Abstract
This work describes the optimization of a photochemically induced method for the detection of eight phenylureas has been developed by response surface methodology (RSM). These pesticides do not show native fluorescence but they were photolyzed into strongly fluorescent photoproducts under UV irradiation. The effect of the main variables affecting the yield of the photoderivatization reaction, and hence the fluorescence intensity, such as solvent, UV irradiation time and pH were optimized for each pesticide. A Doehlert design was applied in order to obtain maximum intensity fluorescence using response surface methodology. In general, a maximum was found for all pesticides using MeOH as organic solvent, except for diuron, whereas the effect of pH and irradiation time was different, according to each pesticide. Finally, the addition of β-cyclodextrin upon the photochemically induced fluorescence intensity was investigate. The fluorescence intensity was only improved for monolinuron at a concentration of 4 × 10−3 M of β-cyclodextrin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phenylureas represent a large and important class of herbicides widely used in agriculture to control weeds in cereals, vegetables and fruit trees. The first urea compound with herbicidal properties was discovered four decades ago, and since then a large number of urea pesticides have been synthesized. These herbicides are water soluble and their soil-based residues can remain for several months following application. From the soil they can migrate to crops and enter the food chain and, the herbicides can also reach ground waters [1].
Presently, phenylurea herbicides residues are mainly determined by chromatographic methods. Gas chromatography (GC) is commonly used because of its high sensitivity and selectivity, however, the thermal instability of most phenylurea pesticides requires preparation of stable derivatives prior detection, which complicates the analysis because of time consuming and tedious manipulations [2, 3]. Therefore, high-performance liquid chromatography (HPLC) with UV diode array detection (DAD) [4–6] and/or mass spectrometric (MS) detection [7] for additional confirmation is often preferred to GC. However, considerable matrix effects consisting of a suppression/enhancement on the analytical signal have been observed in MS detection [8].
Alternatively, fluorimetric detection is generally more sensitive than the classical UV absorption detection. In addition, fluorescence detectors are very selective, overcoming matrix interference [9].
Although few compounds are fluorescents, some of them possess the necessary degree of aromaticity and may be converted to fluorescent species by using derivatization methods. Recently, several authors have demonstrated the usefulness of room temperature photochemically induced fluorescence (PIF) for the determination of aromatic pesticides [10–15]. The PIF methods are based on the direct UV irradiation of stationary liquid solutions or dynamic flow containing a non fluorescent or weakly fluorescent analyte and photoconversion into strongly fluorescent photoproducts. The main advantages of this methodology are its simplicity, rapidity, low cost and possibility of coupled on-line with chromatographic techniques in contract with the sophisticated equipment and time-consuming procedures such as LC-MS or GC-MS. This methodology has been applied to clinical, pharmaceutical, biochemical and environmental analysis [9].
The development of a PIF method for the determination of analytes involves the optimization of some parameters such as solvent, UV irradiation time, pH and the presence of other substances, such as surfactants or cyclodextrins. The general goal in an optimization is to discover the conditions which produce the best response. Conventional optimization is univariate methodology (one factor at a time) which requires a great amount of experiments, reagents and is time consuming; moreover, interactions between variables are not considered. An alternative method entails multivariate techniques whose main advantages are that they are economical and effective, allowing more than one variable to be optimized simultaneously; in addition, some of these approaches, such as Doehlert designs, are able to take into account interactions between factors.
The uniform shell design developed by Doehlert [16] has the following characteristics:
-
i)
A uniform distribution of the experimental points are allocated on the surface of a hypersphere
-
ii)
The number of experiments is given by N 2 + N + 1, where N is the number of variables under study
-
iii)
Each factor is analyzed at different number of levels. This particular characteristic is relevant when some factors are subject to some restrictions such as cost or instrumental constraints, so their study with a small number of levels is necessary
-
iv)
Extension of the experimental matrix to another experimental domain may be done by using previous adjacent points.
