Abstract
Based on boron-dipyrromethene (BODIPY), taking 2-hydroxy-N-(2-hydroxyphenyl)benzamide as recognition site, a new fluorescent probe HHPBA-BODIPY aimed at sensitively detecting Cu ions was designed, synthesized and characterized.The emission spectra of HHPBA-BODIPY exhibited an intensive green fluorescence around 510 nm, with a maximum absorption near 500 nm. When Cu2+ ions are present, the fluorescence at 510 nm can be quenched with a good linearity between the copper ion concentrationand the fluorescence intensity and the detection limit is 0.35 μM. HHPBA-BODIPY is also selective toward Cu2+, while other metal ions show no interfere except Fe3+ and Cr3+ ions. In addition, HHPBA-BODIPY also proved efficient to detect Cu2+ in water samples which offers the possibility to detect trace amount of Cu2+ for environmental monitoring. Copper ions; BODIPY; fluorescent probe.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cu2+ is an significant trace element and it acts as catalyst and structural cofactor for enzyme in a series of biochemical reactions and metabolic processes [1,2,3,4]. However, current evidences have pointed out that excessive intake of Cu2+ will increase the risk of neurological diseases including Parkinson’s disease and Alzheimer disease [5,6,7]. In addition, Cu2+ is also a common pollutant for the fishery industry, and its toxicity will threaten the survival of many aquatic organisms [8,9,10]. Therefore, the rapid and simple analysis of trace Cu2+ is of great significance in environmental monitoring.
Comparing to traditional methods, fluorescence analysis has aroused extensive interests in various fields including biochemistry [11,12,13], analytical chemistry [14,15,16], diagnostics [17,18,19] and molecular biology [20,21,22] for its high selectivity, well sensitivity, simple analysis procedure and fast response. Up to now, a series of fluorescent probes based on coordination with Cu2+ have been designed and developed. Various chemical scaffolds such as rhodamine [23,24,25,26,27], naphthalimide [28, 29], dansyl derivatives [30, 31] were applied to design coordinative fluorescent probes and they exhibited excellent properties. Among these scaffolds, 4,4-difluoro-4-bora-3a,4a-diaza-s-indacence(BODIPY) perhaps is the highest potential candidate for its relative high molar absorption coefficients, high fluorescence quantum yields, resistance towards self-aggregation and high intensity emission peaks with narrow bandwidth. Furthermore, the spectroscopic properties of BODIPY can be controllably adjusted through various methods at appropriate positions [32,33,34,35]. Therefore, A fluorescent probe molecule HHPBA-BODIPY was synthesized by introducing amide receptors into the BODIPY framework, and phenolic hydroxyl and carbonyl groups were attached to the skeleton as donors, the coordination between Cu2+ and HHPBA-BODIPY resulted in changes of fluorescence absorption and emission spectra, which indicates the copper content in certain solutions. The fluorescence properties and the detective ability of HHPBA-BODIPY towards Cu2+ was investigated and evaluated in this work.
Experimental
Reagents
In this work all the reagents are of analytical level and used without any further purification. Cation solutions were prepared from NaNO3, KNO3, Ca(NO3)2·4H2O, Mg(NO3)2·6H2O, Fe(NO3)3·9H2O, Cd(NO3)2·4H2O, Cr(NO3)3·9H2O, Zn(NO3)2·6H2O, Ni(NO3)2·6H2O, Co(NO3)2·6H2O, Ba(NO3)2, Pb(NO3)2, Al(NO3)3·9H2O, HgCl2. Deionized water was purified using distillation equipment. HEPES solution was prepared through solving calculated amount of solids in deionized water. Tap water and the Yellow River water were used as water samples. Yellow River water samples were used after being filtered with a 2.5 μm filter membrane, while tap water samples were used directly without any treatment. Column chromatography experiments were conducted over silica gel (200–300 mesh).
