Abstract
An iminocrown ether was synthesized and its fluorescence properties were studied in the presence of a variety of cations and anions in 99 % aqueous medium. The results revealed the interesting ability of the iminocrown ether in discriminately detection of Cr(III) and Cr(VI) ions in addition to detection of Hg2+ ion. Among various environmentally relevant metal ions, Cr3+ and Hg2+ enhanced and quenched the fluorescence emission, respectively and among anions only dichromate ion, Cr(VI), quenched the emission while the rest of ions insignificantly influenced the fluorescence emission. Selectivity of the iminocrown ether was also investigated and proved in the presence of excess of common competing ions. Furthermore, the fluorescence intensity of the iminocrown ether was studied as a function of concentrations of the three ions by performing a titration experiment for each one of them. The detection limits of 5.36 × 10−8, 2.06 × 10−6, and 7.49 × 10−8 mol L−1 were also calculated for Hg2+, Cr3+, and Cr(VI), respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Transition metals are significantly important mostly due to their either hazardous nature or crucial roles in environmental and biological systems [1, 2]. Mercury and chromium are two of these metal-ions which have attracted a great deal of attention. Mercury as Hg2+ inorganic salt is one of the most abundant toxic metals and causes environmental problems [3], in addition to a wide variety of health issues such as severe damage in central nervous system, heart, kidney, and genes even in low concentrations [4–6]. Furthermore, among the different valence states of chromium, Cr(III) and Cr(VI) are the most stable and hence the most prevalent ones in nature. Cr3+ as an essential trace element for biochemical processes, in addition to improving insulin sensitivity plays a vitally important role in metabolism of carbohydrates, proteins, and lipids [7]. Deficiency of chromium leads to a series of health problems such as diabetes and cardiovascular disease [8]. On the other hand, Cr(VI) easily finds its way into the environment as a pollutant because of its widespread utilization in surface industry, batteries, welding, corrosion control, catalysis, and etc. [9, 10]. Cr(VI) is more hazardous to the environment and humans compared to the other valence states of chromium and leads to severe damages in liver, kidney, and DNA [11, 12]. Hence, development of efficient methods for monitoring trace amounts of mercury and chromium ions has gained considerable attention.
A wide variety of methods such as atomic absorption [13, 14], coupled plasma-mass spectroscopy [15, 16], and voltammetry [17, 18] have been used for mercury and chromium detection in solutions. These methods are costly, time consuming and require precise pretreatments, trained operators and complicated instrumentation. Fluorescent sensors have proved to be convenient alternatives for these methods due to fast response times, low costs, easy detection, high sensitivity, and high selectivity [19, 20]. Thus, development of novel fluorescent sensors is of remarkable interest. Though, a series of fluorescent sensors have been reported for individually detection of Hg2+, Cr3+ and Cr(VI) ions [21–26], to the best of our knowledge, no fluorescent sensor has been reported capable of detecting these three ions simultaneously. Moreover, most of the reported sensors for these ions suffer from poor solubility and applicability in aqueous media [27–30]. Also some reports have been published for detection of both Hg2+ and Cr3+ ions, [31, 32] which fail in distinguishing between the two ions. On the other hand, most of the fluorescent sensors reported for Cr3+ are of the quenching type because of its paramagnetic nature. Thus, it is an impressive challenge to develop sensors of enhancing type for Cr3+. Considering the vitally important role of Cr3+ in biological processes and the toxic nature of Cr(VI), distinguishing between the two valence states is significantly important and demanded. As far as we are aware, some reports have been published for distinguishing between Cr3+ and Cr(VI) [33, 34], but no claim has been published reporting a fluorescent sensor capable of discriminating between Cr(III) and Cr(VI) ions, meanwhile differentiating them from rest of the ions. Designing a single sensor capable of discriminately detection of Cr(III) and Cr(VI) in addition to detection of Hg2+ with functionality in mostly aqueous medium can facilitate detection of the three ions and reduce the costs considerably beside saving the time and trouble of several sample preparation.
