Abstract
A new coumarin based Schiff-base chemosensor-(E)-7-(((8-hydroxyquinolin-2-yl)methylene) amino)-4-methyl-2H-chromen-2-one (H 11 L) was synthesized and evaluated as a colorimetric sensor for Fe3+ and fluorescence “turn on-off” response of Zn2+ and Cu2+ using absorption and fluorescence spectroscopy. Upon treatment with Fe3+ and Zn2+, the absorption intensity as well as the fluorescence emission intensity increases drastically compared to other common alkali, alkaline earth and transition metal ions, with a distinct color change which provide naked eye detection. Formation of 1:1 metal to ligand complex has been evaluated using Benesi-Hildebrand relation, Job’s plot analyses, 1H NMR titration as well as ESI-Mass spectral analysis. The complex solution of H 11 L with Zn2+ ion exhibited reversibility with EDTA and regenerate free ligand for further Zn2+ sensing. H 11 L exhibits two INHIBIT logic gates with two different chemical inputs (i) Zn2+ (IN1) and Cu2+ (IN2) and (ii) Zn2+ (IN1) and EDTA (IN2) and the emission as output. Again, an IMPLICATION logic gate is obtained with Cu2+ and EDTA as chemical inputs and emission as output mode. Both free ligand as well as metal-complexes was optimized using density functional theory to interpret spectral properties. The corresponding energy difference between HOMO-LUMO energy gap for H 11 L, H11L-Zn2+ and H11L-Cu2+ are 2.193, 1.834 and 0.172 eV, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Development of fluorescent chemosensor for sensing of biologically and environmentally important metal ions has been receiving considerable attention in the field of chemical sensors [1, 2]. They are usually very sensitive, low cost, easily performed and versatile, which are also used for real-time monitoring and detection of metal ions at a molecular level and are applicable in many fields such as medical diagnostics, environmental control, living cells and electronics. Nowadays, among the different analyte, special interest is devoted to develop chemosensors for transition metal ions, usually they represent an environmental concern when present in uncontrolled amounts, but at the same time some of them such as iron, zinc, manganese, copper and cobalt are present as essential elements in biological systems. Among biologically important metal ions, iron, copper and zinc ion are the most three abundant and essential trace elements in the human body which play important roles in biology, chemistry and environment. Iron is the most abundant transition metal ion present in the human body. Fe3+ ion is an important and essential for proper functioning of all living cells and acts as a cofactor in many enzymatic reactions to human as well as in specialized transport and storage of proteins [3]. The deficiency of Fe3+ causes anaemia, liver damage, diabetes, hemochromatosis, Parkinson’s disease and cancer [4]. On the other side, zinc is the second most abundant transition metal ion in the human body after iron, and plays a myriad of roles in numerous cellular functions such as regulation of gene expression, apoptosis, co-factors in metalloenzyme catalysis and neurotransmission in biological systems [5, 6]. Many severe neurological diseases, including Alzheimer’s disease, cerebral ischemia and epilepsy [7–9] are associated with the disorder of Zn2+ metabolism. Therefore, estimation of Zn2+ is very important in neurobiology. However, if unregulated, Fe3+ and Zn2+ may cause many severe diseases, such as β-thalassemia, Friedreich’s ataxia, Alzheimer’s disease, Parkinson’s disease and epilepsy [10]. Therefore, there is a great need for developing Zn2+ and Fe3+ selective sensors that can distinguish Zn2+ and Fe3+ from other transition metal ions. Many sensors reports are available for detection of both Fe and Zn with synthetic difficulties which require laborious multistep organic synthesis. However, despite much attention of fluorescent sensors for selective and sensitive detection of both Fe3+ and Zn2+, there is still a huge demand for new sensors with improved properties, low-cost, requirement of less labour and efficient sensors. At the same time, copper is the third most abundant trace element in the human body as well as in many living organisms. Copper plays a vital part in the physiological processes of organisms, including connective tissue development and the formation of bone and blood. However, any alternation in the uptake of copper may lead to neurodegenerative disorders such as Menkes and Wilson diseases [11–13], familial amyotropic lateral sclerosis [14, 15] and Prion diseases [16]. Thus the development of chemosensors for selective and sensitive detection of these trace elements like Fe3+, Cu2+ and Zn2+, still remains a challenge and active field of research.
Research on molecular logic gates has focused mainly in the field of chemistry research for its application in information technology since the first AND logic gate was mimicked with optical signals [17]. Various chemical systems have been developed to exhibit different operations like AND, OR, NOT and their integrated operations [18]. In addition, many useful integrated logic gates such as INHIBIT, half subtractor, half adder, full adder, and full subtractor with various single molecules have been exploited [19, 20]. However, during literature surveys, very few IMPLICATION logic gate are reported so far [21]. Recently, there has been reported several molecular logic gates based on the structure of salicylidine Schiff base [22–25]. Some of the coumarin Schiff base was also reported as molecular switch for dual sensing of different metal ions [26, 27].
The nitrogen atom of azomethine C = N double bond in Schiff base also exhibits a strong affinity for transition metal ions. Therefore, the Schiff base are known to be good ligand for metal ions and used to develop chemosensors. In addition, Schiff base derivatives incorporating a fluorescent moiety are appealing tools for optical sensing of metal ions. Nowadays, designing and synthesis of fluorescent sensors with high selectivity and sensitivity to metal ions is an important and vibrant field. Many excellent metal ions sensors have been contributed significantly but some of the reported synthesis methods are always too complicated. However, only a few coumarin based chemosensors are reported so far for the dual sensing of metal ions [28–34]. As it is well known that coumarin framework exhibits various interesting photophysical properties such as large Stokes shift with visible excitation and emission wavelengths, high quantum yields, good photostability and also has wide application as fluorescent dyes [35–37]. Moreover in the literature, only few coumarin based Schiff base were reported and known to selectively recognize for dual sensing of metal ions [26, 27, 38, 39]. So, in this paper, we have designed and synthesized a new coumarin based Schiff-base fluorescent probe-(E)-7-(((8-hydroxyquinolin-2-yl)methylene)amino)-4-methyl-2H-chromen-2-one (H 11 L) by one step condensation of 7-Amino-4-methyl coumarin and 8-hydroxy quinoline 2-carbaldehyde in methanol solution (Scheme 1) which was selectively and sensitively recognized both Zn2+ and Cu2+ using fluorescence spectroscopy. Herein, the photophysical properties of H 11 L were studied in presence of different metal ions, focusing the attention on their absorption and emission properties using absorption and fluorescence spectroscopy. In the UV-Vis absorption studies, a strong absorption band at 240 nm, 265 nm, 290 nm and 355 nm were observed in ethanol solution. On addition of Fe3+, the absorption intensity was found to increase with a dramatic change in color which can be easily detected with naked eye. This clearly indicates that H 11 L is a highly selective colorimetric sensor for Fe3+ compared to other survey metal ions. It was also seen that the absorption peak at 355 nm disappeared and a new absorption peak at 335 nm appeared with gradual addition of Fe3+ ion. At the same time, we also observed an isosbestic point at 360 nm which clearly indicates complex formation due to metal ion binding. We also observed a pronounced fluorescence enhancement in presence of Zn2+, while there was no change or minimal spectral changes in presence of other metal ions except Cu2+ which shows fluorescence quenching. This chemosensor was used as a fluorescent “turn on-off” detector of Zn2+ and Cu2+ ion, respectively. Importantly, it was able to distinguish Zn2+ from Cd2+ besides having very similar chemical properties often respond together with similar spectral changes. Moreover, the fluorescence emission behavior of H 11 L can be utilized to study as a binary logic function comprising of two INHIBIT logic gates with two different chemical inputs (i) Zn2+ (IN1) and Cu2+ (IN2) and (ii) Zn2+ (IN1) and EDTA (IN2) and the emission as output. Again, an IMPLICATION logic gate is obtained with Cu2+ and EDTA as chemical inputs and emission as output mode. Structure of H 11 L and its metal complexes were fully optimized using BLYP functional and DNP basis sets as implemented in the program DMol3. The corresponding energy difference between HOMO-LUMO energy gap for H 11 L, H11L-Zn2+ and H11L-Cu2+ are 2.193, 1.834 and 0.172 eV, respectively. Chemical hardness for both ligand as well as its metal complexes was also calculated.
