Abstract
Rapid and sensitive detection of heavy-metal ions in natural water environments worldwide is urgently needed because of their severe threats to human health. In the present work, monolayer graphite-like flake C3N4 (g-C3N4) materials were applied as a new fluorescent sensor for the detection of trace silver ion in aqueous solution. The thickness of synthesized g-C3N4 was 0.45 nm and obtained by exfoliating twice with ultrasonic. With the presence of ethylene diamine tetraacetic acid as a screening agent, the highly sensitive sensor reached a low detection limit of 52.3 nmol/L for silver (I) ion and there was no disturbance when silver (I) ion coexisted with other metal ions in water samples. Under the optimal conditions, the monolayer g-C3N4 was successfully used to detect trace silver (I) ion in different environmental water and drinking water samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Silver is not only the most ancient metal with numerous applications in the fields like jewelry, coins, mirrors, anti-corrosive alloys, but also an important element used in electroplating, photographic and imaging industries. Moreover, the products containing silver nanoparticles are the most applicable nanomaterials in the last decade because of their excellent antibacterial activity and have been widely used in various fields including textiles, food containers, and medical devices [1]. The rapidly growing use of silver inevitably releases large amounts of soluble silver species to the environment and may cause environmental problems. It has been found that silver is toxic for humans at a concentration higher than 0.9 mmol/L in drinking water. Excessive intake of silver ions (Ag+) causes argyria in humans and silver deposition has been found in the skin, gingival, cornea, liver, and kidney of patients [2]. For these reasons, determination of trace amount of silver ions with high sensitivity, specificity and rapidity in environmental and industrial samples has great importance.
Various instrumental techniques have been employed for the determination of silver species in different real samples, including atomic absorption spectroscopy (AAS) [3], inductively coupled plasma–mass spectrometry (ICP-MS) [4], inductively coupled plasma optical emission spectrometry and flow injection analysis [5]. However, these techniques need highly sophisticated instruments and a complex preparation process, which are not applicable in most cases. Optical sensing systems have been gained great attention for the effective and sensitive detection. Various organic compounds, quantum dots, nanoparticles, polymers and DNA have been used as chemosensors [6]. For examples, fluorescence switch that consists of DNA-templated silver nanoclusters [7] or nanographene carbon composite potentiometric sensor for the determination of trace amounts of silver (I) ion were recently fabricated and the detection limits were lower to 4.17 × 10−9 mol/L [8]. Room temperature cation exchange reaction in nanocrystals were used to amplify the signal and the detection limit of Ag+ using ICP-MS was 0.3 pg/L [9]. Although these methods show high sensitivity for Ag+ detection, they have several drawbacks such as laborious synthesis of reagents, complex detection system, etc. The development of convenient and direct method for the assay of silver in different real samples is still in an urgent need.
Fluorescence spectroscopy is a commonly method for the detection of organic pollutants [10] or heavy metal ions [11], etc. The majority of fluorescent probes commonly using metal nanoparticles require modification and from their own toxicity considerations. In recent years, low-toxic graphene carbon nitrogen compounds (g-C3N4) fluorescent porous material much attention and numerous studies have been devoted to synthesizing and designing highly functional g-C3N4 materials [12]. Due to their unique catalytic, electrical conductivity and adsorption properties, g-C3N4 materials have been successfully applied as photocatalysts for the degradation of organic dyes in water under light irradiation [13]. It is also an environment-friendly and biocompatibility fluorescence materials for bioimaging and biomedical applications [14]. Unlike other sensors with optical receptor hooking on the porous materials, g-C3N4 itself is the receptor and thus performs higher sensitivity. Lee et al. [15] had studied feasibility of detecting trace amount Cu2+ in aqueous solution with g-C3N4 as optical sensor via observing its photoluminescence quenching effects and the limit of detection (LOD) of Cu2+ in aqueous solution was 12.336 nmol/L. Subsequently, Barman and his coworkers [16] explored g-C3N4 quantum dots as optical sensor to detect mercuric and iodide ions via a mechanism terms namely “ON-OFF-ON” and the lower LOD for Hg2+ was about 10−9 mol/L. Another turn on fluorescence sensor, Cu2+-cubic mesoporous g-C3N4, was also developed for detection of CN− and its LOD in both aqueous solution and human blood serum was 80 nmol/L [17]. However, its excellent chemical resistance emerged in the work done by Gillan [18] showed that g-C3N4 was almost insoluble in water, ethanol, toluene, diethyl ether and THF, which may be due to the van der Waals between its layers stacking. Recently, studies had shown that the use of liquid exfoliating method can also obtained nanosheets structure, but the synthesis needs more than 10 h [19, 20].