Crown ethers, first discovered by Pedersen [35], have been the subject of many studies in various fields such as chromatography [36, 37], phase transfer catalysis [38], ion extraction and transport [39, 40], membranes [41], and electrochemistry [42, 43]. Crown ethers are well-known for selectively formation of complexes with metal ions by electrostatic ion-dipole interaction between metal-ions and electron rich donor atoms of the crown ether. Complex formation of crown ethers is highly influenced by different parameters including the relative size of the crown ether cavity and the metal ion radius, number and type of electron donor atoms in the ring, basicity of the donor atoms, coplanarity of the crown ring, and the electrical charge on the metal ion [35]. These unique properties of crown ethers in complex formation make them promising compounds for selectively detection of metal ions. Nitrogen-containing crown ethers (imino/azacrown ethers) are particularly known for their high affinity toward transition metals [44] and have been the subject of a series of studies in metal recognition area [45]. To date a number of fluorescent sensors based on nitrogen-containing crown ethers have been published for detection of various metal-ions [46, 47] but, only few of them contained imino groups [48]. The interaction of these imino groups with-metal ions result in dramatic changes in the fluorescence properties of the fluorophore which is the key factor in the fluorescent sensors. Herein, we report application of an iminocrown ether as a fluorescent sensor in both cation and anion recognition in 99 % aqueous medium. The three benzo groups, in addition to the quite fixed cavity of the iminocrown ether result in a solid structure and hence a selective sensor. Besides, the two nitrogen atoms in the structure of iminocrown ether enhance its affinity toward transition metals.
Experimental
Materials and Reagents
Salicylaldehyde, O-phenylenediamine (Sigma Aldrich), potassium carbonate, 1,2-diaminobenzene, methanol, dimethylformamide (Merck), and dibromoethane (Acros Organics) were purchased and used without further purification. Stock solutions of all cations were prepared using their nitrate salts, and those of anions were prepared using their sodium or potassium salts.
Aparatus
FT-IR spectra were obtained in KBr disks on a WQF-510A FTIR spectrometer in 400–4000 cm−1 region. Fluorescence measurements were collected on a Cary Eclipse Fluorescent Spectrophotometer. UV-vis absorption spectra were obtained using an Analytik Jena Specord S600 spectrophotometer.
Synthesis of BFE
1,2-Bis(2-formylphenoxy)ethane (BFE) was synthesized based on a previous report [49] with slight modification. Salicylic aldehyde (1.560 g, 12.77 mmol) and 1,2-dibromoethane (1.0 g, 5.32 mmol) were added to dimethylformamide (8.0 mL) containing potassium carbonate (1.765 g, 12.77 mmol), and the mixture solution was stirred for 24 h at 50 °C under nitrogen protection. The resulted dark brown solution was then poured onto 200 mL water while stirring, and 1,2-bis(2-formylphenoxy)ethane was collected by filtration as a brown powder. Finally, colorless crystals were obtained after recrystallization from ethanol and subsequently from ethyl acetate. Yield: 1.09 g (75 %). M.p.: 144–146 °C (lit. [49] 146–148 °C). IR (KBr, cm−1): 3083, 2949, 2870, 1688, 1598, 1483, 1455, 1407, 1250, and 1067.
Synthesis of TBC
3,4:9,10:13,14-Tribenzo-1,12-diaza-5,8-dioxacyclotetradecane-1,11-diene (TBC) was synthesized similar to the procedure reported before [50]. To a solution of BFE (2.7 g, 10 mmol) in 150 mL methanol, (1.08 g, 10 mmol) ortho-phenylene diamine in 30 mL methanol was added and the mixture was refluxed for 3 h. The solvent was evaporated to 30 mL and cooled to room temperature and then 60 mL of water was added while stirring. The suspension was let stand at 0 °C to yield a precipitate which was filtered off and dried at ambient temperature. Yield: 2.4 g (70 %). M.p.: 177–179 °C (lit. [51] 179–180 °C). IR (KBr, cm−1): 3064, 2925, 2880, 1641, 1600, 1585, 1483, 1455, 1407, 1231, and 1052. The overall synthetic procedure of TBC is depicted in Scheme 1.