Experimental Details
Materials
8-hydroxy quinoline 2-carbaldehyde and 7-Amino-4-methyl coumarin was obtained from Sigma-Aldrich Chemical Company. All the spectroscopic grade solvents used were obtained from Sisco Research Laboratory (SRL) Pvt. Ltd. and in some cases, from Aldrich Chemical Company. Chemical reagents were obtained from Lancaster as well as S.D. Fine Chemical Ltd. All experiments were carried out at room temperature.
Synthesis and Characterization of H11L
A portion of the 8-hydroxy quinoline 2-carbaldehyde (0.173 g, 1 mmol) and 7-Amino-4-methyl) coumarin (0.175 g, 1 mmol) was separately dissolved in absolute ethanol and were combined together to get to yellow color. The solution was stirred under reflux conditions for 3 h in presence of 2–3 drops of acetic acid and precipitate was filtrated, washed with cold absolute ethanol three times, then recrystallized with ethanol/chloroform (1/3, v/v) to get deep yellow microcrystal (H 11 L) in 80% yield. m.p. 151 °C. IR (max, cm−1, KBr): 3435(νOH), 3192(ν as(C-H), 2987(ν s(C-H),), 1629(ν(C = N)), 1575(C = C), 1236(ν(C-O)), 1120(ν(CN)),784(ν(C-H)); 1H NMR (400 MHz, CDCl3, TMS, δ, ppm): 10.16 (s, H-1), 7.21 (d, H-2), 7.59 (t, H-3), 7.64 (d, H-4), 8.59 (d, H-5),7.84 (d, H-6), 8.27 (s, H-7), 7.07(s, H-8), 7.29 (d, H-9), 7.74 (d, H-10), 2.42 (s, H-11), 6.18 (s, H-12); 13C NMR (400 MHz, CDCl3, TMS, δ, ppm):112.2, 112.5, 115.5, 117.9, 118.1, 119.0, 119.4, 120.1, 126.9, 128.1, 131.9, 136.1, 137.5, 148.6, 149.6, 151.4, 151.7, 152.7, 153.2, 160.8; Anal. Calc. for C20H14N2O3 (330.3): C, 72.72%; H, 4.27%; N, 8.48%. Found: C, 72.20%; H, 4.24%; N, 8.40%. ESI-MS, m/z: Calcd. for C20H14N2O3 (M + H) +: 331.3, found: 331.5.
Physical Measurements
The IR spectra were measured on a PerkinElmer L 120000A spectrometer with KBr pellets in the range 4000–400 cm−1. 1H and 13C NMR spectra were recorded on Bruker DPX-400 MHz spectrometer with chemical shifts reported as ppm (in CDCl3, tetramethylsilane as internal standard). Elemental analyses were carried out using PE2400 elemental analyzer. pH measurement was recorded on Global Digital pH meter (DPH-500). Absorption and fluorescence emission spectra were recorded on a Shimadzu UV-1601PC absorption spectrophotometer and PerkinElmer LS 45 spectrofluorimeter, respectively. Fluorescence quantum yields (ϕf) were calculated by comparing the total fluorescence intensity under the whole fluorescence spectral range with that of a standard (ϕf = 0.546, quinine sulfate in 1 M sulfuric acid) using the following equation as described before [40].
where F is the total fluorescence intensity under whole fluorescence spectral curve, A i and A s is the optical density of the sample and standard, respectively and η i is the refractive index of the solvent at 298 K. Fluorescence lifetimes were recorded in a nano-LED based time-resolved fluorimeter obtained from Photo Technology International (PTI) using TCSPC technique. The instrument response function (IRF) was obtained at 360 nm using a dilute colloidal suspension of dried non-dairy coffee whitener. The half width of the IRF was ~100 ps. The samples were excited at 360 nm and the fluorescence emission was collected at corresponding emission wavelength. The number of counts in the peak channel was at least 10,000. In fluorescence lifetime measurements, the emission was monitored at the magic angle (54.7°) to eliminate the contribution from the decay of anisotropy.
Computational Methods
Quantum chemical calculations based on density functional theory (DFT) were carried out to investigate the electronic structure of H 11 L, H11L-Zn2+ and H11L-Cu2+ complexes. Full geometry optimization of H 11 L, H11L-Zn2+ and H11L-Cu2+ complexes has been carried out without imposing any constrain using BLYP functional [41, 42] which incorporates Becke’s exchange and Lee-Yang-Parr correlation and double-numerical polarized (DNP) [43] basis sets as implemented in DMol3 program package [44] using Kohn-Sham Theory [45, 46]. In order to confirm the stability of H 11 L, H11L-Zn2+ and H11L-Cu2+ complexes, we performed vibrational frequency calculations at the same level of theory.
Results and Discussion
UV-Vis Titration Studies of H11L
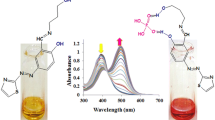
The absorption spectrum of H 11 L shows four different peak positions at 245 nm, 260 nm, 290 nm and 355 nm respectively, in ethanol solution. The stock solution of the ligand and metal ions were prepared in 10 mM concentration in ethanol solution at room temperature. However, the experimental solution of free ligand (H 11 L) was kept to be 2.5 × 10−4 M in all cases. In the absorption spectra, there are no remarkable changes in presence of other different metal ions like alkali (Na+, K+), alkaline earth (Ca2+, Mg2+, Sr2+) and transition metal ions (Cd2+, Ni2+, Co2+, Cu2+, Zn2+) except Fe3+. To further investigate the interaction between H 11 L and Fe3+, UV-Vis absorption spectral variation of H 11 L was monitored in presence of different concentration of Fe3+ in ethanol solution at room temperature. As shown in Fig. 1, gradual addition of Fe3+ (0-55 μM) resulted in an obvious change in absorption intensity as well as in color, from colorless to deep yellow, which can be easily detected with naked eye. This clearly indicates that H 11 L is a highly selective colorimetric sensor for Fe3+ ions. It was also seen that the absorption peak at 355 nm disappeared and a new absorption peak at 335 nm appeared with gradual addition of Fe3+ (fig. 1). At the same time, we also observed an isosbestic point at 360 nm which clearly indicates complex formation due to metal ion binding. Here in presence of Fe3+, an isomerization happened to H 11 L due to intramolecular charge transfer (ICT) from O-H and N = CH which leads to imine bond breaking. Therefore, H 11 L showed selective estimation for Fe3+ ions in UV-Vis absorption studies.