Herein, we prepared monolayer g-C3N4 by using liquid exfoliating method with ultrasonic oscillating for twice. The obtained monolayer g-C3N4 shows good selective fluorescent response to Ag+ and it was then utilized as a fluorosensor to detect Ag+ in aqueous solutions in the presence of other metal ions.
Experiments
Reagents and Apparatus
Reagents
Dicyandiamide (Aladdin, Shanghai, China), ferric nitrate, barium nitrate, beryllium nitrate, mercury nitrate, potassium nitrate, manganese nitrate, zinc nitrate, chromium nitrate, cadmium nitrate, silver nitrate, lead nitrate, copper nitrate, cobalt nitrate, aluminum nitrate, nickel nitrate, magnesium nitrate and ethylene diamine tetraacetic acid (EDTA) were purchased from Guangzhou Chemical Reagent Ltd. (Guangzhou, China). All reagents were used directly. Ultra-pure water used throughout the whole experiments was obtained from the MILLI-Q gradient A10 system (Millipore, Bedford, MA, USA).
Apparatus
The fluorescence intensity (FL) was performed by Hitachi F-2500 fluorescence spectroscopy. UV-vis spectroscopy was recorded on a TianMei UV-VIS 1700 spectrophotometer. The Fourier transform infrared spectroscopy (FT-IR) experiment was carried out on a Shimadzu Prestige-21 FT-IR spectrometer in a KBr pellet, scanning from 4000 to 400 cm−1 at room temperature. Atomic force microscopy (AFM) study was performed by means of Veeco DI nanoscope Multi Mode V system. X-ray diffraction (XRD) patterns of the g-C3N4 was obtained on a Philips X ray diffractometer (PW 3040/60) using Cu Kα target at 40 kV and 40 mA. Scanning electron microscopy (SEM) was performed with a Hitachi S-4800 scanning electron microanalyzer using an accelerating voltage of 15 kV.
Preparation of the Bulk g-C3N4 Porous Materials
The bulk g-C3N4 was prepared by directly pyrolysis of dicyandiamide in a porcelain crucible. In detail, five grams (5.0 g) of dicyandiamide was added into a porcelain crucible and placed in a muffle furnace (Shanghai, China); Then it was heated at 520 °C for 4 h under air condition with a ramp rate of about 4.5 °C/min for both the heating and cooling processes. In order to obtain the finally bulk g-C3N4, the obtained yellow powder was further heated at 500 °C in another porcelain crucible for 2 h with a ramp rate of 2.3 °C/min.
Preparation of Monolayer g-C3N4 Materials
The monolayer g-C3N4 materials were prepared by a liquid exfoliating method. In brief, 50.0 mg of bulk g-C3N4 powder was dispersed in 100 mL of water with ultrasonic dispersion for 2 h. The formed g-C3N4 suspension was then centrifuged at 5000 rpm to remove the unexfoliated bulk g-C3N4 and a stable g-C3N4 suspension can be obtained. And then, the obtained g-C3N4 suspension was further exfoliated with ultrasonic for 2 h. A stable monolayer g-C3N4 material with highly water-dispersity was obtained and it can be kept for months without depositing.
Fluorescence Measurements
The fluorescence measurement of Ag+ was performed at room temperature. 1.0 mL monolayer g-C3N4 was added into an aqueous solution with V1 mL silver ion and V2 mL of water, wherein V1 + V2 = 1.0 mL. And then, it was measured triplicate according to the requirements.
For the interference tests, 1.0 mL of monolayer g-C3N4 were mixed with 0.01 mL 1.65 μmol/L silver ion solution and 0.05 mL interference ions with the same concentration of Ag+, then 0.01 mol/L EDTA was added into this mixed solution and diluted to 2.0 mL to obtain the finally sample solution. After 2.0 min, the PL intensity was measured and all of experiments were test for triplicate.
All of the water samples were filtered with a 0.45 μm membrane filter, and then measured as the same process of interference tests.
The value of LOD was calculated as LOD = 3σ/S, where σ is the standard deviation of PL intensity of monolayer g-C3N4 at 435 nm in the absence of Ag+ and S is the slope between PL intensity at 435 nm versus Ag+ concentration.