Results and Discussion
Cation Sensing
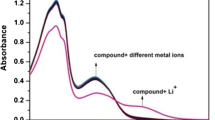
Sensing properties of TBC toward cations were studied in EtOH/H2O (1:99, v/v) solution following excitation at 290 nm which caused an emission with a maximum at 373 nm. The fluorescence emission changes were monitored upon individually addition of 20 equivalents of different cations including Hg2+, Cr3+, Mn2+, Mg2+, Na+, K+, Ca2+, Cd2+, Pb2+, Fe2+, Cu2+, Zn2+, Co2+, and Ni2+ to 3 mL 1 × 10−5 M solution of TBC. The results (Fig. 1) revealed that the fluorescence intensity of TBC quenched in the presence of Hg2+ ions, whereas the presence of Cr3+ ions had an opposite effect on the spectrum and enhanced the intensity. The rest of ions negligibly influenced the fluorescence spectrum of TBC. The quenching effect of Hg2+ ion can be attributed to the interaction of the ion with electron-donor oxygen and nitrogen atoms of TBC and heavy atom effect [52]. On the other hand, the enhancement of the fluorescence intensity in the presence of Cr3+ is probably due to the chelation of TBC with Cr3+ ion and inhibition of lone-pair electrons of the imine nitrogen atoms from taking part in photoinduced electron transfer (PET) process [53]. The strong affinity of Cr3+ to bind with receptors containing sp2 nitrogen atoms is already well-known [54].
The quenching mechanism of the sensor with Hg2+ was further investigated using the following Stern-Volmer equation (1):
where F0 and F are the fluorescence intensities in the absence and presence of the quencher, respectively. [Q] is the concentration of the quencher, i.e. Hg2+ and Ksv is the quenching constant. Plotting the F0/F versus concentration of Hg2+ ion gave a linear plot (Fig. 2). The linearity of the plot indicates that the quenching mechanism is either purely static or purely dynamic. To determine the right mechanism, absorption spectra of TBC was recorded in absence and presence of Hg2+ ion. The absorption spectrum of TBC in presence of Hg2+ is distorted from that of TBC in absence of Hg2+ ion (Fig. 3) which results from a complex formation between TBC and Hg2+ ion before the excitation and suggests that a static quench has taken place. The quenching constant for Hg2+ was calculated to be 5.03 × 104 mol−1 by Stern-Volmer equation.
To determine the stoichiometry between TBC and the two metal-ions, the Job’s plot method was applied (Fig. 4). For both Cr3+ and Hg2+ a 1:1 complexation was obtained.
Binding Constant
The binding constants of TBC to both Hg2+ and Cr3+ were calculated by Benesi-Holdebrand equation (2):
where F0, Fx, and F∞ are the emission intensities in absence, at an intermediate, and at a metal-ion concentration of a complete interaction, respectively. K is the binding constant, [C] is the concentration of the metals, and n is the number of binding metal-ions to TBC (here n = 1). The values of K obtained from the slopes were 1.16 × 105 and 1.32 × 104 mol−1 for Hg2+ and Cr3+, respectively.
Selectivity of the sensor toward Hg2+ and Cr3+ was also investigated in the presence of 5 equivalents of competing metal-ions (except for Hg2+ and Cr3+ competition which was performed in presence of 1 equivalent of each ion) in EtOH/H2O (1:99, v/v) solution. The results (Fig. 5) demonstrated that even much higher concentrations of the competing ions barely affected the fluorescence intensity of Hg2+ + TBC systems and proved the high selectivity of the sensor toward Hg2+ ion. Furthermore, equivalent amounts of Cr3+ did not interfere in Hg2+ detection which means TBC has more affinity toward Hg2+ than Cr3+. The results of selectivity experiment for Cr3+ (Fig. 6) also revealed the selectivity of the sensor toward Cr3+ ion except in presence of Hg2+ ion.
To evaluate the influence of Hg2+ and Cr3+ concentrations on the fluorescence intensity of the TBC, titration experiment was performed in EtOH/H2O (1:99, v/v) solution for each ion. The sensor demonstrated higher sensitivity toward Hg2+ compared to Cr3+ and it was observed that relatively lower starting concentrations of Hg2+ ions were utilized for the experiment. With increasing the ions concentration, the emission intensity of TBC decreased gradually (Fig. 7) and a good linearity was obtained between Hg2+ concentrations and reduction in the fluorescence intensity of the sensor (Fig. 7, inset). As for Cr3+ ions, with the increase of the ion concentration, the fluorescence emission intensity increased (Fig. 8) and resulted in a linear plot of the Cr3+ concentrations versus the fluorescence intensity (Fig. 8, inset). Furthermore, detection limits (DL) were calculated for both Hg2+ and Cr3+ in EtOH/H2O (1:99, v/v) solution on the basis of DL = 3σ/m, where σ is the standard deviation of blank solutions and m is the slope of fluorescence intensity against concentration of the target ion. The detection limit values obtained as 5.36 × 10−8 and 2.06 × 10−6 mol L−1 were for Hg2+ and Cr3+, respectively which show the sensitivity of the sensor toward both ions particularly Hg2+.
