Abstract
A novel fluorescent Ag+ sensor was developed based on the label-free silver (I) specific oligonucleotide (SSO) and Thioflavine T (ThT) monomer-excimer switch. C-rich SSO which contain C-C mismatched base pairs can selectively bind to Ag+ ions and the formed duplexes which constructed by C-Ag+-C structure are thermally stabilized without largely altering the double helical structure. ThT give very weak fluorescent in bulk solution and/or in the presence of SSO. However ThT shows high fluorescence in the presence of SSO and Ag+ at the same time mainly because ThT excimer, which has the high quantum yield, formed and stabilized in the minor or major groove. Based on the discovery, we developed the novel Ag+ sensor. Under the optimum condition, the selectivity of this system for Ag+ over other metal ions in aqueous solution is remarkably high, and Ag+ can be quantified over the dynamic range of 30–450 nM, with a limit of detection of ~16 nM and a linear correlation coefficient of 0.995.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ag+ has been widely employed in the electrical industry, photography/imaging industry, and pharmaceutical industry over the past decades [1, 2]. Given the high toxicity and the ability to accumulate in the body, Ag+ ions are particularly dangerous to aquatic organisms [3, 4]. Thus, the detection of Ag+ is of particular importance [5–12]. A few approaches for the detection of Ag+ have been developed, including inductively coupled plasma-mass spectroscopy (ICP-MS) [13], atomic absorption spectroscopy [14], fluorescence spectroscopy [15], and ion-selective electrodes (ISE) [16]. However, all the aforementioned methodologies show some unavoidable limitations such as low water solubility, poor selectivity toward Ag+, and/or insufficient sensitivity. In this context, the development of novel, simple, and efficient methods for the determination of Ag+ for both environmental and biological samples is of considerable significance.
Recently,the interactions between metal ions and nucleic acids have attracted considerable attention [17]. The interaction between Ag+ and cytosine-cytosine (C-C) mismatches has been extensively studied and numerous oligonucleotide-based fluorescent Ag+ sensors have been developed [9, 18–23]. Ono et al. reported the selective interaction of Ag+ with C-C mismatches, and developed a fluorescent Ag+ sensor for detecting aqueous Ag+ ions using C-rich silver-specific oligonucleotides (SSO) doubly labeled with a fluorophore and a quencher [18]. However, this system required labeled oligonucleotide which is not cost-effective and probed the presence of Ag+ by fluorescence quenching which can be induced by a variety of interfering mechanisms [20]. Single-labeled fluorescent SSO-based Ag+ sensors were developed using deoxyguanosine bases [8], single-walled carbon nanotubes (SWNTs) [21], graphene oxide (GO) [22], nano-C60 [7], and carbon dots [9] as a quencher. Tseng et al. reported the label-free SSO-based fluorescent Ag+ sensor with the use of a double-strand-chelating dye SYBR Green I (SG) [19]. To reduce the high back-ground emission which was the result of the binding of chelating dye to single-stranded DNA, Leung et al. used the G-quadruplex to recognize Ag+ because the binding of chelating dye is even more weakly to the G-quadruplex compared to single-stranded DNA [20]. To reduce the high background emission and in analogy with our previous report [24], we reasoned that the background emission could be further reduced by employing a fluorescent monomer-excimer switch because the non-fluorescent monomer binds even more weakly to the single-stranded DNA (ssDNA) compared to chelating dye and the fluorescent excimer can be formed in the double-stranded DNA (dsDNA). As a continuation of our studies on oligonucleotide-based probe designs based on metal ions-base pairs coordination chemistry [24–27], we present herein the attempt to use the ThT monomer-excimer fluorescent switch and C-Ag+-C coordination chemistry for label-free, turn-on fluorescent detection of Ag+ with low background signal. The foundation of our strategy, as shown in Scheme 1A, comes from the interaction of Ag+ between SSO with mismatched C base pairs which can change its conformation from a random coil to a folded structure and the different fluorescent properties of a fluorescent switch upon the interaction of the switch with ssDNA and dsDNA or hairpin structure DNA, which results in a dramatic fluorescence enhancement upon the Ag+ coordination. In our design, a reported label-free SSO (P, 5′-CTCTCTTCTCTTCATTTTTCAACACAACACAC-3′) was used as the recognition unit which can bind Ag+ and change its conformation from a random coil to a folded structure [18]. To transduce the metal binding event to a measurable fluorescent signal, Thioflavine T (ThT, structure see Scheme 1B) was used as a monomer-excimer fluorescent molecular switch based on the interaction between ThT and DNA [28–30]. The combination of properties would result in fluorescence enhancement which is sensitive to Ag+. Furthermore, our novel design based on simple and cost-effective synthesis, was shown to have a high sensitivity with lower background emission, and exceptional Ag+ binding selectivity.
