Abstract
A novel chromogenic and fluorogenic chemosensor RhB-pMOSal comprising a rhodamine fluorophore and a salicylaldehyde receptor being connected by an iminohydrazine link was synthesized and fully characterized. Its sensing behavior toward various metal ions in neutral aqueous solution was investigated by absorption and fluorescence spectroscopy. RhB-pMOSal exhibited a reversible and sensitive “turn-on” response of absorption and fluorescence toward Cu2+ in aqueous acetonitrile solution. Approximate 65 and 6-fold enhancement in the absorbance at 556 nm and fluorescence intensity at 573 nm were estimated when equivalent Cu2+ was added to the RhB-pMOSal solution. Under the same conditions, RhB-pMOSal displayed more sensitive than a reported analogue RhB-Sal to Cu2+ ion. The competition experiments for Cu2+ mixed with common metal ions exhibited no obvious change in absorption and emission except Cr3+ ion that can induce the fluorescence quenching of RhB-pMOSal to some extent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The design and construction of chemosensory reagents for probing specific analytes, especially the biologically and environmentally-important species, with high selectivity and sensitivity are still of a hot research subject in the communities of chemistry, biochemistry, biology, material science and others [1–9]. Fluorescent sensing, which translates molecular binding events into tangible fluorescence signals, has received much attention in this field [1–9]. Rhodamine-based derivatives are excellent candidates for construction of fluorescent chemosensors due to their well-known spectroscopic properties of large molar extinction coefficient, high fluorescence quantum yield, longer wavelength excitation, and good photostability [6, 10]. The rhodamine moiety in a free molecule takes a spirolactam ring-closed form, which usually shows little absorption and nonfluorescence. However, it transforms to ring-opened amide form upon binding with a specific species, the chelating or reaction of metal ions, which usually becomes highly absorbent and fluorescent [11–17]. Based on such structural character, many off–on-type chromogenic or fluorogenic rhodamine-based chemosensors and reagents for metal ions were fabricated in the past few years [11, 12, 14–16, 18–31]. Among them, considerable attention has been paid to the colorimetric/fluorescent sensing of Cu2+ ion [15, 19, 20, 23–25, 28, 29] due to its known important role in the biological, environmental, and chemical systems [32, 33]. Tong et al. [21] once reported a salicylaldehyde rhodamine B hydrazone (RhB-Sal) that can selectively recognize Cu2+ ion in neutral aqueous solution. In this paper, an analogue of RhB-Sal, in which an electron-donating group −OCH3 was introduced to the 4-position of 2-hydroxyphenyl moiety, was synthesized (RhB-pMOSal, Scheme 1). Its sensing behavior toward various metal ions in aqueous solution was investigated by means of absorption and fluorescence spectroscopy. The results exhibited that RhB-pMOSal displayed more sensitive and selective response than RhB-Sal to Cu2+ ion under the same conditions.
Experimental
Apparatus and chemicals
The absorption and the fluorescence spectra were measured on a Shimadzu UV-2450 spectrophotometer and a JASCO FP-6500 spectrofluorometer equipped with a thermostated cell compartment, respectively. A 1.0 cm quartz cuvette in a volume of 3.0 mL was used for all spectra collection. The pH measurements were carried out on a PHS-3C Exact Digital pH meter equipped with Phonix Ag-AgCl reference electrode (Cole-Paemer Instrument Co.), which was calibrated with standard pH buffer solutions. The mass spectra were obtained on an LCQ electron spray mass spectrometer (ESMS, Finnigan). The 1H NMR spectra were recorded on a Bruker DRX-500 spectrometer with tetramethylsilane (TMS) as an internal standard. All of the measurements were performed at about 298.0 ± 0.2 K. Elementary analysis for C, H, N was performed on a Perkin-Elmer 240C analytic instrument.