Fluorescence emission of TBC upon addition of Hg2+ ions in EtOH/H2O (1:99, v/v) solution, [TBC] = 1 × 10−5 M, [Hg2+] = (0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1, 2, 3, 4, 5, 6, 7, 8, 9 and10 eq.). Inset: fluorescence emission as a function of Hg2+ concentration (0–1 eq.) (λex : 290 nm and λem : 373 nm)
Anion Sensing
The sensing properties of TBC were also investigated toward anions under the same conditions as for cations. Fluorescence emission of TBC was studied in the presence of a variety of anions including Cr2O7 2−, CN−, HCO3 −, CO3 2−, HPO4 2−, CH3CO2 −, NO3 −, H2PO4 −, S2O3 2−, F−, Cl−, Br−, I−, SO4 2−, SCN−, and NO2 − (Fig. 9). It was observed that the fluorescence emission interestingly quenched in the presence of dichromate ions, Cr(VI), which is a stable form of chromium other than Cr(III). The rest of anions either slightly increased or negligibly decreased the intensity, meaning no interference in Cr(VI) detection. The quenching mechanism of TBC with Cr(VI) was further investigated by the same procedure previously applied for Hg2+ ion using the Stern-Volmer equation (1). Results (Fig. 10) show a linear plot of F0/F against the concentration of Cr(VI) ion which indicates an either purely static or purely dynamic quench as the quenching mechanism of TBC emission by Cr(VI) ion. Since the absorption spectrum of TBC in the presence of Cr(VI) ion distorts from that of TBC in absence of the ion, a static quench occurs. The value of quenching constant was also calculated as 2.37 × 105 mol−1. The complexation between TBC and Cr(VI) was also determined by Job’s plot method and results showed a 1:1 stoichiometry (Fig. 11).
Fluorescence emission of TBC in the presence of different anions including Cr2O7 2−, CN−, HCO3 −, CO3 2−, HPO4 2−, CH3CO2 −, NO3 −, H2PO4 −, S2O3 2−, F−, Cl−, Br−, I−, SO4 2−, SCN−, and NO2 − in EtOH/H2O (1:99, v/v) solution, [TBC] = 1 × 10−5 M and [An-] = 1 × 10−4 M (20 eq.) (λex : 290 nm and λem : 373 nm)
Binding Constant
The binding constant of TBC to Cr(VI) was also estimated by the same procedure illustrated before for Hg2+ and Cr3+ ions, using the Benesi-Hidebrand equation (2). The value of the binding constant was obtained as 9.97 × 104 mol−1.
To ensure the lack of interference by common accompanying anions in detection of dichromate, selectivity test was also performed in which the fluorescence emission changes of Cr(VI) + TBC system were examined in the presence of various competing ions. The obtained results (Fig. 12) revealed that competing anions even in much higher concentrations (5 eq.) insignificantly affected the emission of the system and this fact proved high selectivity of the sensor toward Cr(VI) ion.
A titration experiment was also performed to evaluate the effect of Cr(VI) concentration on the fluorescence intensity. As presented (Fig. 13), upon addition of Cr(VI) concentrations to TBC solution, the fluorescence emission intensity decreased gradually. Also, plotting the concentrations of dichromate ions against the emission intensity (Fig. 13, inset), showed a linear relationship between the two factors. The detection limit was then calculated for Cr(VI) ion by a similar method applied for Hg2+ and Cr3+ before and 7.49 × 10−8 mol L−1 was obtained which shows high sensitivity of the sensor toward Cr(VI) ion.