Doehlert design belongs to the category of simultaneous designs, whose basic idea is to record one or more selected experimental responses for a set of experiments carried out in a systematic way, in order to predict the optimum and the interaction effects using regression analysis [17]. These approaches offer advantages in relation to other second order models such as central composite design (CCD) and Box Behnken design (BBD), as they need fewer experiments, which are easier and more efficient. Since the first application of the Doehlert matrix in analytical chemistry [18] a number of articles have appeared involving determinations by spectroscopic, chromatographic, electrochemical and other methods using two, three or even four variables [19–23].
In this paper, the optimization of PIF experimental variables for the determination of eight phenylurea pesticides (monuron, monolinuron, chlorotoluron, isoproturon, diuron, neburon, propanil and linuron) was studied by using Doehlert design. The three optimized variables were pH, organic solvent percentage and UV irradiation time. Two experimental designs were performed for each pesticide using as organic solvent methanol (MeOH) and acetonitrile (AcN) and, finally, the effect of β-cyclodextrin (β-CD) was investigated for the target pesticides in their optimum conditions.
Experimental
Reagents
All experiments were carried out with analytical reagent grade chemicals. The pesticides used in this study, Monuron 99.9%, Linuron 99.5%, Diuron 99.4%, Isoproturon 99.9%, Neburon 99.7%, Chlorotoluron 99.7%, Propanil 99.7% and Monolinuron 99.8% were obtained from Riedel Haën (Seelze, Germany). Organic solvents (AcN and MeOH) were obtained from Merk (Darmstadt, Germany). Demineralized water was obtained with a Milli-Q water purification system (Millipore, Bedford, MA, USA). β-CD was obtained from Cyclolab (Budapest, Hungary).
Apparatus and software
All spectrofluorimetric measurements were carried out using an Aminco Bowman Series 2 Luminescence Spectrometer controlled by a PC Novo Pentium microcomputer.
Fluorescence spectra were recorded by using an AB2 software Version 2.0, running under OS/2 Version 2.0. All spectrofluorimeter measurements were performed at room temperature.
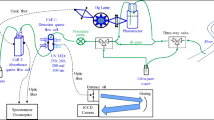
An Oriel 50–500W Model 66901 Xenon lamp (200 W) with an Oriel Model 8500 power supply was used for the photolysis reactions. The photochemical reactor included a light box consisting of a fan, a Xenon lamp and a convenient quartz lens to concentrate the radiation on a standard Hellma 10 mm quartz cell containing an aliquot of the pesticide solution on an optical bench at 30 cm from the Xenon lamp. For the photolysis reactions, the pesticide solution was magnetically stirred during the UV irradiation.
Statgraphics statistical software package was used for data treatment (STATGRAPHICS 6.0, Statistical Graphics Co., 1993).
Procedure
For each pesticide, a stock solution of 200 mg L−1 was prepared by dissolving the exact amount of the corresponding compound in MeOH and preserved in a dark brown bottle at 4 °C in a refrigerator.
Working standard solutions of 4 mg L−1 of each pesticide were freshly prepared by diluting the appropriate volume of each stock solution in a volumetric flask up to a final volume of 10 mL with the appropriate binary aqueous solution. For the study of pH effects, standard solutions of each pesticide were adjusted using 0.02 N HCl and 0.02 N NaOH solutions.
An aliquot of the target solution was placed in a quartz cuvette and irradiated for the fixed time for photolysis reactions by UV light, according to the experimental design. The photoproducts obtained after UV irradiation were then scanned for the fluorescence spectra at the optima excitation and emission wavelengths (λex, λem).
To study the influence of β-Cyclodextrin in the PIF signal, aqueous solutions of 1 × 10−2 M, 5 × 10−3 M, 1 × 10−3 M and 1 × 10−4 M were prepared. An aliquot of each solution was placed into a 10 mL volumetric flask with addition of the appropriate amount of each pesticide solution to give a final concentration of 4 mg L−1 of each pesticide. This solution was irradiated with UV light at 10, 20, 30, 40 minutes. Then each one was scanned to obtain a fluorescence emission spectrum at the optimum λex.
Experimental design
Factors such as pH, solvent composition and irradiation time have been found to show important effects on the fluorescence intensity of photoproducts [11].