Apparatus
1H NMR and 13C NMR spectra were measured on Varian INOVA 600 MHz NMR spectrometer with TMS as internal standard. Ultraviolet-visible absorption spectra were measured by Perkin Elmer Lambda 35 spectrometer. High resolution mass spectra (HRMS) were obtained on a LTQ-Obitrap-ETD (Thermo Scientific) spectrometer, ESI-MS tests were conducted with a Bruker micrOTOF II spectrograph and fluorescence spectra were recorded by an Edingburgh Instruments FLS920 luminescence spectrometer. Absorption and emission spectra were measured in quartz cuvettes with 1 cm path length. The pH data of solutions were determined by a Sartorius pH meter.
Synthetic Procedures
Synthesis of T2 Compound T2 was prepared according to the reported procedure [36]. 836.3 mg 3-nitro-4-hydroxybenzaldehyde and 1 mL 2,4-dimethyl pyrrole were added into a 500 mL round-bottomed flask, 350 mL CH2Cl2 were introduced to the solution then. At room temperature this solution was stirred for 6-7 h in nitrogen atmosphere, then dichloromethane solution containing 1.2323 g tetrachlorobenzoquinone was introduced to the solution and then mixed for 30 min. Finally, a mixed solution of 6.6 mL diisopropylethylamine and 7.5 mL boron trifluoride ethyl ether was added to the solution slowly by using drop funnel, then further stir the mixture for 1 h. Later the reactant was washed by using distilled water for 2–3 times, and the organic phase was dehydrated with anhydrous Na2SO4, and then purified by conducting column chromatography (dichloromethane: ethyl acetate: n-hexane = 1:0.5:5), and 367.3 mg red solids were obtained(yield 19.07%). 1H NMR (CDCl3, 600 MHz), δ(ppm): 1.46 (s, 6H), 2.56 (s, 6H), 6.02 (s, 2H), 7.33 (d, J = 6 Hz, 1H), 7.52 (d, J = 6.0 Hz, 1H), 8.10 (s, 1H), 10.70 (s, 1H); 13C NMR (DMSO-d6, 150 MHz), δ(ppm): 14.65, 15.10, 118.86, 121.21, 121.84, 125.02, 127.22, 131.56, 133.97, 137.25, 142.56, 155.33, 156.63;
Synthesis of T3 T3 was synthesized basing on the method reported by Lu H. et.al. [36]. In 10 mL methanol a certain amount of compound T2 was dissolved, then 564.5 mg iron powder, 4 mL HCl solution (0.6 M) and 6 mL H2O were added into the T2 solution and the resulting mixture was heated and mixed under reflux condition for 2 h. To monitor the process thin-layer chromatography(TLC) was applied. Later the product was cooled to room temperature naturally and centrifuged. After this, it was washed by using methanol several times until the supernatant was clear. Then, by using reduced pressure distillation the washing solution was removed, the products were separated through a silica gel column (dichloromethane: ethyl acetate: n-hexane = 1:1:5). The final product is 154.7 mg red solid with a yield of 77.8%. 1H NMR (DMSO-d6, 600 MHz) and δ(ppm): 1.51 (s, 6H), 2.41 (s, 6H), 4.74 (s, 2H), 6.12 (s, 2H), 6.28 (d, J = 6.0 Hz, 1H), 6.47 (s, 1H), 6.77 (d, J = 6 Hz, 1H), 9.43 (s, 1H); 13C NMR (DMSO-d6, 150 MHz), δ(ppm): 13.98, 14.14, 113.02, 114.76, 115.15, 120.87, 124.81, 131.08, 137.77, 142.77, 143.76, 144.59, 154.0.