Fluorescence emission of TBC upon addition of Cr(VI) ions in EtOH/H2O (1:99, v/v) solution, [TBC] = 1 × 10−5 M, [Cr(VI)] = (0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, and 15 eq.). Inset: fluorescence emission as a function of Cr(VI) concentration (0–1 eq.) (λex : 290 nm and λem : 373 nm)
Conclusion
TBC was synthesized with a two-step procedure. The fluorescence properties of the synthesized iminocrown ether were investigated in the presence of a variety of cations and anions and results revealed its capability in selectively detection of Hg2+, Cr3+, and Cr(VI) in 99 % aqueous media. Quenching mechanisms were also determined to be of static type for Hg2+ and Cr(VI) ions with Ksv = 5.03 × 104 and 2.37 × 105 mol−1, respectively. The binding constants were also estimated as 1.16 × 105, 1.32 × 104, and 9.97 × 104 mol−1 for Hg2+, Cr3+, and Cr(VI), respectively. Furthermore, in the selectivity experiment, even 5 equivalents of the common competing ions did not interfere in recognition of the target ions which proves the high selectivity of the sensor toward the ions. Titration experiments demonstrated a linear relationship between the concentration of the ions and the fluorescence intensity variation. Finally, the limits of detection were calculated as 5.36 × 10−8, 2.06 × 10−6 and 7.49 × 10−8 mol L−1 for Hg2+, Cr3+, and Cr(VI), respectively. Quite low detection limits acquired, showed the high sensitivity of the sensor toward the three ions, particularly Hg2+ and Cr(VI).
References
Sigel H (1983) Metal ions in biological systems: volume 15: zinc and its role in biology and nutrition, vol 15. CRC press, Boca Raton
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manag 92(3):407–418
Tchounwou PB, Ayensu WK, Ninashvili N, Sutton D (2003) Review: environmental exposure to mercury and its toxicopathologic implications for public health. Environ Toxicol 18(3):149–175
Zhang Z, Wu D, Guo X, Qian X, Lu Z, Xu Q, Yang Y, Duan L, He Y, Feng Z (2005) Visible study of mercuric ion and its conjugate in living cells of mammals and plants. Chem Res Toxicol 18(12):1814–1820
Nolan EM, Lippard SJ (2003) A “turn-on” fluorescent sensor for the selective detection of mercuric ion in aqueous media. J Am Chem Soc 125(47):14270–14271
Fitzgerald WF, Lamborg CH, Hammerschmidt CR (2007) Marine biogeochemical cycling of mercury. Chem Rev 107(2):641–662
Prasad MNV (2008) Trace elements as contaminants and nutrients: consequences in ecosystems and human health. Wiley, Hoboken
McRae R, Bagchi P, Sumalekshmy S, Fahrni CJ (2009) In situ imaging of metals in cells and tissues. Chem Rev 109(10):4780–4827
Seiler HG, Sigel H, Sigel A (1988) Handbook on toxicity of inorganic compounds. Marcel Dekker, New York
Plunkett ER (1976) Handbook of industrial toxicology. Chemical Publishing Company, Inc., New York
Lippard S, Berg J (1994) Principle of bioinorganic chemistry. University Science Books, Mill Valley
Costa M (1997) Toxicity and carcinogenicity of Cr (VI) in animal models and humans. Crit Rev Toxicol 27(5):431–442
Hatch WR, Ott WL (1968) Determination of submicrogram quantities of mercury by atomic absorption spectrophotometry. Anal Chem 40(14):2085–2087
Paleologos EK, Stalikas CD, Tzouwara-Karayanni SM, Pilidis GA, Karayannis MI (2000) Micelle-mediated methodology for speciation of chromium by flame atomic absorption spectrometry. J Anal Atom Spectrom 15(3):287–291
Shum SC, Pang HM, Houk R (1992) Speciation of mercury and lead compounds by microbore column liquid chromatography-inductively coupled plasma mass spectrometry with direct injection nebulization. Anal Chem 64(20):2444–2450
Hirata S, Honda K, Shikino O, Maekawa N, Aihara M (2000) Determination of chromium (III) and total chromium in seawater by on-line column preconcentration inductively coupled plasma mass spectrometry. Spectrochim Acta B 55(7):1089–1099
