Abstract
A new 7-nitrobenz-2-oxa-1,3-diazole (NBD) derived fluorescent probe (1) exhibiting high selectivity for Cu2+ detection, produced significant fluorescence quenching in the presence of Cu2+ ion, while the metal ions Ca2+, Cd2+, Co2+, Fe2+, Hg2+, Mg2+, Mn2+, Ni2+ and Zn2+ produced only minor changes in fluorescence. The apparent association constant (K a) for Cu2+ binding in chemosensor 1 was found to be 1.22 × 103 M−1. The maximum fluorescence quenching activity caused by Cu2+ binding to 1 was observed over the pH range 6–10.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ionic copper is the third most abundant essential transition metal ion in the human body, and plays important roles in various biological processes. Many proteins use copper ions as a cofactor for electron transport, O2 transport, and catalysis of oxidation-reduction reactions [1]. Free copper ions in live cells catalyze the formation of reactive oxygen species (ROS) that can damage lipids, nucleic acids, and proteins. Research has connected the cellular toxicity of copper ions with serious diseases including prion disease [2], and Menkes and Wilson’s disease [3, 4]. Due to its extensive applications, the copper ion is also a significant metal pollutant. The limit of copper in drinking water as set by the US Environmental Protection Agency (EPA) is 1.3 ppm (∼20 μM). Several methods for the detection of copper ions have been proposed, including atomic absorption spectrometry [5], inductively coupled plasma mass spectroscopy (ICPMS) [6], and inductively coupled plasma-atomic emission spectrometry (ICP-AES) [7], and voltammetry [8]. These methods all provide good limits of detection (LOD) and wild concentration ranges. However, most of these methods require the use of costly apparatus and are not suitable for assays, because they involve destruction of the samples. Consequently, the development of fluorescent chemosensors for the detection of Cu2+ ions is attracting much research attention [9–23].
A general strategy used in developing metal ion chemosensors is to combine a metal-binding unit with signaling units such as chromophores or fluorophores. Changes in absorption wavelength or emission intensity during interaction with binding units, signals the presence of metal ions. The mechanism of Cu2+ recognition is a key issue for the design of Cu2+ chemosensors. Cu2+ can induce deprotonation of NH groups that are conjugated to aromatic compounds, or in amide bonds, upon Cu2+ binding. This deprotonation process caused by Cu2+ binding can be used for Cu2+ recognition. Peptides developed for copper ion sensing such as gly-his [10], gly-gly-his [9], and his-gly-gly-gly [18], can selectively bind Cu2+ to deprotonated amides.
In this study, we designed a 7-nitrobenz-2-oxa-1,3-diazole (NBD) based fluorescent chemosensor for metal ion detection. Two components make up chemosensor 1; a NBD moiety as a reporter, and N-(2-aminoethyl)picolinamide as a metal ion chelator (Scheme 1). Binding metal ions to the chemosensor 1 causes fluorescence quenching of NBD. The metal ions Ca2+, Cd2+, Co2+, Cu2+, Fe2+, Hg2+, Mg2+, Mn2+, Ni2+ and Zn2+ were tested for metal ion binding selectivity with chemosensor 1, and Cu2+ was the only ion that caused fluorescent quenching upon binding with chemosensor 1.
Experimental Section
Materials and Instrumentation
All solvents and reagents were obtained from commercial sources and used as received without further purification. UV/Vis spectra were recorded on an Agilent 8453 UV/Vis spectrometer. NMR spectra were obtained on a Bruker DRX-300 NMR spectrometer. IR data were obtained on Bomem DA8.3 Fourier-Transform Infrared Spectrometer.
Synthesis of N-(2-(picolinamido)ethyl)-7-nitrobenzo[c][1,2,5]oxadiazol-4-amine (chemosensors 1)
Que et al. reported a synthesis of N-(2-Aminoethyl)picolinamide by the reaction of ethyl picolinate with ethylenediamine [24]. In this study, we developed an alternative process to obtain N-(2-aminoethyl)picolinamide. A mixture containing picolinic acid (246 mg, 2.0 mmol) and thionyl chloride (146 μL, 2.0 mmol) in 30 mL CH3CN was stirred for 5 min at 0°C. Triethylamine (420 μL, 3.0 mmol) and tert-butyl 2-aminoethylcarbamate (160 mg, 1.0 mmol) were added to the mixture and stirred for 12 h. The solvent was removed under reduced pressure, and the residue extracted with dichloromethane. The organic layer was dried over anhydrous MgSO4. After evaporation of solvents, the product was obtained as a white solid. The white solid was dissolved in 15 mL CH2Cl2, and trifluoroacetic acid (1 mL) was added at 0°C. The mixture was allowed to warm to room temperature and stirred for 12 h. After evaporation, the product, N-(2-aminoethyl)picolinamide was obtained as brown oil in 93% yield, according to the amount of picolinic acid.
