Abstract
The interaction between bergenin and human serum albumin (HSA) in AOT/isooctane/water microemulsions was studied by fluorescence quenching technique in combination with UV absorption spectroscopy, circular dichroism (CD) spectroscopy and dynamic light scattering (DLS) technique. Fluorescence data in ω o 20 microemulsions revealed the presence of a binding site of bergenin on HSA and its binding constants (K) were 1.64 × 104, 1.44 × 104, 1.26 × 104 and 1.09 × 104 M−1 at 289, 296, 303, and 310 K, respectively. The binding of bergenin with HSA in microemulsions was stronger than that in buffer solution. The alterations of protein secondary structure in the microemulsions in the absence and presence of bergenin compared with the free form of HSA in buffer were qualitatively and quantitatively analyzed by the evidence from CD spectra. Enthalpy and entropy changes for the reaction were calculated to be −14.45 kJ mol−1 and 30.76 J mol−1 K−1. These results indicated that bergenin bound to HSA mainly by a hydrophobic interaction in microemulsions which was in agreement with the result of the molecular modeling study. The DLS data suggested that HSA may locate at the interface of the microemulsion and bergenin could interact with them.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The microemulsion systems in question typically consist of discrete, nanometer-sized water domains stabilised by a surfactant, and dispersed in a continuous apolar phase [1]. It has been used extensively as biological membrane models to aid in the understanding of membrane chemistry [2]. Water is readily solubilized in the polar core, forming a so-called “water pool”, which is described by the water-surfactant molar ratio ω o (ω o = [H2O] / [S]). The aggregates containing a large amount of water molecules (above ω o = 15) are usually called microemulsions whereas reverse micelles correspond to droplets containing a small amount of water (below ω o = 15) [3]. Reverse micelles have been used as model systems for studying various reactions in confinement, for example, micellar catalysis [4, 5], enzymatic reactions [6, 7], protein purification [8] and so on. By far, the most widely studied without microemulsion systems are the ternary mixture consisting of the anionic surfactant the sodium salt of bis(2-ethylhexyl)sulfosuccinate (AOT)/nonpolar-solvent/water. It is optically transparent and the change in the system could be followed by different spectrophotometric methods.
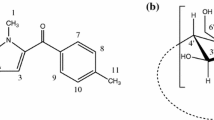
Human serum albumin is the most abundant protein in blood serum with a concentration of 0.63 mM. It is a globular protein composed of a single polypeptide chain of 585 amino acid residues with a large α-helix. Its amino acid sequence contains a total of 17 disulfide bridges, one free thiol (Cys-34) and a single tryptophan (Trp-214). HSA binds a number of the relatively insoluble endogenous compounds such as unesterified fatty acids, bilirubin, and bile acids and thus facilitates their transport throughout the circulation. HSA is also capable of binding a wide variety of drugs and xenobiotics [9, 10]. Binding of drugs to plasma proteins is an important pharmacological parameter, since it frequently affects the distribution and elimination of a drug as well as the duration and intensity of its physiological action [11–13]. Bergenin (structure shown in Fig. 1), is isolated from several medicinal plants and exhibits various biological activities, such as antiarrhythmic [14], hepatoprotective [15], anti-inflammatory [16] and antitumor effects [17] as well as anti-HIV [18], neuroprotective [19], antiulcer [20] and antitussive activities [21]. Given all these, bergenin is very worthy of study.
In previous works, a series of studies concerning the interaction between drugs and proteins in aqueous solution under physiological conditions have been reported [22–25]. Bernard Desfosses et al. [26] compared the behavior of HSA in the presence of three chemically distinct ligands: oxyphenylbutazone, dansylsarcosine, hemin in buffer and reverse micelles. Daniel M. Davis et al. using fluorescence quenching and CD studied the behavior of HSA in AOT reverse micelles [27]. Suzana M. Andrade and Sílvia M. B. Costa investigated the interaction of two water-soluble freebase porphyrins with two drug-carrier proteins (human serum albumin and β-lactoglobulin) in AOT/isooctane/water reverse micelles by steady-state and transient-state fluorescence spectroscopy [28]. Yet the detailed investigation on the binding interaction of bergenin and HSA via diversiform analytical methods in membrane mimetic environments has not been reported.
This study was designed to demonstrate a new array on the interaction of bergenin with HSA in microemulsions. Besides using fluorescence spectroscopy, UV absorption spectroscopy, circular dichroism (CD) and dynamic light scattering (DLS) methods were also applied. Attempts were made to investigate the binding constant (K), the number of binding sites (n) and thermodynamic parameters for the reaction in the membrane mimetic environments. In addition, the molecular modeling was studied with SGI FUEL workstation. These were the first results on bergenin–HSA interactions, which illustrate the nature of the complications in microemulsion.