Change in UV-Vis spectrum of H 11 L (2.5 × 10−4 M) upon gradual addition of Fe3+ in ethanol solution at room temperature. The concentrations of Fe3+ (μM) are: 0.0 (i), 5.0 (ii), 10.0 (iii), 15.0 (iv), 20.0 (v), 25.0 (vi), 30.0 (vii), 35.0 (viii), 40.0 (ix), 45.0 (x), 50.0 (xi) and 55.0 (xii).Inset show the spectra of free ligand in absence (0 μM) and presence (55 μM) of Fe3+ ion concentration
Fluorescence Studies of H11L
In Presence of Different Metal Ion
The interactions of H 11 L with different metal ions have been investigated by evaluating the changes in their fluorescence properties by addition of different metal ions in ethanol solution at room temperature. The fluorescence emission maxima peak of H 11 L appeared at 438 nm upon excitation at 355 nm, which on addition of Zn2+ (~45 μM) gives slightly blue-shifted at 428 nm. However, the fluorescence emission intensity has been drastically increased with almost 6-fold (Fig. 2) in presence of Zn2+ (~45 μM). Similarly, in presence of other different metal ions like Na+, K+, Ca2+, Mg2+ Cd2+, Ni2+, Co2+, Ba2+, Al3+, Hg2+, Mn2+ and Fe3+, H 11 L showed either no change in the fluorescence peak position or a very negligible amount of changes occurs in the fluorescence intensity in case of Cd2+. So, there was no appreciable change in the fluorescence emission behavior of H 11 L with others metal ions except for Zn2+. However, in presence of Cu2+, it also exhibits quenching of fluorescence emission intensity without any interference of other metal ions present in the solution. Fluorescence intensity profile changes of H 11 L in presence of different metal ions were shown in the histogram (Fig. 3) which clearly indicates high selectivity of Zn2+.Therefore, H 11 L shows selective estimation for Zn2+ and Cu2+ ions in fluorescence studies.
Fluorescence intensity profile changes of H 11 L in absence (free ligand) and presence of 45 μM concentration of various metal ions at room temperature. Fluorescence intensity changes that occur upon subsequent addition of Zn2+ ion (45 μM) in presence of different metal ions were also reported. Excitation was done at λexc = 355 nm
Influence of Zn2+ Ion
As shown in Fig. 4, with gradual addition of Zn2+ (0–60 μM), a significant enhancement of the emission band intensity at 428 nm occurred, which resulted in a ~ 10 nm blue-shifted and a large increase in the fluorescence intensity. This effect was not observed in presence of other metal ions even with very high concentration (say ~ 100 μM). The enhancement of fluorescence intensity was attributed to the introduction of Zn2+ and consequently occurrence of the strong complexation with H 11 L via O-H and N heteroatoms which can be explain due to prevention of isomerization by metal ion binding. This coordination can enhance the planarity and rigidity which can also decrease nonradiative decay of the excited state and increased radiative decay which may be explained due to photoinduced electron transfer (PET) process between H 11 L and Zn2+ [47–50].The fluorescence quantum yields were also calculated both in free ligand as well as in presence of Zn2+. Here, the fluorescence quantum yield increases drastically from 0.6 × 10−3 for H 11 L compared to 7.4 × 10−2 for H11L-Zn2+ complex. Further, tolerance of fluorescence intensity due to Zn2+ (45 μM) in presence of 50 times an excess of other metal ions like Na+, K+, Cd2+, Ca2+ and Mg2+ has been successfully verified as shown in Fig. 3. So, all competitive metal ions had no obvious interference with the detection of Zn2+, which also indicates that H11L-Zn2+ system was hardly affected by these coexistent metal ions. Thus, H 11 L can be used as selective fluorescent chemosensor for Zn2+ determination in presence of other competing metal ions. The emission intensity of H 11 L was linearly proportional to Zn2+ ion concentration. This linear dependence of Zn2+ ion concentration suggests that H 11 L could be utilized for the quantitative estimation of Zn2+. The detection limit was calculated using 3*S/M [51] where S is the standard deviation of a blank signal and M is the slope of the regression line. The detection limit was found to be in order of 10−6 M. Hence, H 11 L shows selective estimation for Zn2+ and “turn-on” fluorescent sensor in presence of Zn2+ ion.
Variation of fluorescence intensity of H 11 L against concentration of Zn2+. The concentrations of Zn2+ (μM) are: 0.0 (i), 5.0 (ii), 10.0 (iii), 15.0 (iv), 20.0 (v), 25.0 (vi), 30.0 (vii), 35.0 (viii), 40.0 (ix), 45.0 (x), 50.0 (xi), 55.0 (xii) and 60.0 (xiii). Inset show the double reciprocal plot of 1/(F-F0) against 1/[Zn2+] giving a straight line indicating 1:1 complex formation
Influence of Cu2+ Ion
As it is well known, Cu2+ is a paramagnetic ion with an empty d-shell and can strongly quench the emission of a fluorophore via a photoinduced metal-to-fluorophore, electron or energy transfer mechanism [52, 53]. In addition, among the relevant paramagnetic metal ions, Cu2+ has a particularly high thermodynamic affinity for ligands with “N” or “O” as chelating element and fast metal-to-ligand binding kinetics process [54]. So, in order to evaluate the binding nature between H 11 L and Cu2+, a fluorescence titration with increasing concentration of Cu2+ (0-32 μM) was performed and a significant fluorescence emission intensity decrease with slight red-shifted (Fig. 5). This also clearly indicates complex formation with Cu2+ ion. The decrease in fluorescence emission intensity in our study may be due to strong metal-fluorophore communication compared to other interactions which leads to fluorescence quenching. So, the paramagnetic behavior of Cu2+ and the complex probe (H11L-Cu2+) interaction was the main reason for fluorescence quenching. The emission intensity of H 11 L was linearly proportional to Cu2+ ion concentration. The detection limit was calculated and found to be in order of 10−5 M. Hence, H 11 L shows selective estimation for Cu2+ and “turn-off” fluorescent sensor in presence of Cu2+ ion.
Fluorescence emission spectra of H 11 L in presence of different concentrations of Cu2+ in ethanol solution at room temperature. The concentrations of Cu2+ (μM) are: 0.0 (i), 4.0 (ii), 8.0 (iii), 12.0 (iv), 16.0 (v), 20.0 (vi), 24.0 (vii), 28.0 (viii) and 32.0 (ix). Inset shows the Stern-Volmer plot from titration of H 11 L with different conc. of Cu2+ (0-32 μM)
The fluorescence quenching behavior can be expressed mathematically by the Stern-Volmer relation [55] which allows calculating the quenching constant using Eq. (2).
where, I0 and I are fluorescence intensities in the absence and presence of quencher (Q), Ksv is the Stern-Volmer constant related to the bimolecular quenching rate constant and [Q] is the quencher concentration. This plot exhibits linear relationship indicating that H 11 L is potentially useful for detection of Cu2+ with Ksv value of 4.3 × 104 M−1 (Fig. 5(inset)). To further investigate the quenching mechanism whether static or dynamic, we studied the change in absorption spectrum with an increase in Cu2+ ion concentration which clearly indicates the quenching mechanism to be static in nature. Moreover, we also monitored the fluorescence behavior in presence of ascorbic acid. Here, the fluorescence emission intensity increases drastically in presence of ascorbic acid (~30 μM) which indicates the masking of the quenching effect of copper.
