Abstract
About 75 % of African-Americans (AAs) ages 20 or older are overweight and nearly 50 % are obese, but community-based programs to reduce diabetes risk in AAs are rare. Our objective was to reduce weight and fasting plasma glucose (FPG) and increase physical activity (PA) from baseline to week-12 and to month-12 among overweight AA parishioners through a faith-based adaptation of the Diabetes Prevention Program called Fit Body and Soul (FBAS). We conducted a single-blinded, cluster randomized, community trial in 20 AA churches enrolling 604 AAs, aged 20–64 years with BMI ≥ 25 kg/m2 and without diabetes. The church (and their parishioners) was randomized to FBAS or health education (HE). FBAS participants had a significant difference in adjusted weight loss compared with those in HE (2.62 vs. 0.50 kg, p = 0.001) at 12-weeks and (2.39 vs. −0.465 kg, p = 0.005) at 12-months and were more likely (13 %) than HE participants (3 %) to achieve a 7 % weight loss (p < 0.001) at 12-weeks and a 7 % weight loss (19 vs. 8 %, p < 0.001) at 12-months. There were no significant differences in FPG and PA between arms. Of the 15.2 % of participants with baseline pre-diabetes, those in FBAS had, however, a significant decline in FPG (10.93 mg/dl) at 12-weeks compared with the 4.22 mg/dl increase in HE (p = 0.017), and these differences became larger at 12-months (FBAS, 12.38 mg/dl decrease; HE, 4.44 mg/dl increase) (p = 0.021). Our faith-based adaptation of the DPP led to a significant reduction in weight overall and in FPG among pre-diabetes participants.
ClinicalTrials.gov Identifier NCT01730196.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Overweight/obesity is the risk factor most associated with incident Type 2 Diabetes Mellitus (T2DM) [1]. More than 75 % of African-Americans (AAs) ages 20 or older are overweight and nearly 50 % are obese [2]. AAs also have a 10 % higher prevalence of being overweight and more than 15 % prevalence of being obese than non-Hispanic Whites [2, 3]. This disparity may partly explain the higher percentage of age-adjusted diagnosed diabetes among AAs compared with non-Hispanic Whites (12.6 vs. 7.1 %) [4].
Studies such as the Diabetes Prevention Program (DPP) have demonstrated that lifestyle modification which includes weight loss, increasing physical activity, and healthy eating can significantly reduce the development of T2DM among persons with pre-diabetes with an effect shown to persist for at least 10 years [5]. Adaptations of the DPP including the Group Lifestyle Balance (GLB) program lower diabetes risk factors [6–10]; however, such programs in predominantly AA populations are scarce or limited in scope [5, 11–14]. Successful translation of the DPP into community-based programs for AAs could have the potential of reducing the large number of AAs with diabetes over time [4].
The AA church, by playing a major role in providing spiritual and social support for many parishioners, may be an essential partner in these community efforts to decrease health disparities [15, 16]. Many AA churches also have well-organized multidisciplinary health ministries [17]. Several investigators have used culturally appropriate adaptations of the DPP within AA churches, but these studies have either been non-comparison, non-randomized, or small feasibility trials [11–13, 18–20]. In one of those studies, participants were recruited by and received the DPP through lay community health workers, but the primary outcome measures did not include weight loss [11]. Herein, we report our results of a church-based cluster randomized trial, Fit Body and Soul (FBAS) which was designed to determine if a DPP-modified, culturally appropriate behavioral lifestyle intervention delivered to congregants by church-affiliated trained health advisors could lead to significant reduction in weight.
Methods
Study Design
Details regarding the design and methodology of this trial have been reported elsewhere [21]. This study was conducted in 20 AA churches located in Augusta (Richmond County), Georgia between October 2009 and April 2013. These 20 churches were the first of the 35 churches with ≥200 AA members, pastors who accepted randomization, and an existing health ministry, to agree to participate. Congregation size ranged from 200 to 3000. The target recruitment goal was to enroll up to 40 participants from each church. Each church received monetary compensation for participating in the study and for providing the space for group sessions and data collections. Churches were recruited as pairs in the study based on congregation size. These pairs were included in six cohorts with each cohort including either two or four churches. Each church pair was then randomized to the FBAS behavioral lifestyle intervention or health education (HE) comparison group. The authors designed, conducted, and analyzed data from the study. Our Institutional Review Board (IRB) approved this study. Written informed consent was obtained from all participants before their enrollment. Evaluators were blinded to intervention allocation and to outcome measures, and participants were instructed not to disclose the intervention they received. The authors vouch for the accuracy and completeness of this report to the trial protocol. The study was sponsored and supported by the National Institutes of Health alone. There were no corporate sponsors.
