). Vaccination coverage among persons with asthma—United States, 2010–2011 influenza season. Retrieved September 20, 2014, from http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6248a1.htm.
Lee, T. A., Weaver, F. M., & Weiss, K. B. (2007). Impact of pneumococcal vaccination on pneumonia rates in patients with COPD and asthma. Journal of General Internal Medicine: Official Journal of the Society for Research and Education in Primary Care Internal Medicine, 22(1), 62–67.
American Pharmacists Association. Pharmacist administered vaccines. apha/naspa survey of state iz laws/rules. Retrieved September 25, 2015, from http://www.pharmacist.com/sites/default/files/PharmacistIZAuthority.pdf.
Hogue, M. D., Grabenstein, J. D., Foster, S. L., et al. (2006). Pharmacist involvement with immunizations: A decade of professional advancement. Journal of the American Pharmacists Association, 46(2), 168–179.
Grabenstein, J. D., Guess, H. A., Hartzema, A. G., et al. (2001). Effect of vaccination by community pharmacists among adult prescription recipients. Medical Care, 39(4), 340–348.
Bryan, A. R., Liu, Y., & Kuehl, P. G. (2013). Advocating zoster vaccination in a community pharmacy through use of personal selling. Journal of the American Pharmacists Association, 53(1), 70–77.
Szilagyi, P. G., Albertin, C., Humiston, S. G., et al. (2013) A randomized trial of the effect of centralized reminder/recall on immunizations and preventative care visits for adolescents. Academic Pediatrics. 13(3), 204–213.
Shea, A. K., Shah, B. R., Clark, H. D., et al. (2011). The effectiveness of implementing a reminder system into routine clinical practice: Does it increase postpartum screening in women with gestational diabetes. Chronic Diseases in Canada, 31(2), 58–64.
Zhang, Z., & Fish, J. (2012). Recommended care adherence: Improved by patient reminder letters but with potential attenuation by the healthcare process complexity. Quality in Primary Care, 20(2), 149–164.
Ekedahl, A., Oskarsson, V., Sundgerg, B., et al. (2008) Impact of postal and telephone reminders on pick-up rates of unclaimed e-prescriptions. Pharmacy World & Science, 30(5), 503–508.
Bender, B. G., Pedan, A., & Varasteh, L. T. (2006). Adherence and persistence with fluticasone propionate/salmeterol combination therapy. The Journal of Allergy and Clinical Immunology, 118(4), 899–904.
Wisnivesky, J. P., Lorenzo, J., Lyn-Cook, R., et al. (2008). Barriers to adherence to asthma management guidelines among inner-city primary care providers. Annals of Allergy, Asthma & Immunology, 101(3), 264–270.
Centers for Disease Control and Prevention (CDC). Health insurance coverage. Retrieved August 4, 2017, from http://www.cdc.gov/nchs/fastats/health-insurance.htm.
Internal Revenue Service (IRS). SOI tax stats—individual income tax statistics—2012 zip code data (SOI). Retrieved June 7, 2015, from http://www.irs.gov/uac/SOI-Tax-Stats-Individual-Income-Tax-Statistics-2012-ZIP-Code-Data-(SOI).
Dyda, A., MacIntyre, C. R., McIntyre, P., et al. (2015). Factors associated with influenza vaccination in middle and older Australian adults according to eligibility for the national vaccination program. Vaccine, 33, 3299–3305.
Henrich-Morrison, K., McLellan, S., & McGinnes, U. (2015). An effective strategy for influenza vaccination of healthcare workers in Australia: Experience at a large health service without a mandatory policy. BMC Infectious Diseases, 15, 42.
MacDonald, L., Cairns, G., Angus, K., et al. (2013). Promotional communications for influenza vaccination: A systematic review. Journal of Health Communication, 18(12), 1523–1549.
Dey, P., Halder, S., Collins, S., et al. (2001). Promoting uptake of influenza vaccination among health care workers: A randomized controlled trial. Journal of Public Health Medicine, 23(4), 346–348.
Humair, J. P., Buchs, C. R., & Stalder, H. (2002). Promoting influenza vaccination of elderly patients in primary care. Family Practice, 19(4), 383–389.
Funding
015 American Pharmacists Association Foundation Incentive Grant
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no relevant conflicts of interest or financial relationships.
Rights and permissions
About this article
Cite this article
Klassing, H.M., Ruisinger, J.F., Prohaska, E.S. et al. Evaluation of Pharmacist-Initiated Interventions on Vaccination Rates in Patients with Asthma or COPD. J Community Health 43, 297–303 (2018). https://doi.org/10.1007/s10900-017-0421-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10900-017-0421-9