Experimental Section
Chemicals and Apparatus
HEPES (4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid) and ThT were purchased from Adrich-sigma (U.S.A.). AgNO3 and other nitrate salts used in this work were provided by China National Medicines Co. Ltd. (Shanghai, China). All chemicals in this work were reagent grade and used as received without further purification. All stock solutions of metal ions were prepared from nitrate salts and were dissolved in doubly deionized water. The work solutions of metals were obtained by series diluting the stock solutions with 10 mM HEPES (pH 7.0). C-rich oligonucleotide (P) was prepared by Sangon Biotechnology Company (Shanghai, China) and used as capturing sequence to recognize Ag+. P was dissolved in sterilized Milli-Q ultrapure water (18.2 MΩ) as stock solutions and was kept at 4 °C.
All fluorescence measurements were recorded on a RF-5301 fluorescence spectrophotometer (Shimadzu, Japan). The pH was measured by a model 868 pH meter (Orion). All experiments were performed at room temperature.
Performance of Ag+ Detection
For Ag+ detection, 0.2 μM ThT, 0.1 μM P, and proper amount of Ag+ or interfering metal ions solution were mixed and diluted to 2.5 mL with 10 mM HEPES (pH 7.0). After violently stirring for 1 min, the fluorescence emission spectra were recorded immediately.
Results and Discussion
Assay Mechanism
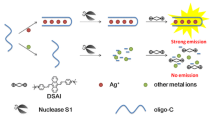
The proposed design takes advantage of the strong and specific interaction between C-C mismatched base pairs and Ag+. Scheme 1 shows the operation principle for Ag+ detection based on C-Ag+-C and ThT. As shown in Scheme 1A, P is ssDNA and is in coiled structure in the absence of Ag+. At the same time, ThT monomer has no/weak emission in the submicromolar concentration range which might be due to quenching effect of smaller order associative states of ThT molecules [31]. And the sensing system is weakly emissive. However, P change its conformation from a random coil to a folded structure in the presence of Ag+ because of the formation of C-Ag+-C [18]. Thus, ThT excimer was formed and stabilized in the hydrophobic environment provided by the folded DNA. The sensing system exhibits an intense emission. Thus, a great fluorescence enhancement is accompanied by the quantitative formation of C-Ag+-C. These results indicated that a novel sensing system for detection of Ag+ could be developed. Compared with the reported label-free fluorescent Ag+ sensors based on C-rich SSO, our design possesses three excellent features. First, by introducing the switch of weak fluorescent monomer and strong emittive excimer, one could successfully reduce the background emission which is induced by the electrostatic interaction between fluorescent dye and ssDNA. Second, ThT is cheap which offers the possibility to apply broadly. Third, ThT has low toxicity, which is different from the common highly toxic intercalator, such as ethidium bromide.
Ag+ Assay Using ThT/P Complex
Figure 1 shows the typical fluorescence response spectra of ThT/P complex in 10 mM HEPES (pH 7.0) with increasing Ag+ concentrations and the background signal of HEPES alone was deducted to evaluate the fluorescent response of the proposed assay. The sensing system exhibits a significant change in fluorescence in the presence of different concentrations of Ag+. The dotted curve was measured in the absence of Ag+, where the sensing system has a very weak emission. When different concentrations of Ag+ were added to the solution of ThT/P, a drastic increase in the fluorescence emission was observed. The intensity of excimer emission increases considerably as an increase in Ag+ concentrations, indicating the formation of excimer of ThT in the Ag+-mediated hairpin structure DNA. Once the concentration of Ag+ is over 0.45 μM, the fluorescence intensity reaches a plateau which suggesting the saturation of the recognition sites by Ag+ binding. And the fluorescence intensity decreased as the Ag+ concentration increased when Ag+ concentration is over 0.6 μM (data not shown) which might be due to its d [10] electronic configuration that is known as fluorescence quencher [6]. As can be seen from Fig. 2, the linear relationship of the emission change of the ThT/P complex and Ag+ concentrations ranging from 30 to 450 nM (F/F 0 = 7.637 C + 0.421, R2 = 0.996). A detection limit that is taken to be 3 times the standard deviation in blank solution was calculated to be 16 nM, which is comparable with Leung’s assays [20] and is lower than similar assays [17].