Study Participants
To participate in the study, eligible persons were required to be self-described AAs, ages 20–64 years, who were planning on remaining in the community for 1 year and to meet the following criteria: non-diabetic [fasting plasma glucose (FPG) <126 mg/dl]; a body-mass index (the weight in kilograms divided by the square of the height in meters) of 25.0 or more; no medical contraindications to physical activity (as determined by the Physical Activity Readiness Questionnaire); no history of gastric weight-loss surgery or weight loss of more than 10 % in the past 3 months for any reason other than childbirth; no physical conditions or medications that might affect glucose metabolism; no behaviors that might interfere with participation; no illnesses that would limit life span; and, for females, no current pregnancy or planned pregnancy within the study period. We defined pre-diabetes as a FPG of 100–125 mg/dl [22]. Those with a FPG of 126 mg/dl or greater at baseline were removed from the study and are excluded from this analysis (FBAS, n = 12; HE, n = 12). Additional eligibility criteria are described elsewhere [21]. Gift cards and non-monetary incentives deemed appropriate by the IRB were provided to participants.
Study Interventions
This study is a single-blinded, cluster-randomized, community-based trial. Investigators allocated churches to two arms: the FBAS (intervention arm) which is a faith-based adaptation of the GLB program [23], and a HE program (comparison arm) developed from the list of topics provided by the Centers for Disease Control and Prevention (CDC) Guide to Community Prevention Services. As the church was the unit of randomization, eligible participants were assigned to the intervention occurring in their church. A community and university advisory board consisting of persons from the Georgia Regents University, from the University of Pittsburgh Diabetes Prevention Support Center, from another church-based trial (Body and Soul), and from six local churches identified as having an active health ministry, provided input for all aspects of the planned project, including recommendations to modify the GLB curriculum by adding selected scriptures, socio-cultural preferences, AA graphics, and quotes from well-known AAs. The HE comparison curriculum addressed key health issues facing AAs in Richmond County, Georgia, and investigators developed the selected health topics into a scripted manual and developed participant handouts from information provided by the American Heart Association, American Cancer Association, American Diabetes Association, Mental Health America, and other national organizations. Equivalent time and effort were provided to each intervention. Curricula for these two interventions have been described in detail elsewhere [21].
FBAS was aimed at achieving a weight loss of at least 7 % of baseline by week-12, and to maintain the weight loss at 12-months post-baseline through six booster sessions. Secondary aims initially included: FPG with a mean reduction of at least 3 mg/dl, and physical activity of moderate intensity for at least 150 min per week. Adults who meet this leisure-activity physical activity guideline may, however, underestimate the aerobic or muscle-strengthening activities associated with occupation, transportation or household chores per Department of Health and Human Services (HHS) guidelines [24]. Therefore, we altered the physical activity aim prior to participant enrollment to include an increase in overall physical activity by at least 150 MET-min/week.
Each intervention arm involved participants attending 12-weekly group 1-h core sessions at their respective church. For FBAS, the 12 core sessions comprised the key components of successful weight loss programs such as strategies to reduce calories and dietary fat consumption, encouraging physical activity, and behavioral modification such as stimulus control, goal setting, and problem solving. For HE, the 12 core sessions included information and risk improvement strategies about mental health and stress, heart disease and stroke, diabetes, cancer, smoking, injury and violence, asthma, nutrition, physical activity, HIV/AIDS, and communicating with one’s health provider. Church health advisors (CHAs) were members of their respective church’s health ministry (e.g., nurses, pharmacists, physicians) and were trained by a co-investigator certified to perform GLB training. CHAs delivered the weekly sessions followed by six monthly 1-h post-core “booster” sessions. To conduct fidelity monitoring, a research team member attended each group session to record participant attendance and used an investigator-developed fidelity tool to verify that the content delivered by CHAs was delivered as designed with all core components and in the appropriate manner and context to the appropriate group.
Study Outcomes and Assessments
The primary outcome of FBAS was change in weight. Secondary outcome measures included FPG and physical activity levels during the same time periods [21].