Further reaction of N-(2-aminoethyl)picolinamide (243 mg, 1.0 mmol) with NBD-Cl (245 mg, 1.2 mmol) was carried out with 15 mL of dichloromethane and triethylamine (169 μL, 1.2 mmol). The mixture was stirred at room temperature for 1 h. Thereafter, the solvent was evaporated under reduced pressure, and the crude product was purified by column chromatography (ethyl acetate: hexane = 1:1) to give 1 as a brown solid. Yield: 64%; mp: 225°C. MS (EI) m/z (rel intensity) 328 (M+, 1%), 135 (91%), 123 (86%), 105 (100%), 78 (93%). HRMS m/z calcd for C14H12N6O4 [M]+ 328.0920; m/z found 328.0913. 1H NMR (300 MHz, DMSO-d 6 ) δ 9.54 (1H, brs, NH), 9.18 (1H, brs, NH), 8.65 (1H, d, J = 4.0 Hz, PyH), 8.50 (1H, d, J = 8.9 Hz, H a ), 8.05–.97(2H, m, PyH), 7.63–7.59 (1H, m, PyH), 6.53 (1H, d, J = 8.9 Hz, H b ), 3.67 (4H, s, CH 2 ); 13C NMR (75 MHz, DMSO-d 6 ) δ 164.2, 149.6, 148.3, 145.2, 144.4, 137.7, 126.5, 121.8, 120.8, 99.3, 43.0, 37.1.
Metal Ion Binding Study by Fluorescence Spectroscopy
Chemosensor 1 (100 μM) was added with different metal ions (1 mM). All spectra were measured in 1.0 mL methanol-water solution (v/v = 1/1, 10 mM Hepes buffer, pH 7.0). The light path length of cuvvet was 1.0 cm.
The pH Dependence on Cu2+ Binding in Chemosensor 1 Studied by Fluorescence Spectroscopy
Chemosensor 1 (100 μM) was added with Cu2+ (1 mM) in 1.0 mL methanol-water solution (v/v = 1/1, 10 mM buffer). The buffers were: pH 1∼2, KCl/HCl; pH 2.5∼4, KH2PO4/HCl; pH 4.5∼6, KH2PO4/NaOH; pH 6.5∼10 Hepes.
Determination of the Binding Stochiometry and the Stability Constants K a of Cu(II) Binding in Chemosensor 1
The binding stochiometry of 1-Cu2+ complexes was determined by Job plot experiments [25]. The fluorescence intensity at 544 nm was plotted against molar fraction of 1 under a constant total concentration. The concentration of the complex approached a maximum absorbance when the molar fraction was 0.5. These results indicate that both chemosensor 1 forms a 1:1 complex with Cu2+. The stability constants K a of a 1:1 1-Cu2+ complexes were determined by the Benesi-Hilderbrand equation [26, 27]:
, where F is the fluorescence intensity at 544 nm at any given Cu2+ concentration, F 0 is the fluorescence intensity at 544 nm in the absence of Cu2+, and F max is the maxima fluorescence intensity at 544 nm in the presence of Cu2+ in solution. The association constant K a was evaluated graphically by plotting 1/(F–F 0 ) against 1/[Cu2+]. Typical plots (1/(F–F 0 ) vs. 1/[Cu2+]) are shown in Fig. 6. Data were linearly fitted according to Eq. 1 and the K a value was obtained from the slope and intercept of the line.