Materials and methods
Materials
Sodium bis(2-ethylhexyl) sulfosuccinate (AOT) (Fluka, ≥96% purity) was used without further purification. Human serum albumin (HSA), purchased from Sigma Chemical Company, was used as received and its molecular weight was assumed to be 66,500 to calculate the molar concentrations. All HSA stock solutions were prepared in the pH 7.40 buffer solution and were kept in the dark at 4°C. Bergenin was of analytical grade, and obtained from the National Institute for Control of Pharmaceutical and Bioproducts (Beijing, China), and the stock solution was prepared in deionized water. Buffer solution (pH 7.40) consisted of Tris (0.2 M) and HCl (0.1 M). Isooctane and other reagents were of analytical grade and deionized water was used throughout all the experiments. All pH were checked with a PHS-10A acidity meter (Xiaoshan Science Instrumentation Factory, Zhejiang, China).
AOT reverse micelle preparation
Microemulsion solutions of desired ω o (ω o = [H2O] / [AOT]) were prepared by adding protein stock solutions, drug solutions or plain buffer (pH = 7.40) to a 0.1 M AOT solution in isooctane. Volume of additivity was assumed in calculating AOT concentration and water AOT molar ratios. The samples were gently shaken until complete clarification. The final sample concentration was calculated according to total mole of sample injected and the total volume of the microemulsion.
Apparatus and methods
UV absorption and fluorescence spectroscopic measurements
The absorption and steady state fluorescence measurements were performed using a CARY-100 UV–visible spectrophotometer (Varian, USA) and a RF-5301PC spetrofluorophotometer (Shimadzu), respectively. The fluorescence emission spectra were recorded from 280 to 500 nm (excitation wavelength 280 nm) using 5/5 nm slit widths. Synchronous fluorescence spectra of HSA encapsulated in microemulsion in the absence and presence of increasing amount of bergenin (0–4.95 × 10−5 M) were recorded λ ex: 280–500 nm.
Fluorometric titration experiments: 2.0 ml microemulsions containing appropriate concentration of HSA were titrated manually by successive addition of a 1.91 × 10−4 M of corresponding microemulsions with bergenin encapsulated (to give a final concentration of 4.65 × 10−6–44.0 × 10−5 M) with trace syringes. The fluorescence intensities were recorded at excitation and emission wavelength of 280 and 311 nm. All fluorescence intensities were measured at four temperatures (289, 296, 303, and 310 K). An electronic thermo regulating water-bath (NTT-2100, EYELA, Japan) was used for controlling the temperature. The fluorescence quantitative data obtained were analyzed by the Scatchard equation [29] to calculate the binding parameters
where r represents the number of moles of bound drug per mole of protein, D f represents the molar concentration of free drug, n and K are the number of binding sites and binding constant, respectively. The quantitative analysis of the binding drug−HSA was performed by fluorescence quenching method, which was done by titrating with drug at a given protein concentration. In the drug−HSA system, the concentration of drug was much higher than the concentration of HSA, so the concentration of drug bound to HSA was little, the free concentration of drug can be seen as the total concentration of drug. The total concentration of drug was calculated for the Scatchard plot instead of the free concentration of drug (D f). On the other hand, drug almost had no fluorescence at 311 nm when the excitation wavelength was kept at 280 nm as shown in the following section, so the fluorescence quenching of HSA can be seen as the decrease of the protein fluorescence (ΔF) owing to the interaction of drug with HSA. Therefore, the value of r can be replaced by the content of the decrease of the fluorescence of protein, that is, ΔF / F 0 (F 0 is the fluorescence of HSA in the absence of drug). To eliminate the inner-filter effect, all the fluorescence intensities were corrected according the relationship [30]:
where F cor and F obs are the corrected and observed fluorescence intensities of HSA, while A ex and A em are the absorbance of bergenin at the excitation (280 nm) and the emission (311 nm) wavelengths of HSA in microemulsion, respectively.
For the anisotropy measurements the excitation and emission band widths were both 5 nm. Steady state anisotropy (r) is defined by
where I VV and I VH are the intensities obtained with the excitation polarizer oriented vertically and the emission polarizer oriented in vertical and horizontal orientation, respectively. The G factor is defined as: G = I HV / IHH, similarly, I HV and I HH are the intensities obtained with the excitation polarizer oriented horizontally and the emission polarizer oriented in vertical and horizontal orientation.