Rhodamine B was purchased from TDI, and 2-hydroxy-4-methoxybenzaldehyde, 2-hydroxybenzaldehyde and Tris(hydroxymethyl)aminomethane (Tris) were from Sigma-Aldrich. Silica gel (300–400 mesh) that used for thin layer and column chromatography were purchased from Qingdao Ocean Chemicals (Qingdao, China). All metal salts used in the spectroscopic experiments were obtained from Shanghai Chemical Reagent Corporation (Shanghai, China) and used without further purification. Acetonitrile in chromatographic grade and newly double-distilled water were used throughout the experiments as solvents. Aqueous Tris-HCl (50 mmol L−1)-NaCl (0.10 mol L−1) solution was used as buffer to keep pH value (pH 7. 0), and to maintain the ionic strength of all solutions in experiments. The stock solution of Cu2+ and other metal cations (1.0 × 10−3 mol L−1) were prepared from chloride salts using acetonitrile, respectively. The stock solution of RhB-pMOSal (1.0 × 10−4 mol L−1) was prepared by dissolving the accurately weighed RhB-pMOSal in acetonitrile. The working solution of RhB-pMOSal (10 μmol L−1) was prepared by diluting stepwise the stock solution with Tris buffer/acetonitrile (1/1, v/v).
General methods
An aliquot of CuCl2 stock solution was continuously syringed into 3 mL of RhB-pMOSal working solution in quartz cuvette. After full mixture and 5 min equilibration, the absorption spectra were collected by scanning the solution within 400–650 nm. The fluorescence spectra were recorded by the same procedures with excitation light of 520 nm. The slit width of the excitation and the emission were both set at 5 nm. The volume change for all spectroscopic experiments did not exceed 1%. All experiments were run in triplicate, and the average values were adopted. The stoichiometry of RhB-pMOSal and Cu2+ was determined by Job’s method from the obtained absorption spectroscopic data, which was verified by ESI-MS results and Benesi-Hildebrand method [34]. In the determination, the sum of concentration of Cu2+ and RhB-pMOSal was kept at 40 μmol L−1 and the molar ratio of Cu2+ was changed from 0 to 1.0. Each solution of RhB-pMOSal/Cu(II) was prepared by adding appropriate volume of RhB-pMOSal and Cu2+ solution into a 10 mL volumetric flask, and diluting to the scale mark with the Tris buffer and then mixing fully.
Syntheses
Compound RhB-pMOSal was prepared by coupling rhodamine B hydrazide [35] with 2-hydroxy-4-methoxybenzaldehyde referring the reported procedures for RhB-Sal [21] with some modifications (Scheme 1). To 20 mL anhydrous ethanol containing rhodamine B hydrazide (0.23 g, 0.5 mmol), an excessive 2-hydroxy-4-methoxybenzaldehyde (0.6 mmol) was added and the mixture was stirred vigorously at room temperature for 24 h. The reaction progress was monitored by thin-layer chromatography (TLC). After completion of the reaction, the formed precipitate was filtered, washed with cold ethanol (3 × 10 mL) and then dried in vacuum, affording 0.17 g crude product, which was further purified by silica gel column chromatography using petroleum ether/ethyl acetate (2/1, v/v) containing 1% (v/v) triethylamine as eluent. 0.16 g of RhB-pMOSal as white powder solid was obtained, yield: 61.0%. ESI-MS for C36H38N4O4: m/z, calcd. (M + H+) 591.29, found 591.42 (100%) (Supporting Information, Figure S1). 1H NMR (CDCl3): δ (ppm) 1.18 (12H, t, NCH2 CH 3 ), 3.34 (8H, q, NCH 2CH3), 3.76 (3H, s, −OCH3), 6.27 (2H, d, Xanthene-H), 6.38 (2H, s, Xanthene-H), 6.48–6.51 (4H, t, Phen-H), 7.02 (1H, d, Phen-H), 7.18 (1H, d, Phen-H), 7.54 (2H, t, Ar-H), 7.99 (1H, d, Ar-H), 9.23 (1H, s, N = C-H), 11.084 (1H, s, Phen-OH) (Supporting Information, Figure S2). Elemental analysis. Found (calcd.) (%): C, 73.23 (73.20); H, 6.43 (6.48); N, 9.41 (9.48).