Synthesis of HHPBA-BODIPY HHPBA-BODIPY was synthesized according to reported literature [37]. 81.1 mg carbonyl diimidazole was dissolved in 15 mL anhydrous tetrahydrofuran, then 69.1 mg salicylic acid was added. At room temperature the mixture reacted for 0.5 h under N2 protection, then 195.3 mg compound T3 was added. After reacting at room temperature for 1 h, the mixture were heated under reflux condition, then the reaction continued around 24 h. To monitor the process TLC method was applied. When reaction finished, via rotary evaporation the solvent was removed, then the product was separated through a silica gel column (ethyl acetate: dichloromethane: petroleum ether = 1:1:5), and the component having a Rf of 0.13 was collected. Again to remove the solvent rotary evaporation was conducted and then the product was recrystallized (dichloromethane: n-hexane = 1:1) to afford compound HHPBA-BODIPY(75.5 mg with a yield of 35.16%). 1H NMR (DMSO-d6, 600 MHz), δ(ppm): 1.49 (s, 6H), 2.43 (s, 6H), 6.16 (s, 2H), 6.88 (d, J = 12 Hz, 2H), 6.95 (t, J = 6.0 Hz, 1H), 6.99 (d, J = 7 Hz, 1H), 7.06 (d, J = 6 Hz, 1H), 7.39 (t, J = 6 Hz, 1H), 7.96(d, J = 12 Hz, 1H), 8.28 (s, 1H), 10.56 (s, 1H), 10.91 (s, 1H), 11.71 (s, 1H); 13C NMR (DMSO-d6, 150 MHz), δ/ppm: 14.14, 14.17, 115.20, 116.80, 118.64, 119.57, 119.67, 121.13, 123.03, 124.31, 127.87, 130.67, 131.06, 133.33, 142.45, 142.69, 147.41, 154.47, 156.16, 163.48; HRMS (ESI), found:[M-H] + 474.1857, HHPBA-BODIPY requires [M-H] + 474.1801.
Results and Discussions
Synthesis
Based on the synthetic route showed in Scheme 1 HHPBA-BODIPY was synthesized and the product compound was characterized through HRMS, 1H NMR and 13C NMR (Fig. S1, S2, S3). These results attested that the amide, phenolic hydroxyl and carbonyl groups have been successfully attached to the BODIPY scaffold.
Spectroscopic Properties
The absorption and emission spectra of HHPBA-BODIPY are determined in EtOH solution, and its spectral shape is similar to that of most BODIPY fluorescent dyes. Normalized absorption and emission spectra of HHPBA-BODIPY are presented in Fig. S4. It has a strong absorption band at 499 nm (ε = 8.44 × 104 cm−1•M−1), and there is a weak absorption shoulder peak near 470 nm. The fluorescence emission spectrum showed a maximum intensity at 510 nm with a small Stokes shift of 11 nm, which corresponds to the S1-S0 transition of HHPBA-BODIPY. The quantum yield of the HHPBA-BODIPY fluorescent molecule, determined by using the integrating sphere measurement, was 0.06, and the fluorescence lifetime was 2.03 ns (45.46%), 0.4878 ns (54.54%).
Responses towards Cu2+
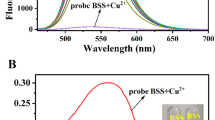
When a certain amount of Cu2+ is added to the HHPBA-BODIPY solution, the spectral change is presented in Fig. 1, and the fluorescence emission at 510 nm is quenched. The absorption spectrum of HHPBA-BODIPY also changed after Cu2+ addition. The absorption peak at 499 nm was attenuated, and the maximum absorption wavelength blue-shifted to 497 nm. In ultraviolet region, the absorption band of HHPBA-BODIPY at 310 nm was attenuated, and the absorption peak at 330 nm is enhanced. These spectral changes indicate that the HHPBA-BODIPY probe molecule may coordinated with Cu2+.
The fluorescence intensity at 510 nm shows a good linear relationship with the copper ion content (Fig.2). When the Cu2+ concentration is between 0 and 7.5 μM, the linear correlation coefficient R2 = 0.995 (n = 15).