Thermodynamic data
Thermodynamic parameters were calculated based on the temperature dependence of the binding constant in ω o 20 microemulsion for bergenin–HSA binding. The temperatures were used 289, 296, 303, and 310 K. The enthalpy change (ΔH 0) was calculated from the slope of the Van’t Hoff relationship:
K is the binding constant at temperature T and R is gas constant. The value of ΔS 0 was obtained from linear Van’t Hoff plot. The value of ΔG 0 was calculated from the equation:
CD measurements
Circular dichroism (CD) spectra were measured with an Olis DSM 1000 Circular Dichroism (American), using a 1 mm cell at 296 K. CD spectra (200–300 nm) were taken in microemulsions with 3.0 μM HSA encapsulated (ω o 20) and the results were taken as millidegrees. The α-helical content of HSA was calculated from the molar ellipticity ([θ]) at 208 nm according the equation \(\% {\text{helix}} = \left\{ {{{\left( { - \left[ \theta \right]_{208} - 4000} \right)} \mathord{\left/ {\vphantom {{\left( { - \left[ \theta \right]_{208} - 4000} \right)} {\left( {33000 - 4000} \right)}}} \right. \kern-\nulldelimiterspace} {\left( {33000 - 4000} \right)}}} \right\} \times 100\) [31]:
DLS data
Dynamic light scattering experiments were performed to determine hydrodynamic diameter of AOT microemulsions at room temperature. The measurements were done using a BI-200SM Static and Dynamic laser light scattering system (Brookhaven, America) coupled with a 4 W laser.
Molecular modeling studies
The crystal structure of HSA was taken from the Brookhaven Protein Data Bank (entry codes 1h9z) [32]. The potential of the 3-D structure of HSA was assigned according to the Amber 4.0 force field with Kollman-all-atom charges. The initial structure of all the molecules was generated by molecular modeling software Sybyl 6.9 [33]. The geometry of the molecule was subsequently optimized to minimal energy using the Tripos force field with Gasteiger–Marsili charges. The FlexX program was used to build the interaction modes between the bergenin and HSA.
Results and discussion
Determination of ω o value for microemulsions with HSA encapsulated
To choose a suitable ω o value of microemulsions for HSA encapsulated, in which microemulsion HSA’s stability is better, a series of microemulsions with different ω o from 10 to 50 were prepared. When ω o equaled to 35 to 50, the clear microemulsions were prone to turbid. When the ω o changed from 10 to 25, the microemulsions maintained pellucid which were more stable than ω o 30. According to the research result of Susana Andrade et al. [34], HSA is a spherical protein, the radius of its sphericity r p (Angstrom) = 0.7 × Mr1/3, an empirical equation for radius of AOT micelle inner core is given by r m (Angstrom) = 4 + 1.5 × ω o, when r p = r m, the optimal ω o for HSA is expected to be around 16. Therefore, 20 was chosen as the optimized ω o of the microemulsion for the main fluorometric titration experiments and spectroscopic measurements in which microemulsion HSA had the higher fluorescence intensity in Fig. 2. Furthermore, 20 was close to the calculated value 16 and literature values 21 [27], 22.4 [26], respectively.
Fluorescence and UV spectra of HSA in microemulsions of different ωo
The fluorescence spectra of HSA in microemulsions with various ω o were measured. In Fig. 2 there was no significant shift in the fluorescence emission maximum (λ max) with the change of ω o. When ω o increased from 10 to 50 the λ max were all about 309 nm. There can be two possible reasons for this result: (a) the microenvironment of HSA remains essentially invariant with ω o, or (b) change in microenvironment does occur, but is not reflected through λ max values. And the effect of ω o on the relative fluorescence intensity was not large. It is noticed that the properties of reverse micelle solubilized water are different from those of bulk water, even at higher ω o values, and its apparent microviscosity is six to nine times greater than that of free aqueous solution [35]. Compare 309 nm with λ max 332 nm of HSA in buffer, it may indicate a less polar environment of amino acid residues.
Figure 3 showed the UV absorption spectra of HSA in different ω o microemulsions. There is an absorbance intensity band of HSA with a maximum at around 210 nm in aqueous solutions. In AOT microemulsions there was a general tendency towards a red shift from 210 to 239 nm for UV absorption spectra when compared with that in water solution. The relative absorption intensities of peaks at 277 nm which character the conjugated double bond of tryptophan, tyrosine and phenylalanine residue to peaks at 239 nm were stronger than that in buffer solution. It means that there is some difference between microenvironment of HSA in microemulsions and in water. Variations of water quantity in microemulsions influenced the absorbance of HSA with a constant peak positions. The absorption of the HSA in ω o 25 microemulsion was relatively weaker. The possible reasons were that the conformation of HSA was affected most by the microenvironment in ω o 25 microemulsion or the stability of HSA in this microemulsion was comparatively lower.