Fluorescence enhancement of ThT/P complex upon the addition of different Ag+ concentrations at 30, 40, 50, 80, 100, 150, 200, 250, 300, 350, 400, 450, 480, 500 and 520 nM in 10 mM HEPES (pH 7.0) after stirring for 1 min. Spectra were acquired with excitation at 440 nm at room temperature. The up-arrow indicates the increase of free [Ag+] from 0 to 4.5 μM. Inset: Ratio of fluorescence intensities (F/F 0) as a function of Ag+ concentration, where F and F 0 represent the fluorescent intensity at 480 nm in the presence or absence of Ag+, respectively. The excitation was at 440 nm
Sensing Parameters Optimization
The effect of several important parameters on the response of the proposed sensing system to Ag+ was studied. First of all, the effect of molar ratio of P and ThT was investigated in order to select the optimum conditions for the presented Ag+ assay. The molar ratio of the ThT/P was found to strongly affect the fluorescence response toward Ag+. The molar ratio was optimized first to enhance the performance of the assay by fixing ThT at 0.1 μM and varying P concentrations. As shown in Fig. 3, the fluorescence enhancement (F/F 0) response of ThT/P complex to Ag+ for three composition ratios was plotted as functions of the concentration of Ag+, where F and F 0 are the fluorescence intensities at 480 nm in the absence and presence of increasing amount of Ag+. Obviously, depending on the molar ratio of the ThT and P, there are different response characteristics (linear dynamic range and response sensitivity) of the proposed sensing system. A higher concentration of P would increase the dynamic working range of the measurement. However, the amount of P in the system should not be too high because the response sensitivity is significantly smaller than that obtained using lower concentrations of P. On the other hand, lower concentrations of P result in the narrow linear range, though there is a good sensitivity. Thus, it is a contradiction between the sensitivity and response range of the measurement. Therefore, the present experiment showed the best response in terms of both the sensitivity and linear range when the molar ratio of ThT to P is 2:1, compared with that obtained when the molar ratio is 1:2 and 1:1, respectively.
The fluorescence response of the sensing system to Ag+ was influenced by the acidity of the solution. Figure 4 depicts the pH dependence of fluorescent ratio in the absence and presence of Ag+. The experiment was carried out by adding HEPES with different pH to ThT/P complex in the presence of 0.2 μM Ag+ ions. And it is obvious that F/F 0 is independent of pH in the range of 6.8–7.2. We chose HEPES (10 mM, pH 7.0) as optimum buffer system in our experiments.
Selectivity
To establish the specificity for the sensing of Ag+, we also investigated the response of ThT/P towards the other divalent or trivalent competing metal ions such as Ca2+, Mg2+, Co2+, Ni2+, Cu2+, Zn2+, Pb2+, Ba2+, Fe3+, Al3+ under the same conditions. The inherent selectivity of C-C mismatched base pairs toward its specific target Ag+ is also reflected in the designed sensing system. The fluorescent ratio (F/F 0) was greatly enhanced in response to 0.4 μM Ag+, while all above metal ions have no significant response, as illustrated in Fig. 5. The results mean that the high selectivity for Ag+ is obtained by the presented sensing system which are important and helped in validation of the method for the determination of Ag+.
Conclusion
In conclusion, we proposed here a design for novel, simple, remarkably highly sensitive and efficient probe for monitoring Ag+ using ThT monomer-excimer fluorescent switch as signal reporter and a C-rich SSO as molecular recognition unit which exhibits excellent selectivity for Ag+ over competing metal ions. This novel assay method is simple in design, avoiding any oligonucleotide modification compared to other labeled DNA-based Ag+ methods. And the obtained high sensitivity as far as 16 nM is concerned and compares well with those of other Ag+ assays. The preparation of the ThT/P can be realized in 100% aqueous solution, and the enhancement of the ThT fluorescence is fast, and the degree of the enhancement is proportion to Ag+ concentration. Finally, other than metal ions, this sensing platform can also be used to detect amino acids such as cysteine (Cys) which can bind Ag+ through thiol-Ag+ interaction based on the competition mechanism [21, 32, 33]. The further study was conducted in our laboratory.
