Abstract
The number of individuals receiving genetic counseling for hereditary breast and ovarian cancer syndrome has steadily risen. To triage patients for genetic counseling and to help reduce the amount of time needed by a genetic counselor in direct patient contact, many clinics have implemented the use of family history questionnaires. Although such questionnaires are widely used, scant literature exists evaluating their effectiveness. This article explores the extent to which family history questionnaires are being used in Ontario and addresses the utility of such questionnaires in one familial cancer clinic. By comparing the pedigrees created from questionnaires to those updated during genetic counseling, the accuracy and effectiveness of the questionnaires was explored. Of 121 families recruited into the study, 12% acquired changes to their pedigree that led to a revised probability estimate for having a BRCA1 or BRCA2 mutation and 5% acquired changes that altered their eligibility for genetic testing. No statistically significant difference existed between the eligibility for genetic testing prior to and post counseling. This suggests that family history questionnaires can be effective at obtaining a family history and accurately assessing eligibility for genetic testing. Based on the variables that were significantly associated with a change in probability estimate, we further present recommendations for improving the clarity of such questionnaires and therefore the ease of use by patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most prevalent cancer in women, with approximately 22,500 new cases diagnosed each year in Canada alone, and over 5,000 deaths annually (NCIC 2007). Likewise, approximately 2,400 women will be diagnosed with ovarian cancer each year, and approximately 1,700 women will die annually from this disease (NCIC 2007). It is well established that approximately 5–10% of all breast and ovarian cancer cases are hereditary in nature; the majority attributed to mutations in the cancer susceptibility genes BRCA1 (Miki et al. 1994) and BRCA2 (Wooster et al. 1995). Women with mutations in BRCA1 or BRCA2 have a high lifetime risk of developing both breast and ovarian cancer, with reports of breast cancer risk from 36–87%, and ovarian cancer risk from 11–66% (Antoniou et al. 2003; The Breast Cancer Linkage Consortium 1999; Ford et al. 1994; King et al. 2003; Marroni et al. 2004; Satagopan et al. 2001; Thorlacius et al. 1998; Whittemore et al. 1997).
As the knowledge of hereditary breast and ovarian cancer syndrome (HBOCS) has become more widespread, the numbers of individuals seeking genetic counseling and genetic testing has steadily increased. Armed with the knowledge of their genetic testing results many patients seek to modify their cancer screening practices or to opt for more invasive risk reducing strategies. While striving to keep patient waiting lists to a minimum and maximizing the use of a genetic counselors time, many hereditary cancer clinics have adopted the use of a family history questionnaire (FHQ) to obtain medical and family histories prior to scheduling patients for genetic counseling.
While it has traditionally been felt that obtaining a family history, in person, provides an opportunity to contract with the patient and thereby learn more about the patient’s family dynamics and social context, there is no evidence directly supporting that this cannot be accomplished in the absence of obtaining the family history. In fact, by briefly reviewing or updating the patient’s family history as obtained from a FHQ, the opportunity still exists to establish the same rapport to serve as a foundation for genetic counseling. Further, studies indicate that women prefer to know prior to genetic counseling exactly what details of their family history are needed (Hallowell et al. 1997). Those who had gaps in the information they knew about their family history at the time of counseling felt that the risk assessments they were given were not accurate (Hallowell et al. 1997). This data suggests that if women know prior to their appointments what information is required of them they can then provide a more accurate family history and will have greater confidence in their risk assessment and screening recommendations.
In a related study, Ziogas and Anton-Culver found that during personal interviews, patients gave significantly more accurate information regarding first-degree relatives versus second and third-degree relatives (Ziogas and Anton-Culver 2003). This suggests the need for patients to confirm their family history with other family members or family historians prior to genetic counseling. In addition to these studies, Chalmers et. al. mailed out FHQs to patients and discovered that none of the respondents strongly disliked using a FHQ. Further, 50% of these respondents preferred the mailed out questionnaires as it allowed them the opportunity to research their family history in advance of their appointment (Chalmers et al. 2001). Together, these preliminary studies support the notion that providing women with a FHQ prior to counseling, is desirable and may result in more accurate information.