Stoichiometry of Complexation
The stoichiometric ratio and apparent binding constant for ligand-metal ions complex was also determined by analyzing the changes in fluorescence emission intensity with different metal ions concentration. The apparent binding constant of the complex for 1:1 binding between ligand and metal ions can be obtained using modified form of Benesi-Hildebrand relation [56] written as
where, F0 and F are the fluorescence intensities in the absence and presence of metal ions respectively. Fα is the fluorescence intensity in the presence of excess amount of metal ions. Therefore, for 1:1 complex formation, the double reciprocal plot of 1/(F-F0) against 1/[M2+] should give a straight line; from the slope and intercept of which, the equilibrium constant (K) can be calculated. Figure 4(inset) shows the representative linear fitting using Eq. (3) and confirms 1:1 stoichiometry between H 11 L and Zn2+ with association constant of 2.6 × 104 M−1. At the same time, we also studied Job’s plot analyses for determination of stoichiometry between H 11 L and Zn2+. The method is that keeping total concentration of H 11 L and Zn2+ at 50.0 μM and changing the molar ratio of Zn2+ from 0.1 to 1.0. From Fig. 6(a) when molar fraction of Zn2+ was 0.5, the fluorescence emission maxima at 438 nm got to maximum, indicating that forming a 1:1 complex between H 11 L and Zn2+. Moreover, this further corroborated 1:1 complex formation based on B-H relationship. Similarly, in case of H 11 L and Cu2+ complex formation, it was confirmed to be 1:1 stoichiometry between H 11 L and Cu2+ with association constant of 8.6 × 104 M−1. Figure 6(b) shows 1:1 complex formation between H 11 L and Cu2+ based on Job’s plot analyses.
We also determine the nature of binding ratio and binding sites of metal ions to H 11 L using other form of B-H equation as given below.
where I0 and I are the fluorescence intensities in the absence and presence of metal ions respectively. Imax is the fluorescence intensity in presence of excess amount of metal ions. From the plot of log((I-I0)/(Imax-I)) versus log [M2+] in Eq. (4), the slope and intercept were obtained giving the slope value, n = 1.28 indicating the binding ratio of Zn2+ ions to H 11 L as 1:1 and log Kd was found to be 1.785. Similarly, in case of H 11 L and Cu2+ complex formation, the slope and intercept were found to be slope value, n = 1.32 and log Kd = 1.62 indicating 1:1 binding nature of Cu2+ ions to H 11 L.
Effect of pH
We also studied the effect of pH both in presence and absence of Zn2+ and Cu2+ (Fig. 7). Over a wide range of pH, there was no obvious change in the fluorescence intensity of free ligand alone which clearly indicates insensitivity to pH. However, in presence of Zn2+, H 11 L has a strong pH dependent even though it had a weak fluorescence response to Zn2+ in acidic environment because of protonation of phenolic hydroxyl [57] leading to a weak coordination ability of Zn2+ [58]. However, satisfactory Zn2+ sensing abilities were exhibited with increasing pH. Thus, H 11 L indicates a good fluorescence sensing ability to Zn2+ over a wide range from pH 5.5 to pH 11. Moreover, on addition of Cu2+, quenching behavior was observed in the wide pH range from pH 4.0 to pH 11.5. These results also clearly indicate that H 11 L can be employed as a selective fluorescent probe to recognize and distinguish Zn2+ and Cu2+ in presence of other survey metal ions.
Effect of Different Substituents
Metal-ligand complexes have been studied showing both the affect on the fluorescence emission wavelength and intensity of the ligand through metal coordination. The luminescent properties of Zn2+ complexes were reported to be determined by the organic ligand because of the electronic configuration of Zn2+ (3d104s0) where the d-shell are completely filled which causes lack of intrinsic spectroscopic or magnetic signal [59]. Herein, the fluorescence behaviors of H 11 L and complexes with different substituent of Zn2+ were studied in the solution phase at room temperature. Here, the emission spectra of complexes are very similar with H 11 L except for the fluorescence intensity and peak position, indicating that the fluorescence of complexes is L-based emission. Meanwhile, the fluorescence emission for complexes were slightly blue-shifted compared to free ligand which is considered to mainly arise from the coordination of Zn2+ centre to H 11 L. The incorporation of Zn2+ effectively increases the conformational rigidity of the ligand and enhanced fluorescence intensities of all five complexes [60]. Moreover, the different in fluorescence intensities of the complexes with different substituent’s like CO3, SO4, Cl, NO3 and CH3COO, can be explain due to bigger conformational rigidity for a 3D supramolecular network, as well as hydrogen bonds and π….π packing interactions [61].
Real Water Sample Assay
As we all know that zinc is the second most abundant and essential trace elements in the human body which play important roles in biology, chemistry and environment. Moreover, zinc is also a mineral that naturally occurs in rocks and soil and is a normal constituent of the human diet. So, in order to evaluate the application feasibility to determine Zn2+ ion in real water samples, several water samples from different water sources were selected including tap water, mineral water, river water, pond water, well water and distilled water. The changes in fluorescence emission intensity were measured after addition of 20% of the water samples to ethanol solution of the probe (Fig. 8). Here, the fluorescence intensity of the probe upon addition of 20% pond and well water indicates significant increase compared to other water samples, which indicates that the content of Zn2+ ion in pond and well water is high. However, in case of river and tap water, the fluorescence intensity is weak compared to pond and well water which indicates low content of Zn2+. The possible reason might be due to low content of Zn2+ in river and tap water or due to the presence of different interfering metal ions compare to pond and well water. For mineral and distilled water, there is almost negligible enhancement of fluorescence intensity after addition of 20% of the water samples with suggests that the amount of Zn2+ ion in these two samples is very low.
Effect of EDTA
Reversibility nature of H 11 L was also studied using EDTA as a coordinating ligand. Here, we have selected EDTA as a ligand of choice as it is available in abundance and relatively low cost. The fluorescence emission behavior of the complex probe (H11L-Zn2+) was monitored in presence of EDTA (~35 μM) and the fluorescence intensity was found to decrease and returned to lower level for H 11 L indicating regeneration of free H 11 L which became constant and corresponds to the fluorescent intensity of the free ligand. A similar type of reversibility nature for fluorescent sensor has already been reported earlier [62, 63]. This may be due to the lack of affinity of EDTA with H 11 L as well as its ability to form a preferential complex with Zn2+ ions. Thus, using EDTA, free H 11 L was regenerated from the complex probe (H11L-Zn2+) which can be reused for further Zn2+ sensing. Again on subsequent addition of Zn2+ and EDTA, the change in fluorescence emission intensity occurred reversibly even after several cycles (Fig. 9(a)). These results show that H 11 L can be used as reversible fluorescent chemosensor using EDTA as coordinating ligand. Similarly, upon alternatively gradual addition of Cu2+ and EDTA, the change in fluorescence emission intensity shows reversibility to its original state up to several cycles as shown in fig. 9(b). Overall, these experiments confirm the reusability, recyclable and stability nature of the ligand, which may be used for wide practical applications.