Detection Limit
Figure 3 shows the relationship between the copper ions concentration and the fluorescence intensity of HHPBA-BODIPY at low concentration. By measuring the standard deviation (Sb = 1450) of HHPBA-BODIPY without adding Cu2+, the detection limit of HHPBA-BODIPY is 0.35 μM by using the equation [38](LOD = K × Sb/S), which indicates that HHPBA-BODIPY possesses good detection ability towards copper ions.
Reaction Mechanism
According to the variations of emission spectra and absorption spectra, the HHPBA-BODIPY coordination with Cu2+ may be the cause of the fluorescence quenching. Figure 4 exhibits that the probe molecule fluorescence intensity changes with Cu2+ equivalent added in HHPBA-BODIPY solution. When the Cu2+ concentration is greater than 1.5 times of the probe molecule concentration, with the copper ion addition, the probe molecule fluorescence intensity is essentially unchanged, and it can be inferred that the probe molecule is likely to be 1:1 coordinated with Cu2+.
The method of equivalent molarity is often used to determine the coordination constant of a complex. For fluorescence quenching systems, the coordination number of the complex can also be determined using the deformation of the method of equivalent molarity [39]. Figure 5 shows the experimental results. It can be seen that the value of I0[1-f(Cu2+)]-I is maximized when the relative content of Cu2+ is between 0.5 and 0.6, that is, the composition of the complex is ML or M3L2.The probe molecule HHPBA-BODIPY may form a 1:1 complex or a 2:3 complex during the identification of copper ions. ESI-MS analysis of the complex revealed a composition with a coordination ratio of 1:1 in the spectrum (Fig. S5) [Cu(HHPBA-BODIPY)] + (Cal:538.1175; Found: 538.1840), and the isotope analysis is also basically consistent with the calculated values.Therefore, HHPBA-BODIPY is most likely to coordinate with copper ions with a coordination ratio of 1:1 in the process of recognizing copper ions.
pH and Time Effect
pH value is an extremely important parameter for probe molecules. In this part the pH effect of HHPBA-BODIPY towards Cu2+ detection was explored. The results are presented in Fig. 6. The fluorescence intensity at 510 nm varies greatly with pH. Under acidic conditions, the probe molecule has a strong fluorescence emission, and the fluorescence intensity basically does not change with pH. When pH > 7, the fluorescence of the probe molecule gradually attenuates as the increase of solution alkalinity, and is finally almost completely quenched. After adding a certain amount of Cu2+, the probe molecule has almost no response to Cu2+ when pH < 6, while the addition of Cu2+ can quench the fluorescence of HHPBA-BODIPY when pH > 6. The protonation of phenolic hydroxyl groups of HHPBA-BODIPY under acidic conditions may be related to this result. Under acidic conditions, the protonated phenolic hydroxyl group has weaker coordination ability and cannot chelate with Cu2+, resulting in the consequence that the fluorescence of HHPBA-BODIPY molecules does not change with the addition of Cu2+. As the alkalinity of the solution increases, phenolic hydroxyl groups begin to dissociate, and the ability of HHPBA-BODIPY to chelate with Cu2+ is enhanced, thereby quenching the fluorescence of HHPBA-BODIPY.
The response time is also an important indicator of the performance of the probe molecule. In general, it is desirable that the probe molecule respond as quickly as possible to the analyte. HHPBA-BODIPY responds very rapidly to Cu2+, as shown in Fig. 7, after Cu2+ was added, the fluorescence of HHPBA-BODIPY is quickly quenched and remains stable during the measurement time. This characteristic of the probe can be used to real-time monitor the environmental samples.