Analysis of fluorescence quenching of HSA by bergenin in microemulsion
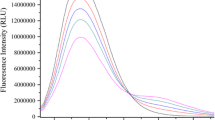
Fluorescence quenching of the single tryptophan residue in HSA was used to measure drug-binding affinity. The fluorescence spectra of HSA in microemulsion before and after addition of bergenin compared with the native HSA in the pH 7.40 Tris buffer were measured with the excitation wavelength at 280 nm and their representative spectra were shown in Fig. 4. HSA had a strong fluorescence emission with a peak at 311 nm in ω o 20 microemulsion, a large shift of emission to a shorter wavelength from 332 nm of HSA in aqua. It indicated perturbations of the amino acid residue microenvironment. The intensity of HSA in microemulsion was much higher than in aqueous solutions. Bergenin had a weak fluorescence emission at 380 nm under the presence experiment conditions in microemulsion. With gradual increase in drug concentrations, typical dual fluorescence behavior was observed. It can be seen that the binding of HSA and bergenin in microemulsion quenched the intrinsic fluorescence of the HSA. Moreover, the occurrence of an isoactinic point at 375 nm might also indicate the existence of bound and free bergenin in equilibrium [24]. This behavior was more visible in the succeeding synchronous fluorescence spectra of HSA in the presence of bergenin in ω o 20 microemulsion.
The fluorescence spectra of bergenin–HSA system in ω o 20 microemulsion. The concentration of HSA was 3.0 μM while the bergenin concentration corresponding to 0, 9.09, 17.3, 24.9, 31.8, 38.2, 44.0, 49.5 μM from a to h; (j) [bergenin] = 49.5 μM in ω o 20 microemulsion; T = 296 K; λex = 280 nm, λem = 311 nm; (i) 3.0 μM HSA in Tris buffer, pH = 7.40; λex = 280 nm, λem = 332 nm
The synchronous fluorescence spectra are frequently used to characterize the interaction between fluorescence probe and proteins since it can provide information about the molecular microenvironment in a vicinity of the chromospheres molecules. According to Miller [36], with large Δλ values such as 60 nm, the synchronous fluorescence of HSA is characteristic of tryptophan residue. Synchronous fluorescence spectral changes of HSA upon addition of bergenin with varied concentration in ω o 20 microemulsion when Δλ = 60 nm were displayed in Fig. 5. Compared with HSA in the Tris buffer, protein encapsulation immediately resulted in a shift of emission to a shorter wavelength from 339 to 333 nm. The addition of the drug led to a dramatic decrease in the fluorescence intensity with a bathochromic shift to 333–339 nm. It is considered that the λ max of the tryptophan residues is relative to the polarity of microenvironment. λ max at 330–332 suggests that tryptophan resides are located in the non-polar region, that is, they are buried in a hydrophobic cavity in HSA; λ max at 350–352 nm indicates that tryptophan residues are exposed to water, that is, the hydrophobic cavity in HSA is disagglomerated and the structure of HSA is looser. Thus Fig. 5 may show that after introducing HSA into microemulsion, there was a less polar environment of tryptophan residues. And bergenin mainly bound to the hydrophobic cavity of HSA, which was in accordance with the results from molecular modeling and the thermodynamics parameters obtained by the experimental data as follows. It was also indicated that the reaction between HSA and bergenin in microemulsion led the polarity around the tryptophan residues increased.
Synchronous fluorescence spectra of HSA (3.0 μM) in ω o 20 microemulsion with Δλ = 60 nm in the absence and presence of increasing amount of bergenin (μM): (a) 0; (b) 9.09; (c) 17.3; (d) 24.9; (e) 31.8; (f) 38.2; (g) 44.0; (h) 49.5; (j) [bergenin] = 49.5 μM in ω o 20 microemulsion; T = 296 K; (i) 3.0 μM HSA in Tris buffer, pH = 7.40
Binding constant and number of binding sites
The quantitative analysis for HSA–bergenin at four different temperature in ω o 20 microemulsions was carried out using the Scatchard equation Eq. 1. In Fig. 6 the satisfactory linearity of Scatchard plots may indicate that bergenin binds to a class of binding sites on HSA and approximate process of the Eq. 1 in “Apparatus and methods” section is logical. The fluorescence quenching data may indicate that the binding site of bergenin on HSA may be the site І in the hydrophobic cavity of sub-domain IIA. It could make the bergenin much closer to the tryptophan residues. The binding constants K decreased with the increasing temperature in microemulsions. Bergenin can be removed by the proteins in the body. The binding constants and the number of binding sites were summarized in Table 1. In Table 2 the binding constants K were listed for bergenin associated with HSA in microemulsions of different ω o and in buffer solution at 296 K. Binding constants K changed a little in different water pools which have the diverse water capacity. So the change of microenvironment in microemulsions had very small effect on binding of bergenin and HSA. While K of the buffer solution was lower to a small degree than the microemulsions’, this may indicate greater accessibility of bergenin toward HSA when HSA was encapsulated in microemulsion. It may imply that HSA associates with the interface of microemulsion and an interesting conformational change is induced in the protein’s structure.