Having a patient’s family history prior to genetic counseling has further benefits from the counselor’s perspective. First, it enables the triage of patients for genetic counseling. A cursory review of the questionnaire can indicate whether a genetic counseling appointment is appropriate, and if so those patients deemed at higher risk scheduled for priority appointments. Secondly, by having the pedigree in advance of the first appointment it allows an opportunity to obtain pathology records, confirming as many cancer diagnoses in the family as possible. By obtaining pathology records prior to the patient’s first visit, the counselor is able to provide the patient with the most accurate risk information and therefore possibly reducing the need for time consuming secondary appointments to review records and reassess patient risks. Third, by having the pedigree prior to the appointment, the genetic counselor is relieved of the time required to obtain the pedigree themselves. The information provided on the questionnaire can easily be translated into a pedigree by a student or support staff, which can then be quickly confirmed with the patient during their visit. Although not directly assessed in this study, it is expected that the use of a FHQ should decrease the amount of time spent in contact with a patient, allowing more patients to be seen, and consequently an increase in counselors’ productivity.
Currently, no evidence exists in the literature supporting the accuracy of a FHQ as a genetic counseling tool. However, limited data suggests that a self-administered FHQ is useful (Brener et al. 1996). Data obtained from this prostate clinic study demonstrated that 47/50 (94%) family histories gathered by FHQ acquired a change post intervention by a study assistant, but that only 2 (4%) had a change to the family’s risk category. This study suggests that while some information may change upon review with the assistant or genetic counselor, the information modified did not have a significant impact on the family’s risk assessment.
Methods
Family History Questionnaire
All new patients seen in the Familial Breast and Ovarian Cancer Clinic (FBOCC) at Princess Margaret Hospital in Toronto, Ontario are mailed a package upon receipt of their doctor’s referral. Included in this package are an introductory letter, the FHQ, a personal medical history questionnaire (PHQ), and two blank release forms to obtain pathology records for family members affected with cancer.
The FHQ itself is a series of questions and tables designed to elicit a three-generation pedigree. Information on some third degree relatives is obtained (first cousins) but not for others (great aunts/uncles or great grandparents). For each person, the table includes a place to provide age or age at death, cause of death, cancer diagnosis, and age at cancer diagnosis. To elicit other family members diagnosed with cancer more distantly related than those asked for specifically by the FHQ, a separate table is provided where these members can be listed, along with an explanation of their exact relationship to the proband. Space is also provided to indicate if the proband has children with more than one partner or if the proband has half siblings. A question regarding ethnicity is also included.
Questions regarding the patient’s personal medical history, including current medications, cancer diagnoses, past surgeries, chemoprevention, and abnormal breast and ovarian cancer screening results are included in the PHQ.
Patients that do not complete their FHQ within 6 months of it being sent are contacted by phone and offered the chance to provide their family history at that time or to have another questionnaire sent by fax, mail, or e-mail. Those patients whose family history was obtained by phone were not eligible to participate in this study.
Frequency of FHQ Use
To determine what proportion of patients return their FHQs, 100 referrals were followed for a 6-month period following the time the FHQ was mailed to them.
Genetic Counseling
At the FBOCC, and in many Canadian familial cancer clinics, genetic counseling is offered as part of a two-step process. During the first appointment, the family history is obtained, a probability estimate for having a BRCA1 or BRCA2 mutation is given to the patient, and a full pre-test counseling session is performed for those patients eligible to provide a blood sample for genetic testing. For those choosing to pursue genetic testing, a second appointment, 4–6 months later, is scheduled for results disclosure. Differences among the Canadian cancer genetics clinics, with respect to these two appointments, are mainly based on when and how the family history is obtained.
Use of FHQs in Ontario
To determine to what extent FHQs are being used in the province, all 22 clinics were contacted by phone to determine what methods they use to obtain a patient’s family history.
Subject Recruitment
To address the effectiveness of the FHQ used by the FBOCC, all new patients whose family histories were obtained by the FHQ and were seen between May 1, 2005 and August 31, 2006 had the opportunity to participate in either the retrospective or prospective arm of the study. Both parts of the study were approved by the hospital’s research ethics board. Between December 1, 2005 and August 31, 2006 participants were recruited prospectively with informed consent at the end of the first counseling session. To increase the number of study participants, a retrospective arm of the study was introduced to capture all new patients that had been previously seen between May 1, 2005 and November 30, 2005. Informed consent was not obtained from patients participating in the retrospective study as the required data was obtained by chart review. For these families, the pedigree printout from Cyrillic (FamyGenetix Ltd 2001) that was created prior to counseling was compared to the handwritten notes on the pedigree added by the genetic counselor during the counseling session.