Study investigators used a common standardized operating protocol to train all data collectors who were blinded to the study-arm assignments and reassessed each data collector periodically for adherence to protocols and measurement drift. At baseline, 12–14 weeks post-baseline and 12-months post-baseline, data collectors obtained demographic data, medication use, and anthropometric and physiological measures [height (baseline only) and waist circumference in centimeters; weight in kilograms; blood pressure]. Data collectors oversaw the participant self-administered International Physical Activity Questionnaire long form (IPAQ-LF) to measure physical activity, the SF-12 version 2 to measure health-related quality of life, and the Euro-Quality of Life (EuroQOL) to determine health utilities. Phlebotomists contracted through a local laboratory collected blood for FPG, hemoglobin A1C, and hemoglobin. Those participants with a hemoglobin below the laboratory’s normal range, or a systolic blood pressure ≥160 mmHg, or a diastolic blood pressure ≥100 mmHg, were notified and provided a letter to be given to their non-study medical provider. Additionally, if participants had a FPG ≥ 126 mg/dl at baseline, they would be withdrawn from the study, and they and their medical provider would be notified. If the participant did not have a medical provider, we sent them a list of free or low-cost providers and the lab results to take to their first appointment.
Statistical Analysis
This controlled cluster-randomized trial included repeated measures (RM) at multiple time points (baseline, 12–14 weeks post-baseline, 12-months post-baseline), as well as clustering of participants who were nested within churches (the clusters) with the congregation size acting as a blocking factor. The study design was therefore hierarchical or nested in nature. Consequently, statistical modeling and hypothesis testing accounted for the hierarchical random effects and the RMs as noted below.
Power and sample size calculations were based on the primary outcome of weight change and the secondary outcome of change in FPG and have been described in detail elsewhere [21]. In December 2011, we conducted a blinded interim analysis using the 415 enrolled participants with 12–14 week post-baseline data to assess our original sample size calculations. Given the statistically significant group differences in the primary outcome measure at that time, the substantially lower variability than originally assumed, and a 6.8 % attrition rate compared with the anticipated 20 %, we are confident that the 604 enrolled subjects provides sufficient power to detect group differences. We used independent-samples t tests to compare arm means and Chi square tests to compare arm proportions for selected baseline characteristics of the study participants by FBAS intervention with HE. We modeled and tested hypotheses using General Linear Mixed Models (GLMM) as implemented in the MIXED procedure of IBM SPSS Statistics (Version 21, Armonk, NY: IBM Corp). Hypotheses were planned a priori; thus, no multiple comparison adjustments were made to the alpha level (Type I error rate). We used the Bonferroni–Sidak adjustment in conducting post hoc comparisons. We conducted all analyses using a 5 % significance level. The dependent variables used in these analyses were weight, FPG, and physical activity. Each of these three dependent variables was analyzed separately using GLMM procedures. The design factors used in each analysis were (1) between-participant factor—Arm (FBAS intervention; HE comparison); (2) within-participant factor—Time (RM: baseline; 12-weeks post-baseline; 12-months post-baseline); and (3) hierarchical/nesting factor—Cluster (church). Since the functional form of the continuous covariates with the dependent variables of interest was not known, we chose to block on the following variables by implementing a median split instead of using them as a continuous covariate in the analyses: age at baseline (≤48; >48); gender (male; female); education (some college or less; college graduate or higher); EuroQOL VAS baseline health status (≤80; >80); SF12 baseline general health status (≤60; >60); congregation size of church (<1000; ≥1000); baseline diabetes status (ADA FPG Definition) [normoglycemic (<100 mg/dl); pre-diabetes (100–125 mg/dl)] [22]; baseline overweight status [CDC Body Mass Index (BMI) Definition] [overweight (25–29.9); obese (≥30.0)] [25]; number weekly sessions attended (≤9; >9; 12 max); number booster sessions attended (≤3; >3; 6 max); number all sessions attended (≤12; >12; 18 max). For session attendance, we used either weekly, booster, or all sessions attended depending on the analysis and the dependent variable.
The number and percentage of participants achieving at least a 3, 5, and 7 % weight loss were calculated by study arm for 12-weeks post-baseline and 12-months post-baseline. Chi square tests of homogeneity of study arm proportions at the two post-baseline times were conducted. We also conducted these analyses for only those participants classified as having pre-diabetes (FPG between 100 and 125 mg/dl) at their baseline assessment. (Note: Because of reduced counts in the pre-diabetes analyses, we used Fisher’s exact test instead of the Chi square tests.) Also, for only those participants classified as pre-diabetes at their baseline assessment, we conducted the GLMM analyses for the FPG analyses detailed above.