RhB-Sal that used as control was synthesized according to the above method using rhodamine B hydrazide and 2-hydroxybenzaldehyde as raw materials. After further purification of the crude product by silica gel chromatography using the same eluent, a white powder was obtained in 71.4% yield. ESI-MS for C35H36N4O3: m/z, calcd. (M + H+) 561.28, found 561.40 (90%); (2 M + Na+) 1143.38, found 1143.25 (100%) (Supporting Information, Figure S3). 1H NMR (CDCl3): δ (ppm) 1.18 (12H, t, NCH2CH3, J = 7.1 Hz), 3.33 (8H, q, NCH2CH3, J = 7.1 Hz), 6.28 (2H, dd, Xanthene-H, J1 = 8.9 Hz, J2 = 2.2 Hz), 6.48 (2H, d, Xanthene-H, J = 2.2 Hz), 6.50 (2H, d, Xanthene-H, J = 8.9 Hz), 6.78 (1H, dd, Phen-H), 6.85 (1H, d, Phen-H), 7.10 (1H, d, Phen-H), 7.17 (1H, s, Phen-H), 7.19 (1H, d, Ar-H), 7.51 (2H, m, Ar-H), 7.98 (1H, d, Ar-H), 9.26 (1H, d, N = C-H), 10.85 (1H, d, Phen-OH) (Supporting Information, Figure S4). Elemental analysis. found (calcd.) (%): C, 74.93 (74.98); H, 6.43 (6.47); N, 9.95 (9.99).
The intermediate rhodamine B hydrazide was prepared referring to the literature [34] with some modifications as follows. To a vigorously stirred solution of rhodamine B (1.20 g, 2.6 mmol) in 100 mL anhydrous ethanol at room temperature, 5.0 mL of hydrazine hydrate (80%) was added dropwise. After the addition, the mixture was heated to reflux in an oil bath. When the reaction finished, the mixture was cooled to room temperature and the solvent was removed under reduced pressure. Dichloromethane (30 mL) and water (30 mL) were added to dissolve the resultant solid. The organic layer was collected, washed twice with water and dried over anhydrous Na2SO4. Filtration of sodium sulfate and evaporation of the solvent gave crude solid product, which was further purified by silica gel column chromatography using petroleum ether/ethyl acetate (1/1, v/v) containing 1% (v/v) triethylamine as eluent. White solid was obtained in 74.0% yield. ESI-MS for C28H32N4O2: m/z, calcd. (M + H+) 457.25, found 457.58 (4 %); (2 M + Na+) 935.17, found 935.58 (100%) (Supporting Information, Figure S5). 1H NMR (CDCl3): δ (ppm) 1.18 (12H, t, NCH2 CH 3 , J = 7.0 Hz), 3.36 (8H, q, NCH 2CH3, J = 7.0 Hz), 3.63 (2H, bs, NH2), 6.30 (2H, dd, Xanthene-H, J1 = 9.0 Hz, J2 = 2.4 Hz), 6.44(2H, d, Xanthene-H, J = 2.4 Hz), 6.48 (2H, d, Xanthene-H, J = 9.0 Hz), 7.13 (1H, dd, Ar-H, J1 = 5.4 Hz, J2 = 3.3 Hz), 7.46 (1H, d, Ar-H, J = 3.3 Hz), 7.47 (1H, d, Ar-H, J = 3.3 Hz), 7.96 (1H, dd, Ar-H, J1 = 5.4 Hz, J2 = 3.3 Hz). (Supporting Information, Figure S6). Elemental analysis. found (calcd.) (%): C, 73.63 (73.66); H, 7.03 (7.06); N, 12.24 (12.27).
Results and discussion
Fluorescence response of RhB-pMOSal to solution pH
Rhodamine-based compounds are usually sensitive to solvent character and solution pH, etc. [17]. A suitable pH scope in which RhB-pMOSal is capable of acting as a chemosensor for metal ions was firstly investigated. As shown in Figure S7, with the solution acidity increasing, the fluorescence of RhB-pMOSal (excited at 520 nm) in 50% aqueous Tris-buffered acetonitrile gradually enhanced along with a clear color changes from colorless to pink. Within the pH values of 10.0 to 7.0, the fluorescence and the solution color could hardly be observed, which suggests that the rhodamine moiety of RhB-pMOSal adopted a spirocyclic form and this tautomer was insensitive to such pH span. When pH was lower than 7.0, a remarkable fluorescence enhancement accompanied by appearance of pink was observed, which implies that the spirolactam ring of RhB-pMOSal was opened due to protonation [36] (Supporting Information, Figure S7). Considering the higher pH range could lead to hydrolysis for transition metal ions, the proper pH span for RhB-pMOSal to sense metal ions in aqueous solution was selected to be ca 7.0. An aqueous Tris buffer with pH value of 7.0 was used throughout the experiments. The ESI-MS result for RhB-pMOSal stayed in neutral Tris-buffered acetonitrile solution for 6 days indicates this compound is highly stable (Supporting Information, Figure S8).