Patients were excluded from either the prospective or retrospective arm of the study if a member of their family previously had genetic counseling either at the FBOCC or another genetics clinic, as a pedigree had already been created. Participants were also excluded if one or both parts of the FHQ were not completed, if the patient required a translator, or if they were unable to provide a family history (for example due to adoption). Patients seen for more than one appointment were also excluded, as it was not possible to determine what changes were made to the pedigree during the first appointment or the second.
Patients eligible to participate in the prospective study were identified during weekly chart review prior to genetic counseling. These patients were introduced to the study at the end of the first genetic counseling session, prior to leaving the clinic. To minimize coercion wherever possible, participants were consented into the study by another member of the study team. All family histories were reviewed and updated as part of their standard genetic counseling appointment, prior to introduction of the study.
A total of 41 patients were recruited from the retrospective study. Of 163 patients eligible to participate in the prospective study, 80 provided consent to participate, 2 declined, and 81 were excluded for reasons described previously.
Pedigrees
Prior to each genetic counseling session, a pedigree was created from the information found in the FHQ by a member of the study team using the pedigree drawing program, Cyrillic (FamyGenetix Ltd 2001). This pedigree was then reviewed and updated by the genetic counselor during the patient’s first appointment. To assess the accuracy of the FHQ, any information added to the pedigree during the counseling session was compared to that provided by the patient on the FHQ. A total of ten different types of changes were noted when comparing pre-counseling pedigrees to post-counseling pedigrees. The changes included a change to cancer diagnosis, age at cancer diagnosis, current age, age at death, cause of death, death of a family member originally indicated as living, ancestry, incorrectly drawn relationship lines, consanguinity, and the prophylactic use of Tamoxifen by the proband.
To determine how the accuracy of the FHQ could be improved, we sought to determine which of the ten variables listed above contributed to a change in the patient’s probability estimate or genetic testing eligibility. The association between these variables and a change in probability estimate or genetic testing eligibility was tested as described under statistical analysis. Those variables that were statistically significant predictors of a change in probability estimate or genetic testing eligibility signified important areas of the FHQ that needed to be addressed in order to improve its accuracy.
Probability Estimates and Genetic Testing Eligibility
Based on the family history as reported by the patient on the FHQ, each family was given a probability estimate for having a BRCA1 or BRCA2 mutation. Three broad probability estimate categories are in use within our clinic. Low risk implies a chance of having a BRCA1 or BRCA2 mutation of 15% or lower, moderate: 16–30% and high: greater than 30%. These three categories serve the purpose of giving the patient a relative appreciation for their family’s chance to have a mutation. A cut-off of 15% was previously chosen as the cut-off for the low risk category, as the literature at the time the categories were developed suggested that an individual with ovarian cancer had a 12–16% chance of having a BRCA1 or BRCA2 mutation, depending on whether the histology was known (Risch et al. 2001). Because our clinic was initially developed as a hereditary ovarian cancer clinic, we have a high population of patients and families with ovarian cancer. Given that we consider an isolated ovarian cancer to be at low risk for having a BRCA1 or BRCA2 mutation, a cut-off of 15% was selected. Similarly, a risk of 30% was chosen as the cut-off for medium risk families as it separated those families considered by our clinic to be at moderate risk from those to be considered at high risk. For example, two first-degree relatives, one with breast and one with ovarian cancer have a 23–28% chance of having a BRCA1 or BRCA2 mutation and could therefore be considered moderate risk (Risch et al. 2006). In comparison, two first-degree relatives with ovarian cancer have a 35–49% chance of having a mutation and therefore were considered high risk (Risch et al. 2006).
The chance of carrying a BRCA1 or BRCA2 mutation was determined using the BRCAPRO model (Berry et al. 2002). From our experience, this model does not accurately assess the chance to have a mutation in the absence of breast cancer, so data from the ovarian cancer literature was used to more accurately assess genetic risk in these families (Risch et al. 2001, 2006). Probability estimates obtained prior to and post genetic counseling were based strictly on the information provided by the patient on the FHQ or verbally during the counseling session, and did not include modifications based on available pathology reports.