Fluorescence Response Time in Presence of Zn2+ and Cu2+ Ions
The time dependent fluorescence intensity profiles of H 11 L was performed in presence of three different concentrations of Zn2+ and Cu2+ ions, respectively. Figure 10(a) shows the fluorescence intensity profile curve showing instant enhancement in presence of Zn2+ ion with almost in no time. As the time increases, fluorescence intensity increases to a certain time ~ 30 s and remain almost unchanged over a period of time ~ 3 min, which may be due to strong complexation behavior of H 11 L with Zn2+ ion via OH and N-heteroatoms due to photoinduced electron transfer. This performance also clearly indicates a good and much faster response compared to other available reports [64, 65] in addition of Zn2+ ion. However, in presence of Cu2+ ion, fluorescence intensity quenching behavior was observed in no time (Fig. 10(b)). Here, fluorescence intensity decreases to a certain time and remains constant over a period of time ~ 2 min. This decrease in fluorescence intensity may be due to strong metal-fluorophore communication which leads to fluorescence quenching. These experiments clearly indicates a good and short response time to monitor both Zn2+ and Cu2+ ions, respectively which can be further use for wide practical applications.
Fluorescence response of H11L in presence of a Zn2+ with different concentrations at different times. The concentrations of Fe3+ (μM) are: (i) 10.0, (ii) 25.0 and (iii) 45.0, respectively. b Cu2+ with different concentrations at different times. The concentrations of Cu2+ (μM) are: (i) 10.0, (ii) 20.0 and (iii) 35.0, respectively
1H NMR Titration and Mass Analysis
To understand the interaction and binding behavior of H 11 L towards Zn2+ ion, 1H NMR titration experiments were carried out in the absence and presence of different concentrations of Zn2+ ion in DMSO-d6 solvent at room temperature. Here, four different NMR tubes containing H 11 L dissolved in DMSO-d6 were prepared and then three different concentrations (0.25 eq., 0.5 eq. and 1.0 eq of Zn2+ ions) of Zn(CH3COO)2 dissolved in DMSO-d6 were also prepared and added to different NMR tubes. After shaking thoroughly for 1 min, 1H NMR experiments were recorded at room temperature. As shown in the Fig. 11, the hydroxyl proton of H 11 L obtained at around 10.16 ppm in the free ligand has experienced a remarkable downfield shift towards 10.52 ppm upon addition of Zn2+ ion. On the other hand, the aromatic protons signal at 8.57, 8.27, 7.84, 7.59, 7.29, 7.07, 6.18 ppm were shifted marginally downfield to 8.73, 8.62, 8.23, 8.04, 7.51, 7.74, 6.24 ppm while the other signals at 7.74, and 7.21 ppm were shifted marginally upfield to 7.16 and 6.97 ppm in presence of Zn2+ ion, respectively. These spectra exhibited the binding nature of H 11 L with Zn2+ ion in 1:1 stiochiometric ratio [66]. Moreover, these overall changes in the chemical shifts of the proton in presence of Zn2+ ions suggested that the binding of H 11 L to Zn2+ forms a rigid system by a strong complexation with H 11 L via O-H and two N-atoms of pyridine and azomethine, respectively. These results also indicated the structural and conformational changes of H 11 L upon Zn2+ ion binding. Again, 1H NMR spectrum of the ligand was also recorded in different pH to confirm the stability of the ligand. Here, in this experiment, we have observed that the peak signals remain almost at same position throughout different pH which indicates insensitivity to pH.
To better understand the binding nature of H 11 L with Zn2+ ion, ESI-mass spectra of H 11 L and its complex (H11L-Zn2+) were also recorded and shown in Fig. 12. The observed molecular ion peak m/z for H 11 L was obtained at 331.5. However, in presence of Zn2+ ion, the molecular ion peak m/z was found at 445.3 which correspond to [H11L + Zn2++Acetate] + (calculated: 445.04). Here it is worth to mention that the complex (H11L-Zn2+) ensemble also involves a coordinated acetate molecule. So, these mass spectra also confirmed the binding nature of H 11 L to Zn2+ as 1:1 stoichiometry [67].
Time-Resolved Fluorescence Measurements
Lifetime data of H 11 L was obtained by nanosecond time-correlated single photon counting technique using 360 nm LED source and monitoring the emission at 428 nm. The fluorescence decay curves were analyzed by non-linear least-square iterative convolution method based on Lavenberg-Marquardt [68] chi-sqare (χ2) minimization algorithm. Here, the fluorescence decay need two exponential function both for free ligand as well as the complex probe (H11L-Zn2+) to reproduce the experimental data points with acceptable statistical parameters like reduced chi-square (χ2) values and Durbin-Watson parameter as demonstrated by visual inspection of the distribution of weighted residuals with time (Fig. 13). The biexponential fluorescence decay parameters for H 11 L: τ1 = 0.63 ns (0.70) and τ2 = 1.91 ns (0.30) and for H11L-Zn2+: τ1 = 1.22 ns (0.29) and τ2 = 3.66 ns (0.71), respectively. Here, it is seen that the amplitude of the short nanosecond component is much larger compared to long nanosecond component in free ligand. However, with addition of Zn2+, the amplitude of the short nanosecond component is much smaller compared to long nanosecond component which may be due to suppression of PET process by metal ion binding. This complex nature of the fluorescence decay parameters is a reflection of the flexibility of the molecules [69]. Here, we calculate the average decay time of H 11 L both in free ligand and complex probe using Eq. (5) to discuss the fluorescence decay behavior.
The calculated average fluorescence decay values are 1.02 ns and 2.95 ns for free ligand and the complex probe (H11L-Zn2+), respectively. It is interesting to note that the average fluorescence decay time in (H11L-Zn2+) is about three times larger than the corresponding values in H 11 L. So, the metal-ligand orbital mixing in the complex may be the reason for longer time passing in the excited state compared to free ligand. Here, we found that the fluorescence decay time was affected by turn-on sensor in presence of Zn2+.
Application as Logic Function
The fluorescence emission behavior of H 11 L can be utilized to study as a binary logic function with dual stimulating inputs as Zn2+ (IN1) and Cu2+ (IN2) and emission as output. With coordination of H 11 L with Zn2+ (IN1) a new fluorescence emission band appears at 428 nm. Upon gradual addition of Cu2+ (IN2), the emission intensity of the band at 428 nm gets quenched. Herein, the coordination complex of H11L-Zn2+ is replaced with Cu2+ due to greater abilities of binding with Cu2+ rather than Zn2+. Actually it represents an AND gate with an inverter [70] in one of its input. Thus the emission change at 428 nm with Zn2+ as well as Cu2+ (with an inverter) as inputs can be interpreted as a monomolecular circuit showing an INHIBIT logic function [24–27]. Hence, the fluorescence behavior of H 11 L can be applied for construction of INHIBIT logic function and its truth table (Fig. 14 (a)).