Results and Discussion
AFM measurement was carried out to understand the thickness of obtained monolayer g-C3N4 and the results are shown in Fig. 1a and 1b. As can be seen from Fig. 1b, the randomly measured nanosheets were nearly 0.45 nm of thickness. This value of the thickness was as same as that of grapheme reported before [21], indicating the exfoliated nanosheets are comprised of only C − N monolayer.
XRD analysis was also carried out to investigate the crystal structure of the samples. As shown in Fig.1c, two characteristic peaks of bulk g-C3N4 could be observed. One is the strong peak at 27.6° corresponding to the (002) plane, which is attributed to the interlayer distance d = 0.33 nm in bulk g-C3N4. The other is the week peak at 13.2° corresponding to the (100) plane, which is related to the inplane structural packing motif of tri-s-triazine units. For the monolayer g-C3N4, the significant decrease in the peak at 27.6° can be attributed to the reduced correlation length introduced by nanostructuring [22].
Further evidence for the g-C3N4 phase comes from the FT-IR spectra and the results are showed in Fig.1d. The board peaks between 3000 to 3500 cm−1 corresponded to the N–H stretching and hydrogen-bonding interactions. The peaks at 1101 and 1633 cm−1 were contributed to the typical stretching vibration of connected units of C–N(−C)–C (full condensation) or C–NH–C (partial condensation), while the absorption band located at about 810 cm−1 was originated from the viabration of triazine rign. It is worth noting that there were no differences of FT-IR peak between the bulk g-C3N4 and the monolayer g-C3N4, which indicated that the chemical structure of the monolayer g-C3N4 was as same as that of the bulk g-C3N4.
In addition, Raman spectra were also carried out to study the structure changes between as-exfoliated monolayer g-C3N4 and bulk counterpart. As can be seen from Fig. 2, the Raman spectrum of as-exfoliated g-C3N4 nanosheets showed nearly identical Raman shifts of bulk g-C3N4, indicating the exfoliated ultrathin g-C3N4 nanosheets retain the same crystal structure of bulk g-C3N4. Comparing with bulk g-C3N4, the Raman spectra of monolayer g-C3N4 nanosheets showed a blue shift of about 4 cm−1 (Fig. 2b), ascribing to the phonon confinement effect of the as-exfoliated nanosheets and inferring their ultrathin thickness. At the same time, both of the Raman spectra of bulk g-C3N4 and monolayer g-C3N4 nanosheets show no shifts of carbon materials, which often located at about 1350 cm−1 and 1580 cm−1, indicating the high purity of the as-exfoliated nanosheets.
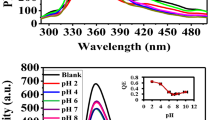
The monolayer g-C3N4 exhibited exceptional solubility in water and the solution were stable for several weeks without any precipitation. Its optical properties were examined in the solution. Figure 3 shows the monolayer g-C3N4 has a strong absorption at 312 nm in UV light; A strong fluorescence peak centered at 435 nm was observed when the aqueous solution was excited at 365 nm. The nearly transparent feature as well as the well-defined Tyndall effect of the monolayer g-C3N4 solution as displayed in the inset of Fig. 3 indicate the presence of highly monodisperse monolayer nanosheets in water.
The selectivity of the monolayer g-C3N4 towards Ag+ and other different metal ions in water was investigated. The change in fluorescence intensity (FL) before and after the addition of interfering ions by five times of molar concentrations to the Ag+ solution of 1.65 μmol/L are shown in Fig. 4a. Comparing to g-C3N4 + Ag(I), the FL differences of g-C3N4 + M(n) (Fe3+, Ba2+, Be2+, Hg2+, K+, Mn2+, Zn2+, Pb2+, Cr3+, Mg2+, Ni2+, Co2+, Ca2+, Cd,2+ and Al3+) were much lower, indicating no significant spectral change of monolayer g-C3N4 observing in the presence of these interface ions. But after the silver (I) ion was added into the solution of g-C3N4 + M(n), significant FL differences (g-C3N4 + M(n) + Ag(I)) were observed and the intensities were similar to that of g-C3N4 + Ag(I). The quenching of FL response of monolayer g-C3N4 with various metal ions clearly shows the selectivity of Ag+ to bind with it. However, it was reported that the PL intensity of g-C3N4 could be gradually quenched with increasing Cu2+ concentration [15]. In the present work, a decrease about 25 % in the PL intensity was also observed when Cu2+ coexisted with other cations.