Results
Description of Study Participants at Baseline
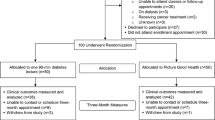
From October 2009 to March 2012, 604 persons were enrolled and analyzed in the twenty churches randomly assigned to receive FBAS (n = 317) or HE (n = 287) (Fig. 1). There were no significant differences between the two study arms in any characteristic at baseline (range: 0.32 < p < 0.91) (Table 1). The average age was 46.5 years, 83 % of the participants were women, 51 % had a college degree, the mean body mass index was 35.7 and the mean waist circumference was 107.3 cm. The mean systolic and diastolic blood pressure was 130.5 and 82.6 mmHg, respectively, and 15.2 % of participants had pre-diabetes. Additional baseline data have been published previously [21]. No harms or unintended effects occurred.
Weight
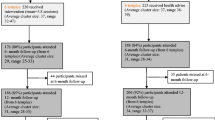
There was a significant (p = 0.001) interaction of arm (FBAS vs. HE) and time (baseline, week-12, and month-12) for weight (Fig. 2a). Those in FBAS lost 2.39 kg over the 12 months compared with a gain of 0.47 kg for those in HE group (Table 2).
Weight, fasting plasma glucose, and physical activity at baseline, week 12, and week 52. Shown are estimated marginal means for those who participated in the Fit Body and Soul (FBAS) intervention arm or the Health Education comparison arm. Means were estimated with the use of general linear mixed models for continuous measures. For weight, there was a significant interaction of arm and time (p = 0.001). There was not a significant interaction of arm and time for either fasting plasma glucose (p = 0.221) or for physical activity (p = 0.667). MET denotes metabolic equivalents (minutes/week)
Weight Loss from Baseline to Week-12
Participants in both FBAS and HE lost weight over the first 12 weeks. Those in FBAS, however, lost significantly more weight (2.62 kg) than those in HE (0.50 kg) (p = 0.001). The number of weekly sessions attended modified the effect of weight loss among study groups (p = 0.006). No other covariate modified the results. Those in FBAS who attended 10 or more weekly sessions lost, on average, significantly more weight compared with those who attended fewer than 10 weekly sessions (3.72; 1.52 kg, respectively) (Bonferroni–Sidak, p < 0.001). For participants in HE, there was no difference in weight loss for the number of weekly sessions attended (Bonferroni–Sidak, p = 0.418).
Weight Loss from Week-12 to 12-Months
Participants in FBAS, on average, nearly maintained the week-12 weight loss through month-12 (0.05 kg weight gain) whereas those in HE gained 0.97 kg, but this difference was not significant (p = 0.334). The number of booster sessions attended modified the effect on weight regardless of study arm (p = 0.033). No other covariates modified the results. Those attending four or more booster sessions maintained their week-12 weight, whereas, those attending fewer than four booster sessions gained approximately 1 kg.
Percentage Weight Loss
The percentage of those participants achieving at least 3, 5, or 7 % weight loss at either 12-weeks or 12-months post-baseline was significantly greater for those in FBAS compared with HE (p < 0.001) (Table 3). By 12 months post baseline, 39, 27, and 19 % of those in FBAS had lost at least 3, 5, or 7 % weight compared with 22, 13, and 8 % for those in HE.
Fasting Plasma Glucose—All Participants
Overall, the change in FPG did not differ by study arm at any of the three time periods (p ≥ 0.468) (Fig. 2b). None of the participants in either FBAS or HE developed diabetes. Participants, on average, had progressively lower FPG at week-12 and month-12 (Table 2). The adjusted mean FPG at baseline, week-12, and month-12 were 90.4, 89.9, and 87.4 mg/dl respectively. Overweight (25 ≤ BMI < 30) participants had lower mean FPG than obese (BMI ≥ 30) participants, 87.6 and 91.0 mg/dl, respectively.