The regeneration of the free ligand was monitored with addition of EDTA as a good chelating agent. Here, the fluorescence emission intensity of the complex probe (H11L-Zn2+) returned to lower level for H 11 L in presence of EDTA which corresponds to the fluorescence emission intensity of the free ligand. The decrease of fluorescence emission intensity and the emission band at 428 nm is almost disappeared indicating regeneration of free ligand. However in the absence of Zn2+, EDTA does not have any effect on the emission intensity of H 11 L. Thus, with two chemical inputs as Zn2+ (IN1) and EDTA (IN2) and the emission as output, the molecular logic function was studied. Here, H 11 L functions as an AND gate with an inverter in the EDTA input by monitoring the emission output. This function can be interpreted as a monomolecular circuit showing an INHIBIT logic function (Fig. 14(b)). As we know, upon addition of Cu2+, the fluorescence emission intensity band of H 11 L at 428 nm gets quenched. However, with addition of EDTA as chelating agent to this complex (H11L-Cu2+), a pronounced fluorescence enhancement is observed which may be due to complex formation of EDTA with Cu2+ making the ligand free. So, in presence of EDTA to this complex, the fluorescence emission intensity is high compare only when Cu2+ is present alone. Thus, this behavior represents an OR gate with an inverter in one of its input which is also called an IMPLICATION logic gate [71–73] (Fig. 14 (b)).
Quantum Chemical Calculation
Initial structure of H 11 L and its metal complexes (H11L-Zn2+ and H11L-Cu2+) were both generated from the available experimental data in DFT calculations. The structure of H 11 L and its metal complexes were fully optimized using BLYP functional and DNP basis sets as implemented in the program DMol3 [44]. In order to confirm the stability of the complexes, we performed vibrational frequency calculations at the optimized structure with the same level of theory. The DFT optimized geometries of H 11 L and its metal complexes are shown in Fig. 15. In the vibrational frequency calculations, no imaginary frequency is observed for H 11 L and its metal complexes which clearly suggest that the optimized compounds signify stable structures i.e., it shows local minima in the potential energy surfaces. It is observed from Fig. 15 that both zinc and copper metal centre are tri coordinated to the ligand through two nitrogen atoms and one OH group of ligand. It is noted from DFT generated data that selected bond lengths (Å) and bond angles (0) for both Zn-complex and Cu-complex has been evaluated at B3LYP level. In Zn-complex, the bond length (Å) of Zn―N(1), Zn―N(2) and Zn―O are 2.698 Å, 2.452 Å and 3.021 Å, respectively. However, in case of Cu-complex, the bond length (Å) of Cu―N(1), Cu―N(2) and Cu―O are 1.983 Å, 1.981 Å and 2.783 Å, respectively. Here, metal-OH bond lengths in both complexes are longer than the other bond lengths. Moreover, the bond angles N(1)―Zn―N(2), N(1)―Zn―O and N(1)―Cu―N(2) and N(1)―Cu―O are found to be 61.2240, 53.1350, 89.0720 and 70.3250, respectively, around the metal atom. A distortion from 900 bond angle occurs for both H11L-Zn2+ and H11L-Cu2+ complexes, respectively. Here, H11L-Zn2+ has more distorted compared to H11L-Cu2+ as shown above which clearly indicates deviation from planar geometry.
The HOMO and LUMO energies of H 11 L are calculated and found to be −5.211 eV and −3.018 eV, respectively. However, in case of metal complexes, the HOMO and LUMO are −5.018 eV and −3.184 eV for H11L-Zn2+ as well as −3.829 eV and −3.657 eV for H11L-Cu2+ complexes, respectively. The HOMO and LUMO isosurface diagrams of H 11 L and its complexes (H11L-Zn2+ and H11L-Cu2+) are shown in Fig. 16. The corresponding energy difference between HOMO-LUMO energy gap for H 11 L, H11L-Zn2+ and H11L-Cu2+ are 2.193, 1.834 and 0.172 eV, respectively. Chemical hardness for both ligand as well as its metal complexes was also calculated. The chemical hardness value for H 11 L is found to be 1.096 eV and that for its metal complexes are 0.917 eV and 0.086 eV for H11L-Zn2+ and H11L-Cu2+, respectively. It is observed that Zn-complex has much higher stability compare to Cu-complex due to higher HOMO-LUMO energy gap as well as chemical hardness value.
Conclusions
In this work, we have designed and synthesized a new Schiff-base fluorescent probe -(E)-7-(((8-hydroxyquinolin-2-yl)methylene)amino)-4-methyl-2H-chromen-2-one (H 11 L) which has been evaluated as a colorimetric sensor for Fe3+ and fluorescence “turn on-off” response for Zn2+ and Cu2+. The UV-Vis absorption studies of H 11 L indicate high selectivity for Fe3+ ion over other survey metal ions with a distinct color change, from colorless to deep yellow, which provide naked eye detection. Fluorescence studies of H 11 L shows very high selectivity to Zn2+ and Cu2+ whereas other common alkali, alkaline earth and transition metal ions failed to induce response. The stoichiometric ratio and binding constant were evaluated using Benesi-Hildebrand relation giving 1:1 stoichiometry. This further corroborated 1:1 complex formation based on Job’s plot analyses. These binding behaviors were also confirmed by 1H NMR titration as well as ESI-Mass spectral analysis. This chemosensor exhibits a very good fluorescence sensing ability to Zn2+ over a wide pH range. An efficient way for the regeneration of free ligand from the complex probe was achieved using EDTA as a coordinating ligand which further can be reused for Zn2+ sensing. This chemosensor can be used as an important application for detection of Zn2+ in real water samples. H 11 L exhibits two INHIBIT logic gates with two different chemical inputs (i) Zn2+ (IN1) and Cu2+ (IN2) and (ii) Zn2+ (IN1) and EDTA (IN2) and the emission as output. Again, an IMPLICATION logic gate is obtained with Cu2+ and EDTA as chemical inputs and emission as output mode. The corresponding energy difference between HOMO-LUMO energy gap for H 11 L, H11L-Zn2+ and H11L-Cu2+ are 2.193, 1.834 and 0.172 eV, respectively. It is observed that Zn-complex has much higher stability compare to Cu-complex due to higher HOMO-LUMO energy gap as well as chemical hardness value. Moreover, this work provides an insight with a new approach for selective, sensitive and quantitative detection of these three most abundant and essential traces elements in the human body.