Binding mode
There are several types of non-covalent interactions modes such as hydrogen bond, van der Waals force, hydrophobic interaction force, and electrostatic force, etc [37]. The thermodynamic parameters, enthalpy (ΔH) and entropy (ΔS) of reaction are important for confirming binding mode. For this reason, the temperature-dependence of the binding constant of microemulsion (ω o 20) was studied. The temperatures chosen were 289, 296, 303, and 310 K so that HSA does not undergo any structural degradation. According to the binding constants of bergenin to HSA obtained at the four temperatures in ω o 20 microemulsions above, the values of ΔH 0 and ΔS 0 were obtained from linear Van’t Hoff plot (Fig. 7) and presented in Table 1. The free energy change ΔG 0 was estimated from the Eq. 3. As shown in Table 1, ΔH 0 and ΔS 0 for the binding reaction between bergenin and HSA were found to be −14.45 kJ mol−1 and 30.76 J mol−1 K−1. Thus, the negative sign for ΔG 0 means that the binding process was spontaneous and the formation of bergenin–HSA coordination compound was an exothermic reaction accompanied by positive ΔS 0 value. For typical hydrophobic interactions, both ΔH 0 and ΔS 0 are positive, while negative ΔH 0 and ΔS 0 changes arise from van der Waals force and hydrogen bonding formation in low dielectric media. However, negative ΔH 0 might play a role in electrostatic interactions [38]. Therefore, it is not possible to account for the thermodynamic parameters of bergenin–HSA coordination compound on the basis of a single intermolecular force model. The binding process of bergenin to HSA in the microemulsions might involve hydrophobic interaction strongly as evidenced by the positive values of ΔS 0, but the electrostatic interaction could also not be excluded.
Molecular modeling study of the interaction between HSA and bergenin
Descriptions of the 3-D structure of crystalline albumin have revealed that HSA comprises three repeating domains (I–III): I (residues 1–195), II (196–383), III (384–585), and each of which is divided into two sub-domains (A and B). Sudlow et al. [39] have suggested two main distinct binding sites on HSA, site I and site II, which locate in the hydrophobic cavities of sub-domains IIA and IIIA, respectively, and one tryptophan residue (Trp-214) of HSA is in sub-domain IIA [40]. There is a large hydrophobic cavity present in subdomain IIA that many drugs can bind to.
SGI FUEL workstation was used to calculate the partial binding parameters to study the binding site of the HSA–bergenin system, and the best energy ranked result was presented in Fig. 8. It can be seen that bergenin was situated within subdomain IIA in Sudlow’s site I formed by helices. The bergenin molecule was located within the binding pocket, and it was important to note that the only tryptophan residue (Trp-214) of HSA was in close proximity to C- and B-rings, suggesting the existence of hydrophobic interaction between them. Further, this finding provided a good structural basis to explain the efficient fluorescence quenching of HSA emission in the presence of bergenin. The interaction between bergenin and HSA was not exclusively hydrophobic in nature since there were many polar and charged amino acid residues on the protein surface, which play a subordinate role in stabilizing the bergenin molecule via electrostatic interaction. It was in agreement with the binding mode study. On the other hand, there were hydrogen bonding interactions between 6-O of bergenin and the residue Arg-218 of HSA; 15-O and Arg-218, Arg-222; 20-OH and Lys-195, Asp-451; 21-OH and Ala-291; 23-OH and Glu-450; The results suggested that the formation of hydrogen bond decreased the hydrophilicity and increased the hydrophobicity to stability in the bergenin–HSA system. The calculated binding Gibbs free energy (ΔG 0) was −19.72 kJ mol−1, which was not very close to the experimental data (−23.55 kJ mol−1) to some degree. A possible explanation may be that the X-ray structure of the protein from crystals differs from that of the microemulsions system used in this study. Therefore, the results of modeling indicated that the interaction between bergenin and HSA was dominated by hydrophobic force, which was in accord with the binding mode study.