Absorption and fluorescence variation of RhB-pMOSal in the presence of Cu2+
The electronic absorption and fluorescence spectra of RhB-pMOSal in Tris-buffer/acetonitrile (1/1, v/v, pH 7.0) solution are presented in Fig. 1. As shown in the figure, RhB-pMOSal (10 μmol L–1) shows only a very weak absorption and emission (excitation at 520 nm), which is ascribed to its spirolactam form dominating in the solution [6]. The characteristic absorption band appears in the range of 450–600 nm with a λ max of 556 nm (ε = 3.37 × 102 L mol−1 cm−1). Upon addition of Cu2+ to the RhB-pMOSal solution, the absorption enhanced dramatically accompanied by an appearance of a new shoulder peak at ca 521 nm. The absorbance increased gradually with increasing Cu2+ concentration and the solution color turned instantaneously from colorless to clear pink (Fig. 1a, inset).
Similar to the absorption response, a significant enhancement of fluorescence corresponding to the delocalization in the xanthenes moiety of rhodamine [37] occurred (Fig. 1b). Meanwhile, the maximum emission wavelength underwent a slight red shift from 573 to 576 nm. When the concentration of Cu2+ reached to 10 μmol L−1, approximate 65 and 6-fold enhancements in the absorption (at 556 nm) and fluorescence (at 576 nm) were observed, respectively, which were much more sensitive than the known compound RhB-Sal for Cu2+ (ca 30 and 2-fold, respectively) under the same conditions (Fig. 2). The spectroscopic variations suggest that Cu2+ ion coordinated to RhB-pMOSal, resulting in the spirolactam ring open and concomitant formation of RhB-pMOSal-Cu(II) complex. Similar to the previously reported [21], copper(II) could chelate with the carbonyl oxygen, imino nitrogen, and phenol oxygen of RhB-pMOSal. The sensitivity difference between RhB-pMOSal and its analogue RhB-Sal for Cu(II) may be ascribed to the additional methoxy group at para-position of benzene ring of RhB-pMOSal, which could enhance the conjugation effect, and thus enhance the binding capacity of RhB-pMOSal to Cu(II). Fitting of the Job’s plot evaluated from the absorption spectra of RhB-pMOSal and Cu2+ at 556 nm gave rise to a 1:1 stoichiometry for the complex (Fig. 3), which was confirmed by the ESI-MS result (Fig. 4) and the Benesi-Hildebrand method [34].
When assuming a stoichiometry of 1:1 for RhB-pMOSal-Cu(II), the association constant (K a) of RhB-pMOSal with Cu2+ was determined using the Benesi-Hildebrand equation as follow.
A and A 0 is the absorbance of RhB-pMOSal solution in the presence and absence of Cu2+, respectively; Amax is the saturated absorbance of RhB-pMOSal in the presence of excess amount of Cu2+; [Cu2+] is the concentration of Cu2+ ion added (mol L−1).
Plotting of 1/(A–A 0 ) versus 1/[Cu2+] showed a liner relationship (Fig. 5), which suggests that RhB-pMOSal bound with Cu2+ in a 1:1 stoichiometry. The association constant (K a) was determined from the slope to be 3.09 × 104 L mol−1.