Physical Activity
Participants, on average, increased their physical activity from baseline to week-12 and decreased their physical activity from week-12 to month-12, to below baseline levels, but there was no difference between study arms (p = 0.685) (Fig. 2c). The adjusted mean physical activity per minute at baseline, week-12, and month-12 were 2766, 4106, and 2100 MET-min/week, respectively. There were no significant differences in physical activity between those in the FBAS and those in the HE from baseline to week-12, week-12 to month-12, and baseline to month-12 (p ≥ 0.196) (Table 2).
Pre-diabetes Participants
Of the 92 participants (51 FBAS; 41 HE) with pre-diabetes at baseline, 85 (50 FBAS; 35 HE) had 12-week and 81 (49 FBAS; 32 HE) had month-12 FPG values. Pre-diabetes participants in FBAS had, on average, progressively lower FPG at week-12 and month-12 than those in HE (p = 0.003). For FBAS participants, mean FPG at baseline, week-12, and month-12 was 105.3, 94.5, and 91.9 mg/dl, respectively, whereas, for HE participants, mean FPG increased from baseline, to week-12, and to month-12 (104.4, 108.8, and 110.2 mg/dl, respectively). From baseline to week-12, participants in FBAS had a significant decline in FPG (10.93 mg/dl) compared with the 4.22 mg/dl increase by those in HE (p = 0.017). Moreover, from baseline to month-12, differences between those in FBAS and HE became larger (FBAS, 12.38 mg/dl decrease; HE, 4.44 mg/dl increase) (p = 0.021). For those participants in FBAS compared with those in HE, the percentage achieving at least 3 or 5 % weight loss at 12-weeks post-baseline was significantly greater (p ≤ 0.008) as were those achieving at least 3 or 7 % weight loss at 12-months post-baseline (p ≤ 0.023) (Table 3). At 12-months, 61.2 % (n = 30) of the FBAS and 46.9 % (n = 15) of the HE participants with pre-diabetes at baseline had a FPG < 100 mg/dl, but the difference in the two arms was not significant (p = 0.445). Regardless of arm, for pre-diabetes participants, the mean FPG improvement from baseline to week-12 was significantly (p = 0.01) greater for those with a college degree or higher (6.59 mg/dl) than for those with some college or less (0.12 mg/dl). Except for arm, no other main effects or interactions were significant. No main effects or interactions were significant in the mean FPG improvement from week-12 to month-12, and, except for arm, no main effects or interactions were significant in the mean FPG improvement from baseline to month-12.
Discussion
The results from this community-based participatory study show that the 12-week FBAS program led to a significant reduction in weight compared with HE over the 12-week period. More than one-quarter of those in the FBAS program lost at least 5 % of their initial body weight, and FBAS participants were more than four times as likely to lose 7 % of their initial body weight than those in HE. This weight loss was modified by the number of sessions attended with about 3.7 kg average weight loss for those who attended ten or more FBAS sessions. Moreover, the weight loss in the FBAS participants was maintained over the 12-month period whereas those in HE had some weight gain.
Overall, FPG did not differ over the 12-week and 12-month periods between participants in the two arms. When analyses were, however, restricted to only persons with pre-diabetes, the FPG for FBAS participants was statistically significantly reduced by about 11 mg/dl, on average, over the 12-week period and by more than 12 mg/dl, on average, over the 12-month period whereas those in HE had a more than 4 mg/dl, on average, increase in both analysis periods. Of those with pre-diabetes in the FBAS intervention, 22 % achieved a 7 % weight loss at 12 months.
AAs are less likely to benefit from lifestyle programs for weight loss than other racial/ethnic groups [26, 27]. This lack of benefit has been attributed to social and cultural barriers including different body-image ideals and food attitudes, to having fewer models and safe areas for physical activity, and to normative views of overweight and obesity [28–30]. Churches and other faith-based organizations have sparked considerable interest as settings in which to conduct health promotion programs and to disseminate the DPP into the community [27]. Working with AA churches may help to counter those social and cultural barriers. Many AAs are likely to identify themselves as religious, attend church or other organized religious venues, which make these settings ideal for reaching and recruiting potential participants for public health programs [15–18, 28]. Many AA churches include health as part of their mission, create health committees, and participate in community outreach activities [17, 27]. Churches also provide an attractive venue to recruit and retain participants because they tend to be stable institutions with members who attend the same church over many years.