Selectivity of HHPBA-BODIPY
Selectivity is another important performance indicator for fluorescent probe molecules. The selectivity of HHPBA-BODIPY probe towards Cu2+ was compared to other metal cations and was evaluated in this part. The results are shown in Fig. 8, that HHPBA-BODIPY showed no obvious response to cations including, Zn2+, Mg2+, Cd2+, Al3+, Pb2+, Ni2+, Hg2+, K+. The introduction of these metal ions does not result significant changes to the fluorescence emission spectra and absorption spectra of HHPBA-BODIPY. The results of competitive experiments (Fig.9a and 9b) show that the presence of Zn2+, Mg2+, Cd2+, Al3+, Pb2+, Ni2+, Hg2+, K+, etc. exhibited no interference, except Fe3+ and Cr3+.
(a) HHPBA-BODIPY Fluorescence intensity (5 μM) in EtOH-HEPES solution (10 mM, 1/1, v/v, λex = 470 nm, pH = 7.0) after adding 1 eq different metal cations 9(b) Fluorescence intensity of the probe molecule after adding of 1 eq Cu2+ in the presence of 10 eq of various cations. 1: HHPBA-BODIPY 2: Cu2+ 3: Na+ 4: K+, 5: Ca2+ 6: Mg2+ 7: Fe3+ 8: Cd2+ 9: Cr3+ 10: Zn2+ 11: Ni2+ 12: Co2+ 13: Ba2+ 14: Pb2+ 15: Al3+ 16: Hg2+
After adding Cu2+ in the presence of Fe3+, the fluorescence intensity of HHPBA-BODIPY was weakened, but the degree of attenuation is significantly lower than that without iron ions. The appearance of Cr3+ also showed a significant impact. In the presence of 10 times of Cr3+ ions, HHPBA-BODIPY has little response to Cu2+.
Cu2+ Detection in Water Samples
To investigate whether HHPBA-BODIPY can be used for real-time analysis of environmental samples, tap water and yellow river water were selected in this section. By adding a certain concentration of Cu2+ solution, the detection ability of HHPBA-BODIPY fluorescent molecular probe was measured.The results are shown in Table 1. Obviously that the probe molecule exhibits a good yield of copper ions added to tap water sample between 4 and 12 μM. Though the detection sensitivity towards Cu2+ in the Yellow River water is not as good as that in tap water, the probe still showed good yields in the low concentration range. According to the experimental results, HHPBA-BODIPY probe possesses the potential for environmental sample analysis and monitoring.
Conclusion
In this work, the fluorescent molecular probe HHPBA-BODIPY possessing phenolic hydroxyl group and amide group was synthesized by using fluoroboron pyrrole fluorophore as skeleton and 2-hydroxy-N-(2-hydroxyphenyl)benzamide as the recognition unit.Then, the photophysical properties of the HHPBA-BODIPY fluorescent probe molecule were determined. The experimental results of Cu2+ recognition showed that the HHPBA-BODIPY probe resulted in fluorescence quenching by coordination with Cu2+. Thereby the Cu2+ concentration can be determined through measuring the fluorescence emission intensity of HHPBA-BODIPY, which showed a good linear relationship. The HHPBA-BODIPY probe can respond to Cu2+ rapidly under near-neutral conditions, and showed good detection ability towards Cu2+ in the concentration range of 0.35-7 μM. Furthermore, the presence of most metal cations does not influence Cu2+ recognition, except Fe3+ and Cr3+. The analysis results of water samples also showed that HHPBA-BODIPY possesses the potentiality in real sample determination.
References
Kim B-E, Nevitt T, Thiele DJ (2008) Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol 4(3):176–185
Pena MM, Lee J, Thiele DJ (1999) A delicate balance: homeostatic control of copper uptake and distribution. J Nutr 129(7):1251–1260
Jomova K, Valko M (2011) Advances in metal-induced oxidative stress and human disease. Tosicology. 283(2):65–87
Svetlana L, Barnes NL, Bartee MY, Dmitriev OY (2007) Function and regulation of human copper-transporting ATPases. Physiol Rev 87(3):1011–1046
Elena G, Henryk K, Daniela V, Gianni V (2010) Copper homeostasis and neurodegenerative disorders (Alzheimer's, prion, and Parkinson's diseases and amyotrophic lateral sclerosis). Chemical Reviews; 37(37):no-no.