Selective FL response of monolayer g-C3N4 without EDTA, upon adding 1.65 μmol/L Ag+, and upon adding 8.25 μmol/L other metal ion solutions (a); FL response of monolayer g-C3N4 with 0.01 mol/L EDTA, upon adding 1.65 μmol/L Ag+, 8.25 μmol/L Cu2+ solutions, respectively, and upon adding both Ag+ and Cu2+ solutions (b)
EDTA is the most widely used chelator in analytical chemistry, which forms strong 1:1 complexes with many metal ions. Most of the formation constants for EDTA complexes are larger than 1010 and trend to be larger for more positively charged cations [23]. For example, the formation constant for Cu2+ of 1018.8 is much larger than that for Ag+ (107.3), indicating the interference of Cu2+ in the present work can be eliminated by EDTA. Figure 4b presents the quenching results of monolayer g-C3N4 with Ag+ and Cu2+ in the presence of EDTA. For the tests, 1.65 μmol/L Ag+ ion and 8.25 μmol/L Cu2+ were used to quench the PL intensity of monolayer g-C3N4 in the presence of 0.01 mol/L EDTA. The PL intensity of monolayer g-C3N4 was significantly quenched when Ag+ was added into the system, and similar results were also observed when the EDTA was present as chelator, suggesting that the added EDTA showed slight influence on the quench of PL intensity for Ag+. On the other hand, although the PL intensity of monolayer g-C3N4 was also obviously quenched when Cu2+ was added into the system, a slightly quenching was obtained in the presence of EDTA. Further experiments on the test of PL intensity of monolayer g-C3N4 with Ag+, Cu2+ and EDTA also confirmed that the interference of Cu2+ can be successfully eliminated.
In order to evaluate the sensitivity, the PL changes were monitored with increasing concentration of Ag+ ion. As shown in Fig. 5, the intensity of the PL emission centered at 435 nm is highly sensitive to Ag+ and decreases with an increasing concentration of Ag+. The relative PL intensity (F′ = F 0 -F, where F 0 and F are fluorescence intensities at 435 nm in the absence and presence of Ag+ ions) showed good linear correlation when the concentration of Ag + increased from 0.16 to 1.65 μmol/L. The correlation coefficient (R2) was more than 0.99 and the relative standard deviation (RSD) was lower than 5 %. The limit of detection was as low as 52.7 nmol/L at a signal-to-noise ratio of 3.0, which is about 8 times lower than the maximum level of Ag+ in drinking water permitted by the World Health Organization (WHO).
In order to evaluate whether the monolayer g-C3N4 based fluorescent sensor was applicable as a real diagnostic system, different samples including environmental water and drinking water samples were analyzed. The samples were analyzed immediately after filtration with a 0.45 μm membrane filter. The results showed that no Ag+ was detected in these samples probably because their concentrations were far below the limit of detection of the proposed method. Good recoveries of the spiked Ag+ in these samples with different levels ranging from 80.8 % to 115.8 % were obtained and the results are shown in Table 1.
Conclusions
In summary, although the bulk g-C3N4 had showed good sensitive PL response to Cu2+ without interference by other metal ions including Ag+, the monolayer g-C3N4 showed more selective and sensitive to the Ag+ than the Cu2+. On the application of monolayer g-C3N4 fluorescence sensor, the interference of Cu2+ coexisting in the samples can be simply screened by adding EDTA chelant. Under such an optimal conditions, the monolayer g-C3N4 can be successfully used to detect trace Ag+ in real water samples.