Results and Discussion
Synthesis
Chemosensor 1 comprises two parts: an NBD moiety and N-(2-aminoethyl)picolinamide. Reaction of picolinoyl chloride with tert-butyl 2-aminoethylcarbamate in equimolar quantities, and deprotection with trifluoroacetic acid (TFA),furnished the chelator N-(2-aminoethyl)-picolinamide. The reaction of N-(2-aminoethyl)picolinamide with NBD-Cl provided Chemosensor 1 (Scheme 1). Chemosensor 1 is yellow, with an absorption band centered at 472 nm, and the sensor exhibits a green emission band centered on 544 nm with quantum yield, Φ = 0.03.
Cation-sensing Properties
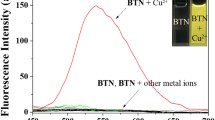
We tested the sensing ability of chemosensor 1 by mixing it with the metal ions Ca2+, Cd2+, Co2+, Cu2+, Fe2+, Hg2+, Mg2+, Mn2+, Ni2+ and Zn2+. Figure 1 shows that addition of most metal ions did not cause a change in intensity; Cu2+ was the only ion that caused significant fluorescent quenching in chemosensor 1. Upon binding with Cu2+, the sensors green emission was completely quenched (Fig. 2). During Cu2+ titration with chemosensor 1, the intensity of the 544 m emission band decreased (Fig. 3). After addition of greater than one molar equivalent of Cu2+, the emission intensity reached a minimum. These observations suggest that Cu2+ is the only metal ion that readily binds with chemosensor 1, causing significant fluorescence quenching, and permitting highly selective detection of Cu2+.
To study the influence of other metal ions on Cu2+ binding with chemosensor 1, we performed competitive experiments with other metal ions (100 μM) in the presence of Cu2+ (100 μM) (Fig. 4). Fluorescence quenching caused by the Cu2+ solution with most metal ions was similar to that caused by Cu2+ alone. This indicated that the other metal ions did not interfere significantly with the binding of chemosensor 1 with Cu2+.
In order to understand the binding stoichiometry of chemosensor 1-Cu2+ complexes, we carried out a series of Job plot experiments. Figure 5 plots the emission intensity at 544 nm against chemosensor 1 molar fraction, under a constant total concentration of 1. Maximum fluorescent quenching occurred for a 0.5 mole fraction. This result indicates a 1:1 ratio for 1-Cu2+ complexes, in which one Cu2+ ion binds with one chemosensor 1. Evaluating the association constant, K a, graphically by plotting 1/ΔF against 1/[Cu2+] produces Fig. 6. Linearly fitting the data to the Benesi–Hilderbrand equation, allows K a to be determined from the slope and intercept of the plot. The apparent association constant, K a, for Cu2+ binding in chemosensor 1 was determined as 1.22 × 103 M−1. The detection limit of chemosensor 1 as a fluorescent sensor for the analysis of Cu2+ was determined from the plot of fluorescence intensity as a function of the concentration of Cu2+ (Fig. 7). It was found that chemosensor 1 has a detection limit of 9.6 μM, which is allowed for the detection of micromolar concentration range of Cu2+.
Calibration curve of Cu2+-1 (100 μM) in a methanol-water (v/v = 1/1, 10 mM Hepes buffer, pH 7.0). The excitation wavelength was 473 nm, and the monitored emission wavelength was 544 nm. The detection limit (DL) of Cu2+ ions using chemosensor 1 was determined from the following equation: DL = K * SD/S, where K = 3; SD is the standard deviation of the blank solution; S is the slope of the calibration curve. DL = K * SD/S = 3 * 49.26011/1.53405*107 = 9.6*10−6 M (9.6 μM)
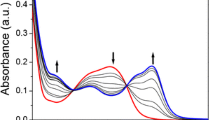
To gain a clearer understanding of the structure of 1-Cu2+ complexes, 1H NMR and Infrared (IR) spectroscopy were employed. Cu2+ is a paramagnetic ion and can affect the proton signals that are close to Cu2+ binding site. In the 1H NMR spectra of chemosensor 1 (Fig. 8), adding Cu2+ caused the proton (amide NH) signal at 9.2 ppm and the proton (at pyridine) signals at 7.6, 8.0, 8.65 ppm to almost completely disappear. Other peaks (protons at NBD) at 6.5, 8.5 ppm became broad upon Cu2+ addition. These observations indicated the binding of Cu2+ with an amide group, pyridine and an amine attached to the NBD motif. The IR spectra were primarily characterized by bands in the double-bond region (Fig. 9). The band 1,633 cm−1 was associated with double-bond (C=O) absorption in chemosensor 1. Binding of Cu2+ with chemosensor 1 resulted in a shift from 1,633 cm−1 to 1,629 cm−1 in the double-bond absorption region, due to the amide group in chemosensor 1. The Job plot indicates that the binding ratio for chemosensor 1-Cu2+ complexes was 1:1. Cu2+ was bound to one nitrogen atom from pyridine, one nitrogen atom from amide and one nitrogen atom attached to the NBD motif (Fig. 10).