Rossi L, Arciello M, Capo C, Rotilio G (2006) Copper imbalance and oxidative stress in neurodegeneration. Ital J Biochem 55(3–4):212
Sharma AK, Pavlova ST, Kim J, Kim J, Mirica LM (2013) The effect of Cu2+ and Zn2+ on the Aβ42 peptide aggregation and cellular toxicity. Metallomics Integrated Biometal Science 5(11):1529–1536
Zhang Z, Liu Y, Wang E (2019) A highly selective "turn-on" fluorescent probe for detecting Cu2+ in two different sensing mechanisms. Dyes Pigments 163:533–537
Lushchak VI (2011) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101(1):13–30
Schamphelaere KAC (2002) De, Janssen CR. A biotic ligand model predicting acute copper toxicity for Daphnia magna: the effects of calcium, magnesium, sodium, potassium, and pH. Environmental Science & Technology 36(1):48–54
Xiaowei C, Weiying L, Kaibo Z, Longwei H (2012) A near-infrared fluorescent turn-on probe for fluorescence imaging of hydrogen sulfide in living cells based on thiolysis of dinitrophenyl ether. Chem Commun 48(85):10529–10531
Liu J, Yue Y, Wang J, Yan X, Liu R, Sun Y, Li X (2015) Study of interaction between human serum albumin and three phenanthridine derivatives: Fluorescencespectroscopy and computational approach. Spectrochim Acta A Mol Biomol Spectrosc 145:473–481
Domaille DW, Que EL Chang CJ. Synthetic fluorescent sensors for studying the cell biology of metals
Chen H, Sun T, Qiao XG, Tang QO, Zhao SC, Zhou Z (2018) Red-emitting fluorescent probe for detecting hypochlorite acid in vitro and in vivo. Spectrochimica Acta Part A Molecular & Biomolecular Spectroscopy. 204:196–202
Young-Keun Y, Keun-Jeong Y, Jinsung T (2005) A rhodamine-based fluorescent and colorimetric chemodosimeter for the rapid detection of Hg2+ ions in aqueous media. J Am Chem Soc 127(48):16760–16761
Zhaochao X, Juyoung Y, Spring DR (2010) Fluorescent chemosensors for Zn2+. Chem Soc Rev 39(6):1996–2006
Weissleder R, Tung CH, Mahmood U, Bogdanov A (1999) In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat Biotechnol 17(4):375–378
Hisataka K, Mikako O, Raphael A, Choyke PL, Yasuteru U (2010) New strategies for fluorescent probe design in medical diagnostic imaging. Chem Rev 110(5):2620–2640
Yue Y, Yin C, Huo F, Chao J, Zhang Y (2016) Thiol-chromene click chemistry: a turn-on fluorescent probe for specific detection of cysteine and its application in bioimaging. Sensors & Actuators B Chemical. 223(2):496–500
Vernekar SKV, Hallaq HY, Guy C, Thompson AJ, Linda S, Lummis SCR et al (2010) Toward biophysical probes for the 5-HT3 receptor: structure-activity relationship study of granisetron derivatives. J Med Chem 53(5):2324–2328
Belousov V, Fradkov A, Lukyanov K, Staroverov D, Shakhbazov K (2006) Av, Lukyanov S. genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods 3(4):281–286
Liu J, Liu C, He W (2013) Fluorophores and their applications as molecular probes in living cells. Curr Org Chem 17(6):564–579
Dujols V, Czarnik AW (1997) A Long-wavelength fluorescent Chemodosimeter selective for cu(II) ion in water. J Am Chem Soc 119(31):7386–7387
Yu X, Zifan L, Xiaotong C, Aijun T (2008) Highly sensitive and selective optical chemosensor for determination of Cu2+ in aqueous solution. Talanta. 74(5):1148–1153