Our results showing a significant reduction in weight among those in our faith-based DPP are consistent with those found in other weight reduction programs, including those that were clinic-based [31, 32]. Other faith-based DPP studies are few and have shown variable results, perhaps due to small sample size, participant attrition, and choice of comparison groups. Boltri, et al., used its research team to implement a 6-week compared with a 16-week DPP among 37 pre-diabetes participants [13]. They, however, combined these two groups and reported a 1.7 kg weight loss and reduction of FPG of 6.4 mg/dl at 6-months post intervention with a 0.9 kg regain at 12 months and further reduction of FPG of 1.4 mg/dl. Yeary et al. [12] conducted a 16-week feasibility study without a comparison group among 26 participants using lay health advisors and found a mean weight change of −2.7 %. Gutierrez et al. [14], implemented a 12-week program without a comparison group within AA and Latino churches showing an average weight change of −2.11 % among the 107 AA participants. Faridi et al. [11], using trained lay health leaders in a non-randomized trial to implement the DPP in 13 churches compared with six churches that received no intervention, found no differences in weight change or physical activity among the 65 % of 246 participants who completed the trial. Evidence showing that the addition of faith concepts enhance the DPP intervention are few and non-conclusive [33].
Whereas most previous studies included only those persons with “pre-diabetes” or metabolic risk factors in addition to being overweight [31, 32], we included all persons who were overweight but not diabetic. Given the 16 mg/dl difference in FPG between FBAS and HE at 12 months among those with pre-diabetes, it might be tempting to screen for pre-diabetes among future FBAS participants. Obesity is associated, however, with significantly higher all-cause mortality relative to normal weight [34]. Thus, from a public health perspective, focusing on reducing obesity in the AA community may have a larger population impact than focusing on pre-diabetes alone.
Our findings showed no statistically significant differences in physical activity between the two arms over the 12-week and 12-month periods; however, participants in both FBAS and HE increased their physical activity over the first 12-week period and subsequently reverted back to baseline levels at 12-months. Regular leisure-time physical activity among AAs is lower than in other races and only about 30 % of AA women are physically active [35]. Our results showing no difference in physical activity between FBAS and HE differ from those of the DPP that showed that participants lost weight through dietary changes and increased physical activity [5]. The reasons for this difference are unclear although motivation for clinic-based pre-diabetic patients may be greater than for community-based participants. Moreover, it appears that this AA cohort was relatively active at baseline perhaps since the IPAQ-long form includes more than just leisure time physical activity [36]. HHS guidelines for physical activity are not restricted to leisure-time activity [24]. Finally, self-reported physical activity questionnaires are valid for individual ranking, but accurate and precise reporting of activity levels is scarce [37].
There are several limitations to our findings. We used a specific faith-based approach that focused on the needs of AA congregants in the USA South. Although intervention studies in AA churches have taken place in other regions of the country [11, 38–40], it is unclear if our approach could be successfully translated elsewhere. Second, it is possible that selection bias occurred since one investigator, well known by the target population, helped in church recruitment. We attempted to reduce this potential bias by recruiting churches using several different methods, including in-person presentations by others at the local ministers meeting and identifying churches through other key community members not affiliated with the study. Third, we did use incentives, such as gift cards, pedometers, and t-shirts, for individuals to attract and maintain participation and monetary compensation for churches for providing the space required to carry out the sessions and data collection [21]; it is unlikely that these incentives would be possible for general dissemination and sustainability. We are currently evaluating the costs of our program and how best to disseminate and implement our findings among other faith-based organizations. Fourth, by using a HE control group, we attempted to determine if a faith-based weight loss program could be implemented successfully in an AA community rather than to determine if our program was comparatively effective versus a different weight loss program. Finally, we did not collect dietary information, thus precluding our ability to determine the relative benefits of diet and physical activity on our study groups.
In conclusion, our findings showed, among AA’s, that an intensive faith-based lifestyle intervention can lead to significant reductions in weight overall (in persons with normoglycemia as well as with pre-diabetes) and in FPG among persons with pre-diabetes, as compared with a HE program.
References
Mokdad, A., Ford, E. S., Bowman, B. A., et al. (2003). Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. Journal of the American Medical Association, 289(1), 76–79.
Ogden, C. L., Carroll, M. D., Kit, B. K., & Flegal, K. M. (2014). Prevalence of childhood and adult obesity in the United States, 2011–2012. Journal of the American Medical Association, 311(8), 806–814.
Flegal, K., Carroll, M. D., Kit, B., & Ogden, C. L. (2012). Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. Journal of the American Medical Association, 307(5), 491–497.