The Ministry of Health establishes eligibility for genetic testing in the province of Ontario. In general, genetic testing is available to an unaffected individual who has a greater than 10% chance of having a BRCA1 or BRCA2 mutation. For a proband diagnosed with breast or ovarian cancer, a defined set of criteria are consulted to determine eligibility (Appendix I). At the time of data collection, three types of genetic testing were offered: Specific mutation analysis for those families with an identified BRCA1 or BRCA2 mutation, an Ashkenazi Jewish panel including the three founder mutations, and full analysis performed by protein truncation testing on all exons of BRCA1 and BRCA2 with complementary sequencing of exons 2 and 5 of BRCA1.
Statistical Analysis
Differences in the distribution of probability estimates before and after genetic counseling were tested using the marginal homogeneity test. This is a generalization of McNemar’s test. Comparison of the eligibility for genetic testing before and after genetic counseling was performed with McNemar’s test. Fisher’s exact test was used to test the univariable association between ten explanatory variables (listed elsewhere) and the two outcomes: 1) change in probability estimate and 2) change in genetic testing eligibility. Each association was based on data from a 2 × 2 contingency table. Confidence intervals for proportions are exact and based on the binomial distribution. Statistical analyses were conducted with SAS version 9.1 and StatXact version 4.
Results
The Use of FHQs in Ontario
In the province of Ontario, there are currently 22 familial cancer clinics. Of these, 13 (59%) report the use of questionnaires to obtain family history information. Six (27%) obtain a pedigree by phone, and 3 (14%) obtain their pedigrees in person during the patient consult. A standard questionnaire does not exist among those clinics reporting the use of a FHQ to obtain family history information.
Patient Use of FHQs
One hundred referrals to The Familial Breast and Ovarian Cancer Clinic at Princess Margaret Hospital were followed for 6 months from the time of being mailed their referral package. Fifty three percent returned their package within 6 months of being sent it.
Patient Demographics
One hundred twenty one patients participated in the study (Table 1). Of these, 99% were women, 89% were Caucasian, and 40% had a current or previous diagnosis of any type of cancer. Approximately half of all participants were professionals or semi-professionals with a mean age of 47 (range 21–81).
Changes Made to Pedigrees
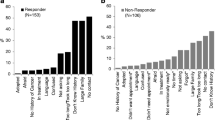
Upon reviewing the changes made to pedigrees during update by the genetic counselor, it was noted that ten different types of changes existed. These changes included: a change to a cancer diagnosis, age at cancer diagnosis, current age of a living family member, age at death, cause of death, death of a family member originally indicated as living, ancestry, incorrectly drawn relationship lines, consanguinity, and the prophylactic use of Tamoxifen by the proband. Of 121 pedigrees, 92% acquired changes or additional information during the genetic counseling session. Distribution of changes made to pedigrees is summarized in Fig. 1. The information most often changed on the pedigree was a change to a family member’s cancer diagnosis (70/121, 58%), followed by a change to any living relative’s age (54/121, 45%).
Probability Estimates
Prior to genetic counseling, each family was given a probability estimate of low, moderate, or high for having a BRCA1 or BRCA2 mutation. Once the pedigree was updated during the genetic counseling session, the family history was reviewed and a second probability estimate was assigned. Any revisions made to the probability estimate were based solely on information provided by the patient and not based on received pathology records. Similarly, each family was assessed as being eligible for genetic testing prior to and post genetic counseling, based on Ministry of Health eligibility criteria in the province of Ontario (Appendix I). Table 2 summarizes the numbers of families with changes to their probability estimate or eligibility for genetic testing, following the counseling session. Of 121 families, 15 (12%, 95% CI 7–20) had changes to their probability estimate, 6/121 (5%, 95% CI 2–10) had changes to their genetic testing eligibility, and 5/121 (4%) had changes to both.