We performed pH titration of chemosensor 1 to investigate a suitable pH range for Cu2+ sensing. As depicted in Fig. 11, the emission intensities of metal-free chemosensor 1 remained unchanged. Only when pH was less than 3, did intensity slightly decreased. This was due to protonation of the bridging amine nitrogen, which bonds to NBD. In the presence of Cu2+, the emission intensity at 544 nm suddenly decreased at pH 5.0 and reached lowest intensity in the range of pH 6 to pH 10. This indicates the formation of the 1-Cu2+ complex at high pH values. This observation also reveals that the formation of the 1-Cu2+ complexes is a deprotonation process (Fig. 10). Cu2+ binding induced protonation of the amide in chemosensor 1. For pH < 5, the emission intensity remained higher due to the protonation of the amine groups, preventing the formation of 1-Cu2+ complexes.
Conclusion
In conclusion, this study developed an NBD-based fluorescent chemosensor for Cu2+ ion sensing. We synthesized chemosensor 1 from the reaction of N-(2-aminoethyl)picolinamide and NBD-Cl, to form a new C–N bond between the two precursors. We observed significant fluorescence quenching with chemosensor 1 in the presence of Cu2+ ion, while, adding Ca2+, Cd2+, Co2+, Fe2+, Hg2+, Mg2+, Mn2+, Ni2+, or Zn2+ to the chemosensor solution caused only minimal changes in fluorescence emission intensity. The optimal pH range for Cu2+ detection by chemosensor 1 was pH 6–10. This NBD-based Cu2+ chemosensor provides an effective, and non-destructive means of Cu2+ ion sensing.
References
Cowan JA (1997) Inorganic biochemistry: an introduction. Wiley-VCH, New York, pp 133–134
Barnham KJ, Masters CL, Bush AI (2004) Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discovery 3:205–214
Waggoner DJ, Bartnikas TB, Gitlin JD (1999) The role of copper in neurodegenerative disease. Neurobiol Dis 6:221–230
Vulpe C, Levinson B, Whitney S, Packman S, Gitschier J (1993) Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase. Nat Genet 3:7–13
Gonzales APS, Firmino MA, Nomura C, Rocha FRP, Oliveira PV, Gaubeur I (2009) Peat as a natural solid-phase for copper preconcentration and determination in a multicommuted flow system coupled to flame atomic absorption spectrometry. Anal Chim Acta 636:198–204
Becker JS, Matusch A, Depboylu C, Dobrowolska J, Zoriy MV (2007) Quantitative imaging of selenium, copper, and zinc in thin sections of biological tissues (Slugs-Genus Arion) measured by laser ablation inductively coupled plasma mass spectrometry. Anal Chem 79:6074–6080
Liu Y, Liang P, Guo L (2005) Nanometer titanium dioxide immobilized on silica gel as sorbent for preconcentration of metal ions prior to their determination by inductively coupled plasma atomic emission spectrometry. Talanta 68:25–30
Ensafi AA, Khayamian T, Benvidi A (2006) Simultaneous determination of copper, lead and cadmium by cathodic adsorptive stripping voltammetry using artificial neural network. Anal Chim Acta 561:225–232
Torrado A, Walkup GK, Imperiali B (1998) Exploiting polypeptide motifs for the design of selective Cu(II) ion chemosensors. J Am Chem Soc 120:609–610
Zheng Y, Huo Q, Kele P, Andreopoulos FM, Pham SM, Leblanc RM (2001) A new fluorescent chemosensor for copper ions based on tripeptide glycyl−histidyl−lysine (GHK). Org Lett 3:3277–3280
Zeng L, Miller EW, Pralle A, Isacoff EY, Chang CJ (2006) A selective turn-on fluorescent sensor for imaging copper in living cells. J Am Chem Soc 128:10–11