Benesi-Hildebrand plot (absorbance at 556 nm) of RhB-pMOSal using eq. 1, assuming 1:1 stoichiometry for association between RhB-pMOSal and Cu2+
Moreover, when an appropriate amount of EDTA was added to the RhB-pMOSal-Cu(II) solution, an instant color change from pink to almost colorless and a simultaneous fluorescence quenching were observed. The ESI-MS results provided a direct evidence that RhB-pMOSal molecule was liberated from the RhB-pMOSal-Cu(II) complex (Supporting Information, Figure S9). In Fig. 4, there are three peaks at m/z of 591.30, 652.20 and 773.30 corresponding to [M + H+]+ (calcd. 591.29), [M − H+ + Cu2+]+ (calcd. 652.21), and [M − H+ + Cu2+ + Tris]+ (calcd. 773.28), respectively. While in Figure S9, the intensity of peaks at m/z = 652.20 and 773.30 decreased dramatically whereas the peak at m/z = 591.30 increased. These results indicated clearly that the binding of RhB-pMOSal with Cu2+ and the subsequent spirolactam ring opening are a reversible process, and furthermore, compound RhB-pMOSal may act as a turn-on chemosensor for Cu2+ in aqueous solution.
Selective sensing of Cu2+ ion in aqueous solution
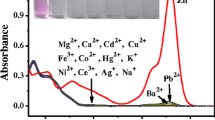
The selectivity is one of the essential requirements for a chemosensor to signal a specific species in a complex system. To validate the selectivity of RhB-pMOSal for sensing Cu2+, common metal ions including alkali, alkaline earth, and transition-metal ions were investigated under the same conditions (50 mmol L−1 Tris-buffer/acetonitrile solution, 1/1, v/v, pH 7.0). When each of the tested metal ions such as Li+, K+, Mg2+, Ca2+, Sr2+, Ba2+, Al3+, Fe3+, Fe2+, Co2+, Ni2+, Zn2+, Hg2+, Cd2+, Cr3+, etc (30 μmol L−1 for each) was added to the RhB-pMOSal solution, respectively, its absorbance and color hardly changed. Only ferrous ion (Fe2+) led to a faint pink and a weak absorption enhancement. However, a strong absorption around 556 nm together with a shoulder peak around 521 nm was observed when 10 μmol L−1 Cu2+ was syringed into the RhB-pMOSal solution containing above mixed metal ions (Fig. 6a). When each of the above metal ions was mixed and reversely added to the RhB-pMOSal solution containing 10 μmol L−1 Cu2+, only slight enhancement in absorption but no further color changes was observed (Fig. 6b), which indicates a good selectivity of RhB-pMOSal for Cu2+ ion in aqueous solution. Such distinct effect was so substantial that it could act as a naked-eye chemosensor for Cu2+.
The results obtained from the fluorimetric response of RhB-pMOSal to all above metal ions corroborated its selectivity for Cu2+. Figure 7 presents the fluorescence spectral changes of RhB-pMOSal (10 μmol L−1) in Tris-buffered acetonitrile solution upon addition of various metal ions (30 μmol L−1 for each). The alkali and alkaline-earth metal cations hardly caused interference, and transition-metal and heavy-metal ions except Cr3+ ion resulted in a weak response of RhB-pMOSal. In contrast, upon addition of Cu2+ (20 μmol L−1) into RhB-pMOSal (10 μmol L−1) solution containing mixed interfering metal ions (30 μmol L−1 for each), a remarkable emission centered at 576 nm in addition to an obvious absorption enhancement was observed (Fig. 7a). Furthermore, the results obtained from an independent experiment indicated that the Cu2+-induced fluorescence enhancement of RhB-pMOSal was not greatly influenced by subsequent addition of other metal ions except for Cr3+ ion (Fig. 7b). Chromium(III) can greatly quench the Cu2+-induced fluorescence of RhB-pMOSal under the same conditions, which could be avoided by previously removing Cr3+ through elevation of solution pH. All of these results reveals that RhB-pMOSal is highly selective for Cu2+ over the competing cations tested in aqueous solution.