Table 3 summarizes the distribution of probability estimates prior to and post genetic counseling. Of 121 families, two were given a probability estimate on both sides of the family, therefore giving a total of 123 probability estimates. A statistically significant difference was seen among the probability estimates prior to counseling versus post counseling (Table 3). Of the 15 families with changes to their probability estimate, one family had an altered probability estimate on both sides of the family, totaling 16 altered probability estimates. When comparing whether the probability estimates increased or decreased for these families, 13/16 (81%, 95% CI 51–91) had a probability estimate that increased (Table 4).
Of the ten variables changed on the pedigrees during counseling, two were statistically significant predictors of a change in probability estimate; these included ancestry (p = 0.00062) and consanguinity (p = 0.01599). Of the 11 families with a change in ancestry, six had a change in risk assessment. Similarly, of the five families with a change to consanguinity, three had a change in risk assessment. While a change in cancer diagnosis was only a marginally significant predictor of a change in probability estimate (p = 0.05760), a subset of this group was a statistically significant predictor of a probability estimate change; in particular, the addition of a new cancer diagnosis in a family member not listed by the patient on the FHQ (p = 0.04675). Of 27 families that had a family member with cancer added to the pedigree, nine had a change in risk assessment. Each of these factors contributed to how the family history information was entered into BRCAPRO therefore altering the probability estimate. If consanguinity was identified in the family, it implied that cancers on both sides of the family could be considered through the common ancestors, thereby resulting in an increased probability estimate. All families with an ancestry change were Ashkenazi Jewish but did not report so on the questionnaire. Adding this information to the risk assessment automatically increased the likelihood of a BRCA1 or BRCA2 mutation. Likewise, if the patient reported during counseling that another family member had cancer, this could also contribute to an increased probability estimate, depending on the type of cancer reported.
Genetic Testing Eligibility
Prior to genetic counseling, each family was assessed as being eligible for genetic testing according to eligibility criteria as set forth by the Ministry of Health in the Province of Ontario (Appendix I). Of the 121 families participating in the study, 83 (69%) were eligible for genetic testing based solely on the information provided in their FHQ. Upon update of their family histories during genetic counseling, one family previously eligible for genetic testing became ineligible and five families previously ineligible became eligible for testing (Table 5). In total, 6/121 families (5%) acquired changes to their family history resulting in a change in their eligibility status for genetic testing. Generally, an increase in genetic testing eligibility was observed post counseling (Table 4); however, no statistically significant difference was seen between genetic testing eligibility prior to and post counseling. Change in genetic testing eligibility was most often accompanied by a change in probability estimate, as seen in 5/6% or 83% of the families.
Discussion
The number of individuals seeking genetic counseling over the years has steadily risen. In order to triage patients for genetic testing and to efficiently make use of a genetic counselors time, many hereditary cancer clinics have adopted the use of a FHQ. Currently, in the province of Ontario approximately 60% of hereditary cancer clinics are using FHQs to obtain a family history in the absence of data evaluating their effectiveness. This study assesses the efficacy of the FHQ in use by one hereditary breast and ovarian cancer clinic and demonstrates that a FHQ is an effective tool for assessing genetic testing eligibility.
To evaluate the effectiveness of obtaining family histories prior to genetic counseling by FHQ, a comparison was made between pedigrees created from 121 completed FHQs to those updated during genetic counseling. The changes made to these pedigrees were analyzed for their impact on the probability estimate and genetic testing eligibility of these patients. While most pedigrees acquired a change during update by the genetic counselor (92%), only a small number (12%) of all families had a change to their probability estimate, with even fewer having a change to their eligibility for genetic testing (5%).
Results of this study demonstrate that while the change in probability estimates prior to and post genetic counseling were statistically significant, there was no statistically significant change in genetic testing eligibility. Because a change to genetic testing eligibility may impact a patient more than a change to their probability estimate, by changing the availability of an appointment or whether or not genetic testing can be offered, the FHQ was considered an efficient and accurate way to triage patients for genetic counseling and genetic testing.
In our clinic, the FHQ is reviewed upon receipt by the genetic counselor. If considered appropriate, the patient is triaged for an appointment based on this information. Those patients offered an appointment for genetic counseling will then have their FHQ translated into a pedigree by one of the clinic’s support staff. It is clear that the benefit of having this information for triage purposes is important in a universal health care system where limited resources for genetic counseling and genetic testing are available, and not every patient can be offered an appointment.