Qi X, Jun EJ, Xu L, Kim S, Hong JSJ, Yoon YJ, Yoon J (2006) New BODIPY derivatives as off-on fluorescent chemosensor and fluorescent chemodosimeter for Cu2+: cooperative selectivity enhancement toward Cu2+. J Org Chem 71:2881–2884
Xiang Y, Tong A, Jin P, Ju Y (2006) New fluorescent rhodamine hydrazone chemosensor for Cu(II) with high selectivity and sensitivity. Org Lett 8:2863–2866
Yang H, Liu Z, Zhou Z, Shi E, Li F, Du Y, Yi T, Huang C (2006) Highly selective ratiometric fluorescent sensor for Cu(II) with two urea groups. Tetrahedron Lett 47:2911–2914
Martinez R, Zapata F, Caballero A, Espinosa A, Tarraga A, Molina P (2006) Rhodamine-diacetic acid conjugate 2-aza-1, 3-butadiene derivatives featuring an anthracene or pyrene unit: highly selective colorimetric and fluorescent signaling of Cu2+ cation. Org Lett 8:3235–3238
Zhang X, Shiraishi Y, Hirai T (2007) Cu(II)-selective green fluorescence of a rhodamine-diacetic acid conjugate. Org Lett 9:5039–5042
Li G, Xu Z, Chen C, Huang Z (2008) A highly efficient and selective turn-on fluorescent sensor for Cu2+ ion based on calix[4]arene bearing four iminoquinoline subunits on the upper rim. Chem Comm 1774–1776
Wu S, Liu S (2009) A new water-soluble fluorescent Cu(II) chemosensor based on tetrapeptide histidyl-glycyl-glycyl-glycine (HGGG). Sens Actuators B 141:187–191
Ballesteros E, Moreno D, Gomez T, Rodriguez T, Rojo J, Garcia-Valverde M, Torroba T (2009) A new selective chromogenic and turn-on fluorogenic probe for copper(II) in water-acetonitrile 1:1 solution. Org Lett 11:1269–1272
Jung HS, Kwon PS, Lee JW, Kim J, Hong CS, Kim JW, Yan S, Lee JY, Lee JW, Joo T, Kim S (2009) Coumarin-derived Cu2+-selective fluorescence sensor:synthesis, mechanisms, and applications in living cells. J Am Chem Soc 131:2008–2012
Zhou Y, Wang F, Kim Y, Kim S, Yoon J (2009) Cu2+-selective ratiometric and “off-on” sensor based on the rhodamine derivative bearing pyrene group. Org Lett 11:4442–4445
He G, Zhao X, Zhang X, Fan H, Wu S, Li H, He C, Duan C (2010) A turn-on PET fluorescence sensor for imaging Cu2+ in living cells. New J Chem 34:1055–1058
Goswami S, Sen D, Das NK (2010) A new highly selective, ratiometric and colorimetric fluorescence sensor for Cu2+ with a remarkable red shift in absorption and emission spectra based on internal charge transfer. Org Lett 12:856–859
Bukowski MR, Zhu S, Koehntop KD, Brennessel WW, Que LJ (2004) Characterization of an FeIII-OOH species and its decomposition product in a bleomycin model system. J Biol Inorg Chem 9:39–48
Senthilvelan A, Ho I, Chang K, Lee G, Liu Y, Chung W (2009) Cooperative recognition of a copper cation and anion by a calix[4]arene substituted at the lower rim by a β-Amino-α, β-unsaturated ketone. Chem Eur J 15:6152–6160
Benesi HA, Hildebrand JH (1949) A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J Am Chem Soc 71:2703–2707
Singh RB, Mahanta S, Guchhait N (2010) Spectral modulation of charge transfer fluorescence probe encapsulated inside aqueous and non-aqueous β-cyclodextrin nanocavities. J Mol Struct 963:92–97
Acknowledgements
We gratefully acknowledge the financial supports of the National Science Council (ROC) and National Chiao Tung University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, SR., Wu, SP. An NBD-based Sensitive and Selective Fluorescent Sensor for Copper(II) Ion. J Fluoresc 21, 1599–1605 (2011). https://doi.org/10.1007/s10895-011-0848-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-011-0848-9