Centers for Disease Control and Prevention. (2011). National diabetes fact sheet. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Retrieved April 9, 2014.
Diabetes Prevention Program Research Group. (2002). Reduction in the incidence of type 2 DM with lifestyle intervention or metformin. New England Journal of Medicine, 346, 393–403.
Ackermann, R. T., & Marrero, D. G. (2007). Adapting the diabetes prevention program lifestyle intervention for delivery in the community: The YMCA model. The Diabetes Educator, 33(1), 69–78.
Katula, J. A., Vitolins, M. Z., Rosenberger, E., et al. (2011). One-year results of a community-based translation of the diabetes prevention program: Healthy-living partnerships to prevent diabetes (HELP PD) project. Diabetes Care, 34(7), 1451–1457.
Seidel, M. C., Powell, R. O., Zgibor, J. C., Siminerio, L. M., & Piatt, G. A. (2008). Translating the diabetes prevention program into an urban medically underserved community: A nonrandomized prospective intervention study. Diabetes Care, 31(4), 684–689.
Kramer, M., Kriska, A. M., Venditti, E. M., et al. (2010). A novel approach to diabetes prevention: Evaluation of the group lifestyle balance program delivered via dvd. Diabetes Research and Clinical Practice, 90(3), e60–e63.
Kramer, M. K., McWilliams, J. R., Chen, H. Y., & Siminerio, L. M. (2011). A community-based diabetes prevention program: Evaluation of the group lifestyle balance program delivered by diabetes educators. Diabetes Education, 37(5), 659–668.
Faridi, Z., Shuval, K., Njike, Y., et al. (2010). Partners reducing effects of diabetes (PREDICT): A diabetes prevention physical activity and dietary intervention through African-American churches. Health Education Research, 25(2), 306–315.
Yeary, K. H., Cornell, C. E., Turner, J., et al. (2011). Feasibility of an evidence-based weight loss intervention for a faith-based, rural, African American population. Preventing Chronic Disease, 8(6), A146. http://www.cdc.gov/pcd/issues/2011/nov/10_0194.htm. Retrieved April, 9, 2014.
Boltri, J. M., Davis-Smith, M., Okosun, I. S., Seale, J. P., & Foster, B. (2011). Translation of the National Institutes of Health diabetes prevention program in African American churches. Journal of the National Medical Association, 103(3), 194–202.
Gutierrez, J., Devia, C., Weiss, L., et al. (2014). Health, community, and spirituality: Evaluation of a multicultural faith-based diabetes prevention program. Diabetes Education, 40, 214–222.
Markens, S., Fox, S. A., Taub, B., & Gilbert, M. (2002). Role of black churches in health promotion programs: Lessons from the Los Angeles mammography promotion in churches program. American Journal of Public Health, 92, 805–810.
Baskin, M. L., Resnicow, K., & Campbell, M. K. (2001). Conducting health interventions in black churches: A model for building effective partnerships. Ethnicity and Disease, 11(4), 823–833.
Carter-Edwards, L., Jallay, Y. B., Goldmon, M. V., Roberson, J. T., & Hoyo, C. (2006). Key attributes of health ministries in African American churches: An exploratory survey. North Carolina Medical Journal, 67(5), 345–350.
Lasater, T. M., Becker, D. M., Hill, M. N., & Gans, K. M. (1997). Synthesis of findings and issues from religious-based cardiovascular disease prevention trials. Annals of Epidemiology, 7(7), S46–S53.
Dodani, S., Kramer, K., Williams, L., Crawford, S., & Kriska, A. (2009). Fit Body & Soul: A church-based behavioral lifestyle program for diabetes prevention in African-Americans. Ethnicity and Disease, 19, 135–141.
Davis-Smith, M. (2007). Implementing a diabetes prevention program in a rural African-American church. Journal of the National Medical Association, 99(4), 440–445.
Williams, L. B., Sattin, R. W., Dias, J., et al. (2013). Design of a cluster-randomized controlled trial of a diabetes prevention program within African-American churches: The Fit Body and Soul study. Contemporary Clinical Trials, 34, 336–347.
American Diabetes Association. (2014). Diagnosis and classification of diabetes mellitus. Diabetes Care, 37, S81–S90.
Kramer, M. K., Kriska, A. M., Venditti, E. M., et al. (2009). Translating the diabetes prevention program: A comprehensive model for prevention training and program delivery. American Journal of Preventive Medicine, 37, 505–511.