By having the patient provide their family history using an FHQ, the amount of time spent by the counselor in direct patient contact is reduced. Although not directly measured in this study, from our experience the time spent by the genetic counselor to briefly review and update the family history is significantly less than the time required to obtain the information by directly questioning the patient. The use of the FHQ does however have further benefit for the genetic counselor as it may allow for a more accurate family history to be provided by the patient (Chalmers et al. 2001; Ziogas and Anton-Culver 2003). By using the FHQ, patients are allowed the time to obtain their family history from knowledgeable relatives and to return the questionnaire when complete. As previously described, our clinic includes blank pathology release forms in the family history package that includes instructions for the patient to obtain signed release from affected family members. By obtaining an accurate family history and pathology if possible, prior to the first genetic counseling appointment, the need for a second appointment to assess this information for genetic testing eligibility is reduced. As a result, the efficiency of the genetic testing process is increased.
In the hopes of improving the accuracy of the FHQ in use by our clinic, we assessed the changes made to the pedigrees that were statistically significant predictors of a change in probability estimate. Given that the overall numbers of families with changes to their probability estimate are small, caution must be used in interpreting this data. However, this data is useful for discerning which areas of the FHQ were not being completed accurately by patients and therefore required improvement or clarification. The information most commonly added during counseling involved a change to a cancer diagnosis, particularly in distant family members such as 3rd or 4th degree relatives. While a change to a cancer diagnosis in itself was not a statistically significant predictor of a probability estimate change, the addition of a previously unreported relative with cancer was. This suggested a need to improve wording on the FHQ that asks about “other” family members diagnosed with cancer. In order to obtain cancer information regarding more distantly related family members that were not directly asked about on the FHQ, a general question at the end of the questionnaire asked the patient to list any other family member diagnosed with cancer. Often this question was left blank or patients listed all family members with cancer that had been previously recorded in other parts of the questionnaire. To clarify what family members should be listed, the wording was rephrased from “other family members with cancer” to “other family members with cancer that are NOT listed above”.
Changes to consanguinity and ancestry were also statistically significant predictors of a probability estimate change. To address this, a question regarding consanguinity was added to the FHQ as none previously existed. Secondly, those families that recorded a change in ancestry were primarily Ashkenazi Jewish, but had only indicated their Eastern European ancestry. It is apparent that many of these families may consider their ethnicity to be Polish or Russian rather than Ashkenazi Jewish, naturally not recognizing the importance for risk assessment purposes. As such, the FHQ has been changed from requesting the patient’s ethnicity (with Ashkenazi Jewish as an example) to requesting the patient’s ethnicity, followed by a yes/no question specifically asking if the patient is Ashkenazi Jewish.
Although a change to the age of a living relative, a change to age at cancer diagnosis, or a change to age at death were not significantly associated with a change in probability estimate, they were the second, third, and forth most common changes made to pedigrees. This suggests a need for improvement in the way information regarding ages is obtained. As the FHQ asked for family member’s dates of birth and dates of death, many patients did not have this information and left these columns blank on the FHQ. To improve the effectiveness of the questionnaire, all questions requesting a date were changed to request an age. Because it is expected that the proband will more likely know their relatives’ approximate age rather than exact age, instructions at the beginning of the FHQ were also added asking the proband to provide an age range if the exact age, age at cancer diagnosis, or age at death are unknown.
On many completed FHQs, it was further noted that patients were not clearly expressing their relationship to more distant family members that had been diagnosed with cancer. To help clarify family relationships, examples provided on the FHQ were changed. Previously the examples on the FHQ asked the patient to describe their relative as a maternal or paternal family member. For example, a great aunt is listed as a maternal great aunt. However, when drawing the pedigree it was not possible to know if the great aunt was a sister to the maternal grandmother or the maternal grandfather. As such, the example now asks the patient to list the great aunt as the maternal grandmother’s sister.
Because some patients were returning their FHQs listing only those family members with cancer, instructions at the beginning of the FHQ were added that included a statement requesting that all family members be listed, even those without cancer. In addition to more general instructions regarding how to complete the questionnaire, it is now recommended in the instructions that patients consult other family members for help in completing the FHQ. To improve ease of use, each question on the FHQ is now numbered and unnecessary questions such as “place where person lives” have been removed.