References
Dastafkan K, Khajeh M, Ghaffari-Moghaddam M, Bohlooli M (2015) Silver nanoparticles for separation and preconcentration processes. TrAC-Trends Anal. Chem. 64:118–126
Greulich C, Braun D, Peetsch A, Diendorf J, Siebers B, Epple M, Koller M (2012) The toxic effect of silver ions and silver nanoparticles towards bacteria and human cells occurs in the same concentration range. RSC Adv 2:6981–6987
Mohammadi SZ, Afzali D, Heshmati Z (2013) Ligand-less in situ surfactant-based solid phase extraction for preconcentration of silver from natural water samples prior to its determination by atomic absorption spectroscopy. Toxico. Environ. Chem. 95:1299–1308
Poitras EP, Levine MA, Harrington JM, Essader AS, Fennell TR, Snyder RW, Black SL, Sumner SS, Levine KE (2015) Development of an analytical method for assessment of silver nanoparticle content in biological matrices by inductively coupled plasma mass spectrometry. Biol Trace Elem Res 163:184–192
Zhang JF, Zhou Y, Yoon J, Kim JS (2011) Recent progress in fluorescent and colorimetric chemosensors for detection of precious metal ions (silver, gold and platinum ions). Chem Soc Rev 40:3416–3429
Singha S, Kim D, Seo H, Cho SW, Ahn KH (2015) Fluorescence sensing systems for gold and silver species. Chem Soc Rev 44:4367–4399
Lee J, Park J, Lee HH, Park H, Kim HI, Kim WJ (2015) Fluorescence switch for silver ion detection utilizing dimerization of DNA-Ag nanoclusters. Biosens. Bioelectron. 68:642–647
Afkhami A, Shirzadmehr A, Madrakian T, Bagheri H (2015) New Nano-composite potentiometric sensor composed of graphene nanosheets/thionine/molecular wire for nanomolar detection of silver ion in various real samples. Talanta 131:548–555
Huang K, Xu KL, Tang J, Yang L, Zhou JR, Hou XD, Zheng CB (2015) Room temperature cation exchange reaction in nanocrystals for ultrasensitive speciation analysis of silver ions and silver nanoparticles. Anal Chem 87:6584–6591
Xu WG, Wang X, Cai ZW (2013) Analytical chemistry of the persistent organic pollutants identified in the Stockholm convention: a review. Anal Chim Acta 790:1–13
Vazquez-Gonzalez M, Carrillo-Carrion C (2014) Analytical strategies based on quantum dots for heavy metal ions detection. J. Biomed. Optics 19:101503
Li XF, Li M, Yang JH, Li XY, Hu TJ, Wang JS, Sui YR, Wu XT, Kong LN (2014) Synergistic effect of efficient adsorption g-C3N4/ZnO composite for photocatalytic property. J. Phy. Chem. Solids 75:441–446
Li YH, Sun YJ, Dong F, Ho WK (2014) Enhancing the photocatalytic activity of bulk g-C3N4 by introducing mesoporous structure and hybridizing with graphene. J. Colloid Interf. Sci. 436:29–36
Zhang XD, Xie X, Wang H, Zhang JJ, Pan BC, Xie Y (2013) Enhanced photoresponsive ultrathin graphitic-phase C3N4 nanosheets for bioimaging. J Am Chem Soc 135:18–21
Lee EZ, Jun YS, Hong WH, Thomas A, Jin MM (2010) Cubic mesoporous graphitic carbon(IV) nitride: an all-in-one chemosensor for selective optical sensing of metal ions. Angew Chem Int Ed 49:9706–9710
Barman S, Sadhukhan M (2012) Facile bulk production of highly blue fluorescent graphitic carbon nitride quantum dots and their application as highly selective and sensitive sensors for the detection of mercuric and iodide ions in aqueous media. J Mater Chem 22:21832
Lee EZ, Lee SU, Heo NS, Stucky GD, Jun YS, Hong WH (2012) A fluorescent sensor for selective detection of cyanide using mesoporous graphitic carbon (IV) nitride. Chem Commun 48:3942–3944
Wang Y, Wang XC, Antonietti M (2012) Polymeric graphitic carbon nitride as a heterogeneous organocatalyst: from photochemistry to multipurpose catalysis to sustainable chemistry. Angew Chem Int Ed 51:68–89
Tian JQ, Liu Q, Asiri MA, Al-Youbi AO (2013) Ultrathin graphitic carbon nitride nanosheet: a highly efficient fluorosensor for rapid, ultrasensitive detection of Cu2+. Anal Chem 85:5595–5599
Zhang XL, Zheng C, Guo SS, Li J, Yan H, Chen G (2014) Turn-on fluorescence sensor for intracellular imaging of glutathione using g-C3N4 nanosheet–MnO2 sandwich nanocomposite. Anal Chem 86:3426–3434
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field effect in atomically thin carbon films. Science 306:666–669
Goettmann F, Fischer A, Antonietti M, Thomas A (2006) Chemische synthese von mesoporösen kohlenstoffnitriden in harten templaten und ihre anwendung als metallfreie katalysatoren in friedel-crafts-reaktionen. Angew Chem 118:4579–4583
Haris DC (2010) Quantitative chemical analysis (8th). Clancy Marshall, NY
Acknowledgments
We are grateful to Dr. Xiao-hua Xiao from Sun Yat-sen University for his helpful advice. This work was supported by the National Natural Science Foundation of China (No.21275056).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cao, Y., Wu, W., Wang, S. et al. Monolayer g-C3N4 Fluorescent Sensor for Sensitive and Selective Colorimetric Detection of Silver ion from Aqueous Samples. J Fluoresc 26, 739–744 (2016). https://doi.org/10.1007/s10895-016-1764-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-016-1764-9