Department of Health and Human Services. (2008). 2008 physical activity guidelines for Americans. http://www.health.gov/paguidelines/pdf/paguide.pdf.
Center for Disease Control, Prevention. (2014). Healthy weight. http://www.cdc.gov/healthyweight/assessing/bmi/adult_BMI/index.html. Retrieved April 9, 2014.
West, D. S., Prewitt, T. E., Bursac, Z., & Felix, H. C. (2008). Weight loss of black, white, and Hispanic men and women in the diabetes prevention program. Obesity, 16, 1413–1420.
Newlin, K., Dyess, S. M., Allard, E., Chase, S., & Melkus, G. D. (2012). A methodological review of faith-based health promotion literature: Advancing the science to expand delivery of diabetes education to Black Americans. Journal of Religion and Health, 51, 1075–1097.
Demark-Wahnefried, W., McClelland, J., Jackson, B., et al. (2000). Partnering with African American churches to achieve better health: Lessons learned during the black churches united for health 5 a day project. Journal of Cancer Education, 15(3), 164–167.
Karanja, N., Stevens, V. J., Hollis, J. F., & Kumanyika, S. K. (2002). Steps to soulful living (steps): A weight loss program for African-American women. Ethnicity and Disease, 12(3), 363–371.
DeHaven, M. J., Hunter, I. B., Wilder, L., Walton, J. W., & Berry, J. (2004). Health programs in faith-based organizations: Are they effective? American Journal of Public Health, 94(6), 1030–1036.
Ali, M. K., Echouffo-Tcheugui, J. B., & Williamson, D. F. (2012). How effective were lifestyle interventions in real-world settings that were modeled on the diabetes prevention program? Health Affairs, 31(1), 67–75.
Dunkley, A. J., Bodicoat, D. H., Greaves, C. J., et al. (2014). Diabetes prevention in the real world: Effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations: A systematic review and meta-analysis. Diabetes Care, 37(4), 922–933.
Fitzgibbon, M. L., Stolley, M. R., Ganschow, P., et al. (2005). Results of a faith-based weight loss intervention for black women. Journal of the National Medical Association, 97(10), 1393–1402.
Flegal, K. M., Kit, B. K., Orpana, H., & Graubard, B. I. (2013). Association of all-cause mortality with overweight and obesity using standard body mass index categories. Journal of the American Medical Association, 309(1), 71–82.
Schoenborn, C. A., Adams, P. F., & Peregoy, J. A. (2013). Health behaviors of adults: United States, 2008–2010. National Center for Health Statistics. Vital Health Statistics, 10(257), 44–59.
Craig, C. L., Marshall, A. L., Sjostrom, M., et al. (2003). International physical activity questionnaire: 12-country reliability and validity. Medicine and Science in Sports and Exercise, 35(8), 1381–1395.
Helmerhost, H. J. F., Brage, S., Warren, J., Besson, H., & Ekelund, U. (2012). A systematic review of reliability and objective criterion-related validity of physical activity questionnaires. International Journal of Behavioral Nutrition and Physical Activity, 9, 103.
Goldfinger, J. Z., Arniella, G., Wylie-Rosett, J., & Horowitz, C. R. (2008). Project HEAL: Peer education leads to weight loss in Harlem. Journal of Health Care for the Poor and Underserved, 19(1), 180–192.
McNabb, W., Quinn, M., Kerver, J., Cook, S., & Karrison, T. (1997). The PATHWAYS church-based weight loss program for urban African-American women at risk for diabetes. Diabetes Care, 20(10), 1518–1523.
Allicock, M., Johnson, L. S., Leone, L., et al. (2013). Promoting fruit and vegetable consumption among members of black churches, Michigan and North Carolina, 2008–2010. Preventing Chronic Disease, 10, 120161. http://www.cdc.gov/pcd/issues/2013/12_0161.htm. Retrieved April 9, 2014.
Acknowledgments
This study was funded by the National Institutes of Health, R18DK082401, which had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We also confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Rights and permissions
About this article
Cite this article
Sattin, R.W., Williams, L.B., Dias, J. et al. Community Trial of a Faith-Based Lifestyle Intervention to Prevent Diabetes Among African-Americans. J Community Health 41, 87–96 (2016). https://doi.org/10.1007/s10900-015-0071-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10900-015-0071-8