The binding mode between bergenin and HSA, only residues around 10 Å of bergenin are displayed. The residues of HSA are represented using gray ball and stick model and the drug structure is represented by a green one. The hydrogen bond between bergenin and HSA is represented using yellow dashed line
Fluorescence anisotropy of HSA in various microemulsions
The fluorescence anisotropy measurements of HSA in AOT microemulsions as a function of increasing amounts of water were carried out when λ ex was 280 and the result was shown in Table 3. It is evident that as ω o increased, very little change in anisotropy (r) was observed, indicating that there was no appreciable additional change in the average mobility of HSA molecules. It is noteworthy that all the r were higher than that obtained for HSA in buffer solution, which showed that the mobility of the chromophores inside microemulsion droplets was more limited than in aqueous solution. This effect could be explained by interactions between amino acid side-chains and AOT sulfonate groups or the structured interfacial water layer in which these were encapsulated. It may also be explained simply by the increase of water microviscosity when water acts as a component of the microemulsion.
Binding studies between bergenin and HSA in microemulsions using UV and CD spectrum
Figure 9 was the UV absorption spectra of HSA with increasing amount of bergenin in microemulsion (ω o 20). As can be seen in Fig. 9, the absorbance of HSA in microemulsion at 239, 279 nm decreased with the addition of bergenin. This result clearly indicated the interaction between bergenin and HSA.
CD spectrum was applied to analyze the changes of HSA secondary structure in the absence and presence of bergenin in microemulsions and compared them with spectrum in buffer; the results were shown in Fig. 10. The CD spectra of HSA exhibit characteristic features of the α-helical structure of protein with negative bands in the ultraviolet region at 208 and 222 nm. In Fig. 10, the CD spectra of free HSA buffer solution had a characteristic of the typical (α + β)-helix structure with negative bands at 208 and 220 nm. After protein encapsulation in microemulsion, the band intensity of negative Cotton effect of HSA at 208 and 220 nm reduced, indicating the considerable changes in the protein secondary structure with the reduction of the α-helical content in HSA. There may be some co-effect factors contributing to the changes: the unusual properties of water localized in the interior of microemulsions could bring about a different chiral environment for HSA; the contact of HSA molecules with isooctane in the process of solubilization can change the structure of HSA; the interaction of AOT and HSA can also change the structure of protein. The binding of bergenin to HSA in microemulsion kept on decreasing the α-helical content in protein and increased the disorder structure content in the HSA. From the above results, there may be the formation of complex between HSA and bergenin in microemulsion. The quantitive CD data exhibited a reduction of α-helices from 55.58% (free HSA in buffer) to 41.10% (free HSA in microemulsion), from 41.10% to 24.74% (bound HSA in microemulsions). This suggested that, in spite of the impact of the environment of microemulsion on the secondary structure of HSA, bergenin could interact with the protein in microemulsion. From CD measurements, Desfosses et al. [26] showed that upon hosted into AOT microemulsions of ω o 22.4, the α-helical content of HSA decreased by approximately 15%.
DLS data
The dynamic light scattering data was presented in order to have insight on the localization of HSA and its interaction with bergenin which occurred after encapsulation in AOT microemulsions. Hydrodynamic diameters (D H) of AOT microemulsions with and without HSA, HSA binding drug at ω o 20 were measured (Table 4). The D H obtained for ω o 20 microemulsion was 10.3 nm, which was comparatively close to the literature’s value [41]. After HSA immersing in AOT microemulsion, D H decreased to 6.3 nm. According to Pileni [3], in microemulsion the contribution of the solute to the interface or to the polar volume induces an increase either in the interfacial area, dΣ, or in the polar volume, dV. The water pool radius expressed as a sphere (R w = 3(V + dV) / Σ+ dΣ) changes by increasing either dV or dΣ. A probe located at the interface induces a decrease in the water pool radius due to an increase in the interface, dΣ, with a constant volume, V. Therefore HSA may locate at the interface inducing a decrease in the water pool, which may have relation to the change of secondary structure in HSA. Upon adding bergenin, the D H decreased to 4.2 nm. The reasons may be that when bergenin entered the water pool of microemulsion which contained the HSA, bergenin interacted with HSA at the interface, so the contribution of HSA–bergenin complex to the interface of water pool was different from the HSA’s. Another possible reason was that after interacting with bergenin, the interaction between HSA and AOT was changed, which may affect the size of water pool.