References
Barriada JL, Tappin AD, Evans EH, Achterberg EP (2007) Trac-Trend Anal Chem 26:809–817
Zhang JF, Zhou Y, Yoon J, Kim JS (2011) Chem Soc Rev 40:3416–3429
Ratte HT (1999) Environ Toxicol Chem 18:89–108
Pedroso MS, Bersano JGF, Bianchini A (2007) Environ Toxicol Chem 26:2158–2165
Wang F, Nandhakumar R, Moon JH, Kim KM, Lee JY, Yoon J (2011) Inorg Chem 50:2240–2245
Huang S, He S, Lu Y, Wei F, Zeng X, Zhao L (2011) Chem Commun 47:2408–2410
Li H, Zhai J, Sun X (2011) Analyst 136:2040–2043
Wang L, Tian J, Li H, Zhang Y, Sun X (2011) Analyst 136:891–893
Li H, Zhai J, Sun X (2011) Langmuir 27:4305–4308
Li D-H, Shen J-S, Chen N, Ruan Y-B, Jiang Y-B (2011) Chem Commun 47:5900–5902
Ho IT, Haung KC, Chung W-S (2011) Chem Asian J 6:2738–2746
Hu Y, Xiao Y, Huang H, Yin D, Xiao X, Tan W (2011) Chem Asian J 6:1500–1504
Jitaru P, Tirez K, Brucker ND (2003) At Spectrosc 24:1
Dadfarnia S, Haji Shabani AM, Gohari M (2004) Talanta 64:682–687
Yang RH, Chan WH, Lee AWM, Xia PF, Zhang HK, Li KA (2003) J Am Chem Soc 125:2884–2885
Zhang X-B, Han Z-X, Fang Z-H, Shen G-L, Yu R-Q (2006) Anal Chim Acta 562:210–215
Ma D-L, Chan D (2011) S-H, Man B Y-W, Leung C-H. Chem Asian J 6:986–1003
Ono A, Cao S, Togashi H, Tashiro M, Fujimoto T, Machinami T, Oda S, Miyake Y, Okamoto I, Tanaka Y, (2008) Chem Commun: 4825-4827
Lin Y H, Tseng W-L, (2009) Chem Commun: 6619-6621
Man BY-W, Chan DS-H, Yang H, Ang S-W, Yang F, Yan S-C, Ho C-M, Wu P, Che C-M, Leung C-H, Ma D-L (2010) Chem Commun 46:8534–8536
Zhao C, Qu K, Song Y, Xu C, Ren J, Qu X (2010) Chem Eur J 16:8147–8154
Wen YQ, Xing FF, He SJ, Song SP, Wang LH, Long YT, Li D, Fan CH (2010) Chem Commun 46:2596–2598
Li B, Du Y, Dong SJ (2009) Anal Chim Acta 644:78–82
Wang YX, Geng FH, Cheng QL, Xu HY, Xu MT (2011) Analyst 136:4284–4288
Wang YX, Li JS, Jin JY, Wang H, Tang HX, Yang RH, Wang KM (2009) Anal Chem 81:9703–9709
Wang YX, Li JS, Wang H, Jin JY, Liu JH, Wang KM, Tan WH, Yang RH (2010) Anal Chem 82:6607–6612
Wang H, Wang YX, Jin JY, Yang RH (2008) Anal Chem 80:9021–9028
Ilanchelian M, Ramaraj R (2004) J Photochem Photobiol A: Chem 162:129–137
Parker CA, Joyce TA (1973) Photochem Photobiol 18:467–474
Cundall RB, Davies AK, Morris PG, Williams J (1981) J Photochem 17:369–376
Khurana R, Coleman C, Ionescu-Zanetti C, Carter SA, Krishna V, Grover RK, Roy R, Singh S (2005) J Stru Bio 151:229–238
Clem GL (1975) Biochimica et Biophysica Acta (BBA) - Protein Structure 386:270–274
Lee J-S, Ulmann PA, Han MS, Mirkin CA (2008) Nano Lett 8:529–533
Acknowledgment
Financial support from the National Natural Science Foundation of China (21105063, 21175091) and Natural Science Foundation of Henan Province (112300410297, 102102210243, 112102210416) are highly acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, Y., Geng, F., Xu, H. et al. A Label-Free Oligonucleotide Based Thioflavin-T Fluorescent Switch for Ag+ Detection with low Background Emission. J Fluoresc 22, 925–929 (2012). https://doi.org/10.1007/s10895-011-1031-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-011-1031-z