Liang H, Xiao W, Guoqiang X, Pinxian X, Zhengpeng L, Min X et al (2010) A new rhodamine-based chemosensor for Cu2+ and the study of its behaviour in living cells. Dalton Trans 39(34):7894–7896
Ying Z, Fang W, Youngmee K, Sung-Jin K, Juyoung Y (2009) Cu2+-selective ratiometric and "off-on" sensor based on the rhodamine derivative bearing pyrene group. Org Lett 11(19):4442–4445
Wang Y, Chang HQ, Wu WN, Peng WB, Yan YF, He CM, Chen TT, Zhao XL, Xu ZQ (2016) Rhodamine 6G hydrazone bearing pyrrole unit: Ratiometric and selective fluorescent sensor for Cu2+ based on two different approaches. Sensors & Actuators B Chemical 228:395–400
Chen Z (2013) Wang, Limin, Zou, gang, et al. highly selective fluorescence turn-on chemosensor based on naphthalimide derivatives for detection of copper(II) ions. Spectrochimica Acta Part A Molecular & Biomolecular Spectroscopy 105(105C):57–61
Cristina S, Giuseppe Trusso S, Maria Emanuela A, Ballistreri FP, Agata C, Maria Laura G et al (2013) A ratiometric naphthalimide sensor for live cell imaging of copper(I). Chem Commun 49(49):5565–5567
Zhang S, Yu T, Sun M, Yu H, Zhang Z, Wang S, Jiang H (2014) Highly sensitive and selective fluorescence detection of copper (II) ion based on multi-ligand metal chelation. Talanta. 126(126):185–190
Hee LM, Hyun Jung K, Sangwoon Y, Noejung P, Jong SK (2008) Metal ion induced FRET OFF-ON in tren/dansyl-appended rhodamine. Org Lett 10(2):213–216
Noel B, Volker L, Wim D (2012) Fluorescent indicators based on BODIPY. Chem Soc Rev 41(3):1130–1172
Kowada T, Maeda H, Kikuchi K (2015) BODIPY-based probes for the fluorescence imaging of biomolecules in living cells. Chem Soc Rev 44(14):4953–4972
Yu G, Tasuku U, Yasuteru U, Hirotatsu K, Tetsuo N (2006) Tunable design strategy for fluorescence probes based on 4-substituted BODIPY chromophore: improvement of highly sensitive fluorescence probe for nitric oxide. Analytical & Bioanalytical Chemistry 386(3):621–626
Wakamiya A, Murakami T, Yamaguchi S (2013) Benzene-fused BODIPY and fully-fused BODIPY dimer: impacts of the ring-fusing at the b bond in the BODIPY skeleton. Chem Sci 4(3):1002–1007
Hua L, Zhaoli X, John M, Zhen S, Xiaozeng Y, Nagao K (2010) Specific Cu2+-induced J-aggregation and Hg2+-induced fluorescence enhancement based on BODIPY. Chem Commun 46(20):3565–3567
Kumar A, Narasimhan B, Kumar D (2007) Synthesis, antimicrobial, and QSAR studies of substituted benzamides. Bioorg Med Chem 15(12):4113–4124
Long GL, Winefordner JD (1983) Limit of detection. A closer look at the IUPAC definition. Anal Chem 55(7):712–724
Chen F, Hou F, Huang L, Cheng J, Liu H, Xi P, Bai D, Zeng Z (2013) Development of a novel fluorescent probe for copper ion in near aqueous media. Dyes Pigments 98(1):146–152
Acknowledgements
This work was supported by National Natural Science Foundation of China (21876073).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 455 kb)
Rights and permissions
About this article
Cite this article
Sun, R., Wang, L., Jiang, C. et al. A Highly Efficient BODIPY Based Turn-off Fluorescent Probe for Detecting Cu2+. J Fluoresc 30, 883–890 (2020). https://doi.org/10.1007/s10895-020-02544-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-020-02544-9