Conclusions
In this paper, we chose AOT/isooctane/water microemulsions as membrane mimetic environments for the binding of bergenin and HSA which was studied by fluorescence quenching technique, UV absorption spectroscopy, CD spectroscopy, dynamic light scattering technique and molecular modeling method. The work demonstrated that the secondary structure of HSA was influenced after encapsulating the protein into microemulsions. Addition of bergenin to the microemulsion with HSA encapsulated kept on changing the conformation of HSA, which was proved by the analysis data of CD spectrum. HSA may locate at the interface of the microemulsion and had the interaction with bergenin according to the DLS data. The main interaction between HSA and bergenin was hydrophobic force but the electrostatic interaction can also not be excluded. The studies presented also clearly indicated that bergenin was a quencher for HSA and bound to HSA with higher affinities in microemulsions than in buffer solution.
References
Burnett GR, Rees GD, Steytler DC, Robinson BH (2004) Fluorescence correlation spectroscopy of water-in-oil microemulsions: an application in specific characterisation of droplets containing biomolecules. Colloids Surf A Physicochem Eng Asp 250:171–178
Andrade SM, Costa SMB, Pansu R (2000) The influence of water on the photophysical and photochemical properties of Piroxicam in AOT/iso-octane/water reversed micelles. Photochem Photobiol 71:405–412
Pileni MP (1993) Reverse micelles as microreactors. J Phys Chem 97:6961–6973
Correa NM, Zorzan DH, D’Anteo L, Lasta E, Chiarini M, Cerichelli G (2004) Reverse micellar aggregates: Effect on ketone reduction. 2. Surfactant role. J Org Chem 69:8231–8238
Pal T, De S, Jana NR, Pradhan N, Mandal R, Pal A, Beezer AE, Mitchell JC (1998) Organized media as redox catalysts. Langmuir 14:4724–4730
Falcone RD, Biasutti MA, Correa NM, Silber JJ, Lissi E, Abuin E (2004) Effect of the addition of a nonaqueous polar solvent (glycerol) on enzymatic catalysis in reverse micelles, hydrolysis of 2-naphthyl acetate by a-chymotrypsin. Langmuir 20:5732–5737
Durfor CN, Bolin RJ, Sugasawara RJ, Massey RJ, Jacobs JW, Schultz PG (1988) Antibody catalysis in reverse micelles. J Am Chem Soc 110:8713–8714
Regalado C, Asenjo JA, Pyle DL (1996) Studies on the purification of peroxidase from horseradish roots using reverse micelles. Enzyme Microb Technol 18:332–339
Sadler PJ, Viles JH (1996) 1H and 113Cd NMR investigations of Cd2+ and Zn2+ binding sites on serum albumin: Competition with Ca2+, Ni2+, Cu2+, and Zn2+. Inorg Chem 35:4490–4496
Tang JH, Qi SD, Chen XG (2005) Spectroscopic studies of the interaction of anti-coagulant rodenticide diphacinone with human serum albumin. J Mol Struct 779:87–95
Kragh-Hansen U, Chuang VTG, Otagiri M (2002) Practical aspects of the ligand-binding and enzymatic properties of human serum albumin. Biol Pharm Bull 25:695–704
Lin JH, Cocchetto DM, Duggan DE (1987) Protein binding as a primary determinant of the clinical pharmacokinetic properties of non-steroidal antiinflammatory drugs. Clin Pharmacokinet 12:402–432
Fichtl B, Nieciecki A, Walter K (1991) Tissue binding versus plasma binding of drugs: general principles and pharmacokinetic consequences. Adv Drug Res 20:117–166
Pu HL, Huang X, Zhao JH, Hong A (2002) Bergenin is the antiarrhythmic principle of Fluggea virosa. Planta Med 4:372–374
Kim HS, Lim HK, Chung MW, Kim YC (2000) Antihepatotoxic activity of bergenin, the major constituent of Mallotus japonicus, on carbon tetrachloride-intoxicated hepatocytes. J Ethnopharmacol 1:79–83
Li RW, Leach DN, Myers SP, Lin GD, Leach GJ, Waterman PGA (2004) New anti-inflammatory glucoside from Ficus racemosa L. Planta Med 5:421–426
Mishima S, Matsumoto K, Futamura Y, Araki Y, Ito T, Tanaka T, Iiluma M, Nozawa Y, Akao Y (2003) Antitumor effect of stilbenoids from Vateria indica against allografted sarcoma S-180 in animal model. J Exp Ther Oncol 5:283–288
Piacente S, Pizza C, De Tommasi N, Mahmood N (1996) Constituents of Ardisia japonica and their in vitro anti-HIV activity. J Nat Prod 6:565–569