References
Silva APD, Gunaratne HQN, Gunnlaugsson T, Huxley AJM, McCoy CP, Rademacher JT, Rich TE (1997) Signaling recognition events with fluorescent sensors and switches. Chem Rev 97:1515–1566
Valeur B, Leray I (2000) Design principles of fluorescent molecular sensors for cation recognition. Coord Chem Rev 205:3–40
Lu Y, Berry SM, Pfister TD (2001) Engineering novel metalloproteins: design of metal-binding sites into native protein scaffolds. Chem Rev 101:3047–3080
Narayanaswamy S, Govindaraju T (2012) Aldazine-based colorimetric sensors for Cu2+ and Fe3+. Sensors Actuators B Chem 161:304–310
Vallee BL, Falchuk KH (1993) The biochemical basis of zinc physiology. Physiol Rev 73:79–118
De Silva JJRF, Williams RJP (2001) The biological chemistry of elements: the inorganic chemistry of life, 2nd edn. Oxford University Press, New York
Bush AI, Pettingell WH, Multhaup G, Paradis M, Vonsattel JP, Gusella JF, Beyreuther K, Masters CL, Tanzi RE (1994) Rapid induction of Alzheimer a beta amyloid formation by zinc. Science 265:1464–1467
Koh JY, Suh SW, Gwag BJ, He YY, Hsu CY, Choi DW (1996) The role of zinc in selective neuronal death after transient global cerebral ischemia. Science 272:1013–1016
Walker CF, Black RE (2004) Zinc and the risk for infectious disease. Annu Rev Nutr 24:255–275
Danuta SK, Des RR (2005) The evolution of iron Chelators for the treatment of iron overload disease and cancer. Pharmacol Rev 579:547–583
Waggoner DJ, Bartnikas TB, Gitlin JD (1999) The role of copper in neurodegenerative disease. Neurobiol Dis 6:221–230
Vulpe C, Levinson B, Whitney S, Packman S, Gitschier J (1993) Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase. Nat Genet 3:7–13
Bull PC, Thomas GR, Rommens JM, Forbes JR, Cox DW (1993) The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet 5:327–337
Valentine JS, Hart PJ (2003) Misfolded Cu Zn SOD and amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A 100:3617–3622
Bruijn LI, Miller TM, Cleveland DW (2004) Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci 27:723–749
Brown DR, Kozlowski H (2004) Biological inorganic and bioinorganic chemistry of neurodegeneration based on prion and Alzheimer diseases. Dalton Trans 13:1907–1917
De silva AP, Gunarane HQN, McCoy CP (1993) A molecular photoionic AND gate based on fluorescent signalling. Nature 364:42–44
Szacilowski K (2008) Digital information processing in molecular systems. Chem Rev 108:3481–3548
De Silva AP, McClenaghan ND (2004) Molecular-Scale Logic Gates. Chem Eur J 10:574–586
Pischel U (2007) Chemical approaches to molecular logic elements for addition and subtraction. Angew Chem Int Ed 46:4026–4040
Rurack K, Trieflinger C, Koval’chuck A, Daub J (2007) An Ionically driven molecular IMPLICATION gate operating in fluorescence mode. Chem Eur J 13:8998–9003
Zhao L, Sui D, Chai J, Wang Y, Jiang S (2006) Digital Logic Circuit Based on a Single Molecular System of Salicylidene Schiff Base. J Phys Chem B 110:24299–24304
Zhao L, Wang S, Wu Y, Hou Q, Wang Y, Jiang S (2007) Salicylidene Schiff Base assembled with Mesoporous silica SBA-15 as hybrid materials for molecular logic function. J Phys Chem C 111:18387–18391
Kaur N, Singh N, Caims D, Callan JF (2009) A Multifunctional Tripodal Fluorescent Probe: “Off−On” Detection of Sodium as well as Two-Input AND Molecular Logic Behavior. Org Lett 11:2229–2232
Kumar M, Dhir A, Bhalla V (2009) A molecular keypad lock based on the Thiacalix[4]arene of 1,3-alternate conformation. Org Lett 11:2567–2570
Sarkar D, Pramanik A, Biswas S, Karmakar P, Mondol TK (2014) Al3+ selective coumarin based reversible chemosensor: application in living cell imaging and as integrated molecular logic gate. RSC Adv 4:30666–30672
Sarkar D, Pramanik A, Mondol TK (2015) A novel coumarin based molecular switch for dual sensing of Zn(II) and Cu(II). RSC Adv 5:7647–7653
Suresh M, Das A (2009) New coumarin-based sensor molecule for magnesium and calcium ions. Tetrahedron Lett 50:5808–5812
Cao L, Jia C, Huang Y, Zhang Q, Wang N, Xue Y (2014) A highly selective fluorescence turn-on detection of Al3+ and Ca2+ based on a coumarin-modified rhodamine derivative. Tetrahedron Lett 55:4062–4066
El-Shekheby HA, Mangood AH, Hamza SM, Al-Kady AS, Ebeid EM (2014) A highly efficient and selective turn-on fluorescent sensor for Hg2+, Ag+ and Ag nanoparticles based on a coumarin dithioate derivative. Luminescence 29:158–167
Hou J, Liu B, Li K, Yu K, Wu M, Yu X (2013) Two birds with one stone: Multifunctional and highly selective fluorescent probe for distinguishing Zn2+ from Cd2+ and selective recognition of sulfide anion. Talanta 116:434–440
Wang H, Feng Y, Meng S (2012) A novel fluorescent sensor for Fe3+ and Cr3+ based on a calix[4]arene bearing two coumarin units. J Chem Res 36:587–589
Wu J, Sheng R, Liu W, Wang P, Zhang H, Ma J (2012) Fluorescent sensors based on controllable conformational change for discrimination of Zn2+ over Cd2+. Tetrahedron 68:5458–5463
Chattopadhyay N, Mallick A, Sengupta S (2006) Photophysical studies of 7-hydroxy-4-methyl-8-(4′-methylpiperazin-1′-yl) methylcoumarin: A new fluorescent chemosensor for zinc and nickel ions in water. J Photoch Photobio A 177:55–60
Liu A, Yang L, Zhang Z, Zhang Z, Xu D (2013) A novel rhodamine-based colorimetric and fluorescent sensor for the dual-channel detection of Cu2+ and Fe3+ in aqueous solutions. Dyes Pigments 99:472–479
Ang JQ, Nguyen BTT, Toh CS (2011) A dual K+–Na+ selective Prussian blue nanotubes sensor. Sensors Actuators B Chem 157:417–423
Goswami S, Das S, Aich K, Sarkar D, Mondal TK (2013) Colorimetric as well as dual switching fluorescence ‘turn on’ chemosensors for exclusive recognition of Zn2+ and image in aqueous solution: experimental and theoretical studies. Tetrahedron Lett 54:6892–6896
Devaraj S, Tsui YK, Chiang CY, Yen YP (2012) A new dual functional sensor: highly selective colorimetric chemosensor for Fe3+ and fluorescent sensor for Mg2+. Spectrochim Acta A 96:594–599
Beltrán OG, Cassels BK, Pérez C, Mena N, Núñez MT, Martínez NP (2014) Coumarin-based fluorescent probes for dual recognition of copper(II) and iron(III) ions and their application in bio-imaging. Sensors 14:1358–1371
Singh TS, Mitra S, Chandra AK, Tamai N, Kar S (2008) A combined experimental and theoretical study on photoinduced intramolecular charge transfer in trans-ethyl p-(dimethylamino)cinamate. J Photochem Photobiol A Chem 197:295–305