Conclusion
The rhodamine-based derivative RhB-pMOSal in Tris-buffered acetonitrile aqueous solution displayed almost no absorption and week fluorescence. Upon addition of Cu2+ ion at micromolar level, significant enhancement of its fluorescence centered at 573 nm accompanied by obvious absorption around 556 nm corresponding to an instantaneous and reversible “turn-on” pink was observed. Approximate 65 and 6-fold enhancement in the absorbance at 556 nm and fluorescence intensity at 573 nm were estimated when 10 μmol L−1 Cu2+ was added to the RhB-pMOSal solution. The results obtained from the disturbance experiments indicated that both changes in the fluorescence and absorption of RhB-pMOSal were remarkably selective for Cu2+ over various competing metal ions, which meets the basic requirements for a chemosensory reagent. Chromium (III) was able to quench the fluorescence to some extent; however, its disturbance could be eliminated by hydrolysis at higher pH before sensing Cu2+ in practice. In comparison with the known RhB-Sal, RhB-pMOSal exhibited higher sensitivity toward Cu2+ in neutral aqueous solution.
References
Wolfbeis OS (2005) Materials for fluorescence-based optical chemical sensors. J Mater Chem 15(26–27):2657–2669
Thomas SW III, Joly GD, Swager TM (2007) Chemical sensors based on amplifying fluorescent conjugated polymers. Chem Rev 107(4):1339–1386
Johnsson N, Johnsson K (2007) Chemical tools for biomolecular imaging. ACS Chem Biol 2(1):31–38
Martí AA, Jockusch S, Stevens N, Ju JY, Turro NJ (2007) Fluorescent hybridization probes for sensitive and selective DNA and RNA detection. Acc Chem Res 40(6):402–409
Nolan EM, Lippard SJ (2008) Tools and tactics for the optical detection of mercuric ion. Chem Rev 108(9):3443–3480
Beija M, Afonso CAM, Martinho JMG (2009) Synthesis and applications of Rhodamine derivatives as fluorescent probes. Chem Soc Rev 38(8):2410–2433
Cho DG, Sessler JL (2009) Modern reaction-based indicator systems. Chem Soc Rev 38(6):1647–1662
Han WS, Lee HY, Jung SH, Lee SJ, Jung JH (2009) Silica-based chromogenic and fluorogenic hybrid chemosensor materials. Chem Soc Rev 38(7):1904–1915
Xu Z, Chen X, Kim HN, Yoon J (2010) Sensors for the optical detection of cyanide ion. Chem Soc Rev 39(1):127–137
Kim HN, Lee MH, Kim HJ, Kim JS, Yoon J (2008) A new trend in rhodamine-based chemosensors: application of spirolactam ring-opening to sensing ions. Chem Soc Rev 37(8):1465–1472
Yang YK, Yook KJ, Tae J (2005) A rhodamine-based fluorescent and colorimetric chemodosimeter for the rapid detection of Hg2+ ions in aqueous media. J Am Chem Soc 127(48):16760–16761
Zhang X, Shiraishi Y, Hirai T (2007) A new rhodamine-based fluorescent chemosensor for transition metal cations synthesized by one-step facile condensation. Tetrahedron Lett 48(31):5455–5459
Zheng H, Shang G, Yang S, Gao X, Xu J (2008) Fluorogenic and chromogenic rhodamine spirolactam based probe for nitric oxide by spiro ring opening reaction. Org Lett 10(12):2357–2360
Xi P, Huang L, Liu H, Jia P, Chen F, Xu M, Zeng Z (2009) Dual-rhodamine urea derivative, a novel chemidosimeter for Hg(II) and its application in imaging Hg(II) in living cells. J Biol Inorg Chem 14(6):815–819
Zhao M, Yang X, He S, Wang L (2009) A rhodamine-based chromogenic and fluorescent chemosensor for copper ion in aqueous media. Sens Actuators B 135(2):625–631
Huang J, Xu Y, Qian X (2009) A Rhodamine-based Hg2+ sensor with high selectivity and sensitivity in aqueous solution: a NS2-containing receptor. J Org Chem 74(5):2167–2170
Arbeloa IL, Ojeda PR (1981) Molecular forms of rhodamine B. Chem Phys Lett 79(2):347–350