Bonfil Y, Brand M, Kirowa-Eisner E (2000) Trace determination of mercury by anodic stripping voltammetry at the rotating gold electrode. Anal Chim Acta 424(1):65–76
Boussemart M, van den Berg CM, Ghaddaf M (1992) The determination of the chromium speciation in sea water using catalytic cathodic stripping voltammetry. Anal Chim Acta 262(1):103–115
Zhang G, Lu B, Wen Y, Lu L, Xu J (2012) Facile fabrication of a cost-effective, water-soluble, and electrosynthesized poly (9-aminofluorene) fluorescent sensor for the selective and sensitive detection of Fe (III) and inorganic phosphates. Sensors Actuators B Chem 171:786–794
Lu L-M, Zhang X-B, Kong R-M, Yang B, Tan W (2011) A ligation-triggered DNAzyme cascade for amplified fluorescence detection of biological small molecules with zero-background signal. J Am Chem Soc 133(30):11686–11691
Jiang X-J, Wong C-L, Lo P-C, Ng DK (2012) A highly selective and sensitive BODIPY-based colourimetric and turn-on fluorescent sensor for Hg2+ ions. Dalton Trans 41(6):1801–1807
Panda S, Pati PB, Zade SS (2011) Twisting (conformational changes)-based selective 2D chalcogeno podand fluorescent probes for Cr (III) and Fe (II). Chem Commun 47(14):4174–4176
Zheng M, Xie Z, Qu D, Li D, Du P, Jing X, Sun Z (2013) On–off–on fluorescent carbon dot nanosensor for recognition of chromium (VI) and ascorbic acid based on the inner filter effect. ACS Appl Mater Interfaces 5(24):13242–13247
Zarabadi-Poor P, Badiei A, Yousefi AA, Barroso-Flores J (2013) Selective optical sensing of Hg (II) in aqueous media by H-acid/SBA-15: a combined experimental and theoretical study. J Phys Chem C 117(18):9281–9289
Zhang Z, Sha C, Liu A, Zhang Z, Xu D (2015) Highly selective detection of Cr (VI) in water matrix by a simple 1,8-naphthalimide-based turn-on fluorescent sensor. J Fluoresc 25(2):335–340
Obali AY, Ucan HI (2012) Aromatic chromophore-tethered Schiff base ligands and their iron (III)/chromium (III) salen and saloph capped complexes. J Fluoresc 22(5):1357–1370
Karak D, Banerjee A, Sahana A, Guha S, Lohar S, Adhikari SS, Das D (2011) 9-acridone-4-carboxylic acid as an efficient Cr (III) fluorescent sensor: trace level detection, estimation and speciation studies. J Hazard Mater 188(1):274–280
Li Y, Yang L-L, Liu K, Zhao F-Y, Liu H, Ruan W-J (2015) Two hexaazatriphenylene-pyrene based Hg2+ fluorescent chemosensors applicable for test paper detection. New J Chem 39(4):2429–2432
Shi L, Song W, Li Y, Li D-W, Swanick KN, Ding Z, Long Y-T (2011) A multi-channel sensor based on 8-hydroxyquinoline ferrocenoate for probing Hg (II) ion. Talanta 84(3):900–904
Lei Y, Su Y, Huo J (2011) A novel fluorescent sensor for Cr3+ based on rhodamine-cored poly (amidoamine) dendrimer. Spectrochim Acta A 83(1):149–154
Das P, Ghosh A, Bhatt H, Das A (2012) A highly selective and dual responsive test paper sensor of Hg2+/Cr3+ for naked eye detection in neutral water. RSC Adv 2(9):3714–3721
Saha S, Chhatbar MU, Mahato P, Praveen L, Siddhanta A, Das A (2012) Rhodamine–alginate conjugate as Self indicating gel beads for efficient detection and scavenging of Hg2+ and Cr3+ in aqueous media. Chem Commun 48(11):1659–1661
Han J, Bu X, Zhou D, Zhang H, Yang B (2014) Discriminating Cr (III) and Cr (VI) using aqueous CdTe quantum dots with various surface ligands. RSC Adv 4(62):32946–32952
Zhang H, Liu Q, Wang T, Yun Z, Li G, Liu J, Jiang G (2013) Facile preparation of glutathione-stabilized gold nanoclusters for selective determination of chromium (III) and chromium (VI) in environmental water samples. Anal Chim Acta 770:140–146
Pedersen CJ (1967) Cyclic polyethers and their complexes with metal salts. J Am Chem Soc 89(26):7017–7036
Cho YJ, Choi HJ, Hyun MH (2008) Preparation of two new liquid chromatographic chiral stationary phases based on diastereomeric chiral crown ethers incorporating two different chiral units and their applications. J Chromatogr A 1191(1):193–198