Based on results of this study we have identified ways to improve ease of use of the FHQ by patients and thereby further improve the accuracy of information obtained. Data from this study has been used to revise the FHQ in use at our hereditary breast and ovarian cancer clinic (Appendix II). However, further research will be required to confirm if these modifications do in fact further improve effectiveness and accuracy of the FHQ. Given that only 53% of patients return their completed questionnaires within 6 months of receiving them, it is important to understand why patients do not return their questionnaires in order to try and maximize this response rate. We are currently exploring this on a larger scale. Although a limitation of our study is the relatively homogeneous nature of the study sample, it accurately reflects the demographics of the patients seen in our clinic. However, to better assess the improvements made to the FHQ, a more diverse population should be sampled.
In summary, we have demonstrated that a FHQ is a valuable pedigree-taking tool that can be easily administered to patients in a familial cancer clinic. The use of a FHQ provides patients with an opportunity to consult family historians to obtain more accurate information than would be provided if they were contacted by phone or asked to give their family history in-person during genetic counseling. Overall, the FHQ is effective at obtaining an accurate family history that can be used for assessing eligibility for genetic testing and can therefore be an important tool for the triage of patients, particularly in a time of reduced health care spending.
References
Antoniou, A., Pharoah, P. D., Narod, S., et al. (2003). Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. American Journal of Human Genetics, 72, 1117–1130. doi:https://doi.org/10.1086/375033.
Berry, D. A., Iversen, E. S,. Jr, Gudbjartsson, D. F., Hiller, E. H., Garber, J. E., Peshkin, B. N., et al. (2002). BRCAPRO validation, sensitivity of genetic testing of BRCA1/BRCA2, and prevalence of other breast cancer susceptibility genes. Journal of Clinical Oncology, 20(11), 2701–2712. doi:https://doi.org/10.1200/JCO.2002.05.121.
The Breast Cancer Linkage Consortium. (1999). Cancer risks in BRCA2 mutation carriers. Journal of the National Cancer Institute, 91(15), 1310–1316. doi:https://doi.org/10.1093/jnci/91.15.1310.
Brener, D., Schulz, C., Schluger, A., & Offit, K. (1996). A self-administered family history questionnaire (FHQ) utilized in an outpatient setting to assess cancer risk. American Journal of Human Genetics, 59, A333.
Chalmers, K. I., Luker, K. A., Leinster, S. J., Ellis, I., & Booth, K. (2001). Information and support needs of women with primary relatives with breast cancer: development of the information and support needs questionnaire. Journal of Advanced Nursing, 35(4), 497–507. doi:https://doi.org/10.1046/j.1365-2648.2001.01866.x.
FamyGenetix Ltd. (2001). Cyrillic 3. Oxford: UK.
Ford, D., Easton, D. F., Bishop, D. T., et al. (1994). Risks of cancer in BRCA1-mutation carriers. Lancet, 343, 692–695. doi:https://doi.org/10.1016/S0140-6736(94)91578-4.
Hallowell, N., Murton, F., Statham, H., Green, J. M., & Richards, M. P. (1997). Women's need for information before attending genetic counselling for familial breast or ovarian cancer: a questionnaire, interview, and observational study. BMJ (Clinical Research Ed), 281, 7076–283.
King, M. C., Marks, J. H., Mandell, J. B., & New York Breast Cancer Study Group. (2003). Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science, 302, 643–646. doi:https://doi.org/10.1126/science.1088759.
Marroni, F., Aretini, P., D’Andrea, E., Caligo, M. A., Cortesi, L., Viel, A., et al. (2004). Penetrances of breast and ovarian cancer in a large series of families tested for BRCA1/2 mutations. European Journal of Human Genetics, 12(11), 899–906. doi:https://doi.org/10.1038/sj.ejhg.5201256.
Miki, Y., Swensen, J., Shattuck-Eidens, D., Futreal, P. A., Harshman, K., Tavtigian, S., et al. (1994). A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science, 266(5182), 66–71. doi:https://doi.org/10.1126/science.7545954.
NCIC. (2007). Canadian Cancer Statistics 2007. Toronto: Canada.