Nguyen T, Francis MB (2003) Practical synthetic route to functionalized rhodamine dyes. Org Lett 5(18):3245–3248
Dujols V, Ford F, Czarnik AW (1997) A long-wavelength fluorescent chemodosimeter selective for Cu(II) ion in water. J Am Chem Soc 119(31):7386–7387
Kwon JY, Jang YJ, Lee YJ, Kim KM, Seo MS, Nam W, Yoon J (2005) A highly selective fluorescent chemosensor for Pb2+. J Am Chem Soc 127(28):10107–10111
Xiang Y, Tong A, Jin P, Ju Y (2006) New fluorescent rhodamine hydrazone chemosensor for Cu(II) with high selectivity and sensitivity. Org Lett 8(13):2863–2866
Wu D, Huang W, Duan C, Lin Z, Meng Q (2007) Highly sensitive fluorescent probe for selective detection of Hg2+ in DMF aqueous media. Inorg Chem 46(5):1538–1540
Zhang X, Shiraishi Y, Hirai T (2007) Cu(II)-selective green fluorescence of a rhodamine-diacetic acid conjugate. Org Lett 9(24):5039–5042
Swamy KMK, Ko SK, Kwon SK, Lee HN, Mao C, Kim JM, Lee KH, Kim J, Shin I, Yoon J (2008) Boronic acid-linked fluorescent and colorimetric probes for copper ions. Chem Commun (45):5915–5917
Xiang Y, Li Z, Chen X, Tong A (2008) Highly sensitive and selective optical chemosensor for determination of Cu2+ in aqueous solution. Talanta 74(5):1148–1153
Shiraishi Y, Sumiya S, Kohno Y, Hirai T (2008) A rhodamine-cyclen conjugate as a highly sensitive and selective fluorescent chemosensor for Hg(II). J Org Chem 73(21):8571–8574
Huang W, Wu D, Guo D, Zhu X, He C, Meng Q, Duan C (2009) Efficient near-infrared emission of a ytterbium(III) compound with a green light rhodamine donor. Dalton Trans (21):2081–2084
Zhou Y, Wang F, Kim Y, Kim SJ, Yoon J (2009) Cu2+-selective ratiometric and “Off–On” sensor based on the rhodamine derivative bearing pyrene group. Org Lett 11(19):4442–4445
Zhao Y, Zhang XB, Han ZX, Qiao L, Li CY, Jian LX, Shen GL, Yu RQ (2009) Highly sensitive and selective colorimetric and Off–On fluorescent chemosensor for Cu2+ in aqueous solution and living cells. Anal Chem 81(16):7022–7030
Liu W, Xu L, Zhang H, You J, Zhang X, Sheng R, Li H, Wu S, Wang P (2009) Dithiolane linked thiorhodamine dimer for Hg2+ recognition in living cells. Org Biomol Chem 7(4):660–664
Huang W, Song C, He C, Lv G, Hu X, Zhu X, Duan C (2009) Recognition preference of rhodamine-thiospirolactams for mercury(II) in aqueous solution. Inorg Chem 48(12):5061–5072
Barnham KJ, Bush AI (2008) Metals in Alzheimer’s and Parkinson’s diseases. Curr Opin Chem Biol 12(2):222–228
Crichton RR, Dexter DT, Ward RJ (2008) Metal based neurodegenerative diseases—from molecular mechanisms to therapeutic strategies. Coord Chem Rev 252(10–11):1189–1199
Benesi HA, Hildebrand JH (1949) A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J Am Chem Soc 71(8):2703–2707
Yang XF, Guo XQ, Zhao YB (2002) Development of a novel rhodamine-type fluorescent probe to determine peroxynitrite. Talanta 57(5):883–890
Valeur B (2001) Molecular fluorescence, principles and applications. Wiley-VCH Verlag GmbH, New York
Weerasinghe AJ, Schmiesing C, Sinn E (2009) Highly sensitive and selective reversible sensor for the detection of Cr3+. Tetrahedron Lett 50(46):6407–6410
Acknowledgments
Financial supports from the Natural Science Foundation of Jiangsu Province (Grant BK2007069) and the Qing Lan Project sponsored by Jiangsu Province (2008) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
ESM 1
(DOC 1797 kb)
Rights and permissions
About this article
Cite this article
Tang, R., Lei, K., Chen, K. et al. A Rhodamine-Based Off–On Fluorescent Chemosensor for Selectively Sensing Cu(II) in Aqueous Solution. J Fluoresc 21, 141–148 (2011). https://doi.org/10.1007/s10895-010-0698-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-010-0698-x