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38:3098–3100
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Delly B, Ellis DE (1982) Efficient and accurate expansion methods for molecules in local density models. J Chem Phys 76:1949–1960
Delly B (1990) An all-electron numerical method for solving the local density functional for polyatomic molecules. J Chem Phys 92:508–517
Hohenberg P, Kohn W (1964) Inhomogeneous electron gas. Phys Rev 136(B):864–871
Kohn W, Sham L (1965) Self-consistent equations including exchange and correlation effects. Phys Rev 140:A1133–A1138
Moorthy S, Amal KM, Sukdeb S, Suresh E, Amit M, Rosa DL (2010) Azine-based receptor for recognition of Hg2+ ion: crystallographic evidence and imaging application in live cells. Org Lett 12:5406–5409
Yin SC, Zhang J, Feng HK, Zhao ZJ, Xu LW, Qiu HY (2012) Zn2+-selective fluorescent turn-on chemosensor based on terpyridine-substituted siloles. Dyes Pigments 95:174–179
Jayabharathi J, Thanikachalam V, Jayamoorthy K (2012) Effective fluorescent chemosensors for the detection of Zn2+ metal ion. Spectrochim Acta A 95:143–147
Reza A, Maisam P, Esmail T, Hassan K, Reza G (2011) Highly selective fluorescent recognition of Zn2+ based on naphthalene macrocyclic derivative. Spectrochim Acta A 82:200–204
Fegade U, Saini A, Sahoo SK, Singh N, Bendre R, Kuwar A (2014) 2,2′-(hydrazine-1,2-diylidenedimethylylidene)bis(6-isopropyl-3-methylphenol) based selective dual-channel chemosensor for Cu2+ in semi-aqueous media. RSC Adv 4:39639–39644
Wu QY, Anslyn EV (2004) Catalytic signal amplification using a heck reaction. An example in the fluorescence sensing of Cu(II). J Am Chem Soc 126:14682–14683
Zeng HH, Thompson RB, Maliwa BP, Fones GR, Moffet JW, Fierke CA (2003) Real-time determination of Picomolar free Cu(II) in seawater using a fluorescence-based fiber optic biosensor. Anal Chem 75:6807–6812
Hu J, Lü FT, Din LP, Zhang SJ, Fang Y (2007) A novel pyrene-based film: preparation, optical properties and sensitive detection of organic copper(II) salts. J Photochem Photobiol A 188:351–357
Lakowicz JR (2006) Principle of fluorescence spectroscopy. Plenum, New York
Benesi HA, Hildebrand JH (1949) A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J Am Chem Soc 71:2703–2707
Roy P, Dhara K, Manassero M, Ratha J, Banerjee P (2007) Selective fluorescence zinc ion sensing and binding behavior of 4-methyl-2,6bis(((phenylmethyl)imino)methyl)phenol: biological application. Inorg Chem 46:6405–6412
Liu Y, Zhang N, Chen Y, Wang L (2007) Fluorescence sensing and binding behavior of Aminobenzenesulfonamidoquinolino-β-cyclodextrin to Zn2+. Org Lett 9:315–318
Kotova OV, Eliseeva SV, Averjushkin AS, Lepnev LS, Vaschenko AA, Rogachev AY (2008) Zinc(II) complexes with Schiff bases derived from ethylenediamine and salicylaldehyde: the synthesis and photoluminescent properties. Russ Chem Bull Int Ed 57:1880–1889
Majumder A, Rosair GM, Mallick A, Chattopadhyay N, Mitra S (2006) Synthesis, structures and fluorescence of nickel, zinc and cadmium complexes with the N,N,O-tridentate Schiff base N-2-pyridylmethylidene-2-hydroxy-phenylamine. Polyhedron 25:1753–1762
Kang B, Weng L, Liu H, Wu D, Huang L, Lu C (1990) Syntheses, structures, and properties of vanadium, cobalt, and nickel compounds with 2-mercaptophenol. Inorg Chem 29:4873–4877
Gao C, Jin X, Yan X, An P, Zhang Y, Liu L (2013) A small molecular fluorescent sensor for highly selectivity of zinc ion. Sensors Actuators B Chem 176:775–781
Zhang D, Wang M, Chai M, Chen X, Ye Y, Zhao Y (2012) Label-free DNA sensor for detection of bladder cancer biomarkers in urine. Sensors Actuators B Chem 168:200–206
Thangaraj A, Gandhi S, Murugan I, Ayyanar S, Duraisamy C (2015) Aminobenzohydrazide based colorimetric and ‘turn-on’ fluorescence chemosensor for selective recognition of fluoride. Anal Chim Acta 876:1–8
Liu J, Meng X, Duan H, Xu T, Ding Z, Liu Y (2015) Two Schiff-base fluorescence probes based on triazole and benzotriazole for selective detection of Zn2+. Sensors Actuators B 227:296–303
Gupta VK, Mergua N, Kumawata LK, Singh AK (2015) Selective naked-eye detection of magnesium (II) ions using a coumarin-derived fluorescent probe. Sensors Actuators B Chem 207:216–223
Kumar V, Kumar A, Diwan U, Shweta R, Srivastava SK (2015) Salicylideneimines as efficient dual channel emissive probes for Al3+: harnessing ESIPT and ICT processes. Sensors Actuators B Chem 207:650–657
Bevington PR (1969) Data reduction and error analysis for the physical sciences. McGraw-Hill Inc, New York
Sarkar M, Banthia S, Samanta A (2006) A highly selective ‘off–on’ fluorescence chemosensor for Cr(III). Tetrahedron Lett 47:7575–7578
López MV, Vázquez ME, Gómez Reino C, Pedrido R, Bermejo MR (2008) A metallo-supramolecular approach to a half-subtractor. New J Chem 32:1473–1477
Fu Y, Feng Q, Jiang X, Xu H, Li M, Zang S (2014) New fluorescent sensor for Cu2+ and S2− in 100% aqueous solution based on displacement approach. Dalton Trans 43:5815–5822
Magri DC, Fava MC, Mallia CJ (2014) A sodium-enabled ‘Pourbaix sensor’: a three-input AND logic gate as a ‘lab-on-a-molecule’ for monitoring Na+, pH and pE. Chem Commun 50:1009–1011
Wang S, Men G, Zhao L, Hou Q, Jiang S (2010) Binaphthyl-derived salicylidene Schiff base for dual-channel sensing of Cu, Zn cations and integrated molecular logic gates. Sensors Actuators B Chem 145:826–831
Acknowledgements
Financial support through Start-Up Research Grant (Chemical Sciences) project No. SB/FT/CS-064/2012 from Science and Engineering Research Board (SERB), Government of India were gratefully acknowledged by Dr. T. Sanjoy Singh. The authors are indebted to Dr. S. itra and his research scholars for their help in TCSPC measurements. The authors are also highly acknowledged to CIF, IIT Guwahati for providing NMR and Mass spectra.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roy, N., Dutta, A., Mondal, P. et al. Coumarin Based Fluorescent Probe for Colorimetric Detection of Fe3+ and Fluorescence Turn On-Off Response of Zn2+ and Cu2+ . J Fluoresc 27, 1307–1321 (2017). https://doi.org/10.1007/s10895-017-2065-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-017-2065-7












 ) of Zn2+ ion and (
) of Zn2+ ion and ( ) of Cu2+ ion
) of Cu2+ ion