Takahashi H, Kosaha M, Watanabe Y, Nakade K, Fukuyama Y (2003) Synthesis and neuroprotective activity of bergenin derivatives with antioxidant activity. Bioorg Med Chem 8:1781–1788
Abe K, Sakai K, Uchida M (1980) Effects of bergenin on experimental ulcers-prevention of stress induced ulcers in rats. Gen Pharmacol Vasc Syst 4:361–368
Xie JX, Wang L, Liu CX, Zhang DY (1981) The identification and total synthesis of aichasu, an antitussive agent. Acta Pharmacol Sin 16:425–428
Zhong L, Zhang YY, Liu H, Yuan JL, Zheng ZL, Zou GL (2007) Transport of a cancer chemopreventive polyphenol, resveratrol: interaction with serum albumin and hemoglobin. J Fluoresc 17:580–587
Li Y, Yao XJ, Jin J, Chen XG, Hu ZD (2007) Interaction of rhein with human serum albumin investigation by optical spectroscopic technique and modeling studies. Biochim Biophys Acta 1774:51–58
He WY, Li Y, Xue CX, Hu ZD, Chen XG, Sheng FL (2005) Effect of Chinese medicine alpinetin on the structure of human serum albumin. Bioorg Med Chem 13:1837–1845
Karnaukhova E (2007) Interactions of human serum albumin with retinoic acid, retinal and retinyl acetate. Biochem Pharmacol 73:901–910
Desfosses B, Cittanova N, Urbach W, Waks M (1991) Ligand binding at membrane mimetic interfaces human serum albumin in reverse micelles. Eur J Biochem 199:79–87
Davis DM, McLoskey D, Birch DJS, Gellert PR, Kittlety RS, Swart RM (1996) The fluorescence and circular dichroism of proteins in reverse micelles: application to the photophysics of human serum albumin and N-acetyl-L-tryptophanamide. Biophys Chem 60:63–77
Andrade SM, Costa SMB (2006) Spectroscopic studies of water-soluble porphyrins with protein encapsulated in bis(2-ethylhexyl)sulfosuccinate (AOT) reverse micelles: aggregation versus complexation. Chem–Eur J 12:1046–1057
Scatchard G (1949) The attractions of protein for small molecules and ions. Ann N Y Acad Sci 51:660–673
Xu Y, Huang HG, Shen HX (1998) Fluorescence quenching behavior of human serum albumin by quinolone antibacterials. Chin J Anal Chem 26:1494–1497
Lu ZX, Cui T, Shi QL (1987) Applications of circular dichroism and optical rotatory dispersion in molecular biology, 1st edn. Science Press, Beijing
Petitpas I, Bhattacharya AA, Twine S, East M, Curry S (2001) Crystal structure analysis of warfarin binding to human serum albumin. J Biol Chem 276:22804–22809
Morris G (2002) SYBYL Software, Version 6.9. Tripos Associates, St. Louis
Andrade S, Kamenskaya EO, Levashov AV, Moura JJG (1997) Encapsulation of flavodoxin in reverse micelles. Biochem Biophys Res Commun 234:651–654
Bermejo R, Tobaruela DJ, Talavera EM, Orte A, Alvarez-Pez JM (2003) Fluorescent behavior of B-phycoerythrin in microemulsions of aerosol OT/water/isooctane. J Colloid Interface Sci 263:616–624
Miller JN (1979) Recent advances in molecular luminescence analysis. Proc Anal Div Chem Soc 16:203–208
Jiang CQ, Gao MX, Meng XZ (2003) Study of the interaction between daunorubicin and human serum albumin, and the determination of daunorubicin in blood serum samples. Spectrochim Acta Part A: Mol Biomol Spectrosc 59:1605–1610
Li Y, He WY, Tian JN, Tang JH, Hu ZD, Chen XG (2005) The effect of Berberine on the secondary structure of human serum albumin. J Mol Struct 743:79–84
Sudlow G, Birkett DJ, Wade DN (1976) Further characterization of specific drug binding site on human serum albumin. Mol Pharmacol 12:1052–1061
Peters T (1996) All about albumin, biochemistry, genetics and medical application. Academic Press, San Diego
Melo EP, Fojan P, Cabral JMS, Petersen SB (2000) Dynamic light scattering of cutinase in AOT reverse micelles. Chem Phys Lipids 106:181–189
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Y., Dong, L., Li, Y. et al. Characterization of Interaction Between Bergenin and Human Serum Albumin in Membrane Mimetic Environments. J Fluoresc 18, 661–670 (2008). https://doi.org/10.1007/s10895-008-0347-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-008-0347-9