Risch, H. A., McLaughlin, J. R., Cole, D. E., Rosen, B., Bradley, L., Kwan, E., et al. (2001). Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. American Journal of Human Genetics, 68(3), 700–710. doi:https://doi.org/10.1086/318787.
Risch, H. A., McLaughlin, J. R., Cole, D. E., Rosen, B., Bradley, L., Fan, I., et al. (2006). Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada. Journal of the National Cancer Institute, 98(23), 1694–1706.
Satagopan, J. M., Offit, K., Foulkes, W., Robson, M. E., Wacholder, S., Eng, C. M., et al. (2001). The lifetime risks of breast cancer in Ashkenazi Jewish carriers of BRCA1 and BRCA2 mutations. Cancer Epidemiology, Biomarkers & Prevention, 10(5), 467–473.
Thorlacius, S., Struewing, J. P., Hartge, P., Olafsdottir, G. H., Sigvaldason, H., Tryggvadottir, L., et al. (1998). Population-based study of risk of breast cancer in carriers of BRCA2 mutation. Lancet, 352(9137), 1337–1339. doi:https://doi.org/10.1016/S0140-6736(98)03300-5. see comment.
Whittemore, A. S., Gong, G., & Itnyre, J. (1997). Prevalence and contribution of BRCA1 mutations in breast cancer and ovarian cancer: results from three U.S. population-based case-control studies of ovarian cancer. American Journal of Human Genetics, 60(3), 496–504.
Wooster, R., Bignell, G., Lancaster, J., Swift, S., Seal, S., Mangion, J., et al. (1995). Identification of the breast cancer susceptibility gene BRCA2. Nature, 378(6559), 789–792. doi:https://doi.org/10.1038/378789a0.
Ziogas, A., & Anton-Culver, H. (2003). Validation of family history data in cancer family registries. American Journal of Preventive Medicine, 24(2), 190–198. doi:https://doi.org/10.1016/S0749-3797(02)00593-7.
Acknowledgments
The authors wish to acknowledge Kara Hitchman for her excellent comments and contributions to reviewing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix I: BRCA1/BRCA2 Genetic Testing Criteria in the Province of Ontario
Testing for affected individuals with breast or ovarian cancer
At least one case of cancer:
-
1.
Ashkenazi Jewish and breast cancer <50 years, or ovarian cancer at any age. Note: testing limited to ethnic specific mutations, unless other criteria given in this list are met.
-
2.
Breast cancer <35 years of age.
-
3.
Male breast cancer.
-
4.
Invasive serous ovarian cancer at any age.
At least two cases of cancer on the same side of the family:
-
5.
Breast cancer <60 years, and a first or second-degree relative with ovarian cancer or male breast cancer.
-
6.
Breast and ovarian cancer in the same individual, or bilateral breast cancer with the first case <50 years.
-
7.
Two cases of breast cancer, both <50 years, in first or second-degree relatives.
-
8.
Two cases of ovarian cancer, any age, in first or second-degree relatives.
-
9.
Ashkenazi Jewish and breast cancer at any age, and any family history of breast or ovarian cancer. Note: testing limited to ethnic specific mutations, unless other criteria given in this list are met.
At least three cases of cancer on the same side of the family:
-
10.
Three or more cases of breast or ovarian cancer at any age.
Testing for unaffected individuals (this should be done only if affected individuals are unavailable e.g. deceased)
-
11.
Relative of individual with known BRCA1 or BRCA2 mutation. Note: specific family mutation only tested.
-
12.
Ashkenazi Jewish and first or second-degree relative of individual with: breast cancer <50 years, or ovarian cancer at any age, or male breast cancer, or breast cancer at any age, with positive family history of breast or ovarian cancer. Note: testing limited to ethnic specific mutations, unless other criteria are met.
-
13.
A pedigree strongly suggestive of hereditary breast/ovarian cancer, i.e. risk of carrying a mutation for the individual being tested is >10%.
Appendix II: Revised Family History Questionaire



Rights and permissions
About this article
Cite this article
Randall Armel, S., McCuaig, J., Finch, A. et al. The Effectiveness of Family History Questionnaires in Cancer Genetic Counseling. J Genet Counsel 18, 366–378 (2009). https://doi.org/10.1007/s10897-009-9228-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10897-009-9228-x