Paik M-J, Kang JS, Huang B-S, Carey JR, Lee W (2013) Development and application of chiral crown ethers as selectors for chiral separation in high-performance liquid chromatography and nuclear magnetic resonance spectroscopy. J Chromatogr A 1274:1–5
Mathias LJ, Carraher CE (1984) Crown ethers and phase transfer catalysis in polymer science, vol 227. Springer, New York
Dai S, Ju Y, Barnes C (1999) Solvent extraction of strontium nitrate by a crown ether using room-temperature ionic liquids†. J Chem Soc Dalton Trans 8:1201–1202
Shinkai S, Nakaji T, Ogawa T, Shigematsu K, Manabe O (1981) Photoresponsive crown ethers. 2. Photocontrol of ion extraction and ion transport by a bis (crown ether) with a butterfly-like motion. J Am Chem Soc 103(1):111–115
Morton-Blake D (2012) An intramembrane ion trap. J Mol Liq 167:57–68
Ekmekci G, Uzun D, Somer G, Kalaycı Ş (2007) A novel iron (III) selective membrane electrode based on benzo-18-crown-6 crown ether and its applications. J Membr Sci 288(1):36–40
Cao Y, Pei Q, Andersson MR, Yu G, Heeger AJ (1997) Light-emitting electrochemical cells with crown ether as solid electrolyte. J Electrochem Soc 144(12):L317–L320
Inoue Y, Gokel G (1990) Cation binding by macrocycles: complexation of cationic species by crown ethers, M. Dekker, New York
Reichenbach-Klinke R, König B (2002) Metal complexes of azacrown ethers in molecular recognition and catalysis. J Chem Soc Dalton Trans (2):121–130
Pond SJ, Tsutsumi O, Rumi M, Kwon O, Zojer E, Brédas J-L, Marder SR, Perry JW (2004) Metal-ion sensing fluorophores with large two-photon absorption cross sections: aza-crown ether substituted donor-acceptor-donor distyrylbenzenes. J Am Chem Soc 126(30):9291–9306
Kim HN, Ren WX, Kim JS, Yoon J (2012) Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem Soc Rev 41(8):3210–3244
Aragoni MC, Arca M, Demartin F, Devillanova FA, Isaia F, Garau A, Lippolis V, Jalali F, Papke U, Shamsipur M (2002) Fluorometric chemosensors. Interaction of toxic heavy metal ions PbII, CdII, and HgII with novel mixed-donor phenanthroline-containing macrocycles: spectrofluorometric, conductometric, and crystallographic studies. Inorg Chem 41(25):6623–6632
Liu Q-X, Wang H, Zhao X-J, Yao Z-Q, Wang Z-Q, Chen A-H, Wang X-G (2012) N-heterocyclic carbene silver (I), palladium (II) and mercury (II) complexes: synthesis, structural studies and catalytic activity. CrystEngComm 14(16):5330–5348
Armstrong L, Lindoy L (1975) Nitrogen-oxygen donor macrocyclic ligands. I. Nickel (II) complexes of a new series of cyclic ligands derived from salicylaldehydes. Inorg Chem 14(6):1322–1326
Singh M, Singh P, Raju MD, Singh A (2007) Remote dianions and their application in the synthesis of macroheterocycles. Indian J Chem Sect B 46(4):694
Li S-H, Chen F-R, Zhou Y-F, Wang J-N, Zhang H, Xu J-G (2009) Enhanced fluorescence sensing of hydroxylated organotins by a boronic acid-linked Schiff base. Chem Commun 28:4179–4181
Lakowicz JR (2013) Principles of fluorescence spectroscopy. Springer Science & Business Media, New York
Chauvin A-S, Frapart Y-M, Vaissermann J, Donnadieu B, Tuchagues J-P, Chottard J-C, Li Y (2003) Synthesis, X-ray crystal structure, and redox and electronic properties of iron (III)-polyimidazole complexes relevant to the metal sites of iron proteins. Inorg Chem 42(6):1895–1900
Acknowledgments
The authors thank the research council of University of Tehran for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Afshani, J., Badiei, A., Karimi, M. et al. A Single Fluorescent Sensor for Hg2+ and Discriminately Detection of Cr3+ and Cr(VI). J Fluoresc 26, 263–270 (2016). https://doi.org/10.1007/s10895-015-1708-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-015-1708-9