A second-order polynomial function, which includes quadratic terms, was used to express the relationship between the fluorescence intensity and the three experimental variables percentage of organic solvent, UV irradiation time and pH, using independently MeOH and AcN or their binary mixtures as solvent. A response surface methodology (RSM) was established to obtain a predictive model for the whole domains of the variables. In order to determine the model coefficients an experimental design Doehlert was chosen.
The three-variables Doehlert matrix has 13 experiments. Five levels were considered for the percentage of organic solvent (A) and the UV irradiation time (B) while for the pH (C) three levels were only considered because previous experiments showed that the two first variables have the stronger effects on the fluorescence intensity of pesticide photoproducts (Y). Table 1 contains the experiments with coded values. Minimum and maximum values of the three variables for each pesticide, listed in Table 2, were chosen according to previous experiments related to their PIF properties. The real values and the corresponding coded values for the three variables were used for setting up the experiments and the model, respectively.
Experimental samples were prepared following the methodology described in the previous section. The mean values of PIF measurements (two replicates for each experiment) obtained at the maxima λex and λem for each pesticide were analysed using the Statgraphics 6.0 software.
Results and discussion
Photochemically induced fluorescence properties
Phenylurea herbicides have no native fluorescence in MeOH and AcN. However, when pesticide solutions (prepared in these organic solvents) were irradiated with a high power UV lamp, they were photolyzed into fluorescent photoproducts in both cases (Fig. 1), except for diuron, neburon and linuron when irradiated in AcN solutions.
In order to investigate the influence of the main variables on the PIF reaction, several experiments involving changes in the percentage of organic solvent in the aqueous mixtures of MeOH and AcN, as well as in irradiation time were performed. The results obtained showed that the PIF signal changes with the percentage of water and this change is different for each pesticide as well as for each organic solvent used. In addition the optimum UV irradiation time increased when the percentage of water in the mixture decreased whereas no photoproducts were obtained for diuron, neburon and linuron in AcN/water mixtures.
Additionally, some experiments were performed in order to determine the pH effect on the fluorescence intensity of photoproducts using binary aqueous mixtures of both organic solvents. The results obtained showed that the PIF intensity and the optimum UV irradiation time changed with the pH of the sample.
In all cases, no significant shift of the λex and λem occurred upon changing the solvent composition and pH at different UV irradiation times, but the fluorescence signal for each pesticide depends on the three variables.
Therefore, in order to obtain the best experimental conditions, it is of interest to evaluate the different variables (percentage of organic solvent, pH and UV irradiation time) affecting the photoderivatization of each pesticide. With this aim an empirical model was developed using RSM to evaluate and optimize their relative significance in the PIF signal even in the presence of complex interactions [24].
Optimisation of the surface response
The optimisation process was carried out using a Doehlert design [16] involving three experimental variables (A, B and C) showed previously in Tables 1 and 2. The surface response that relates the coded variables (A, B and C) with the observed response Y can be calculated as:
where a is the constant term, b, c and d are the coefficients of the linear terms, e, f and g are the coefficients of the quadratic terms and h, i and j are the coefficients of interaction [20].
The analysis of variance (ANOVA) was carried out to identify the variables which have significant effect on the fluorescence intensity of each compound (Tables 3 and 4) at a p-value < 0.05. The results obtained for the F-values using MeOH as solvent indicated that the three variables exhibited statistically significant effects on the PIF signal at the p-value lower than 0.01 for all pesticides except for diuron whose signal was only affected by the irradiation time.
On the other hand, when using AcN as solvent no effect on the PIF signal was observed at different pH except for monuron. As for diuron, linuron and neburon no information was found due to the weak fluorescence signal obtained when using AcN as solvent. Finally, two-level interactions between the three variables also showed significant influence on the analytical responses (Tables 3 and 4).
The results of the multiple regression analysis which provided the estimates of these model coefficients are listed in Table 5, also including the coefficients R2. These were greater than 0.92; therefore the fitted regression equations explain more than 92% of the total variation in the data.
Determination of critical points
From every fitted second-order response surface we studied if there exits a critical point representing the optimal conditions to maximize or minimize the response. Different approaches have been proposed to find them.
Firstly, the signal of the quadratic coefficients (e, f and g) can evidence initial information about the indication of maximum and minimum points. If all of them are negative, the function can show a maximum; if all these coefficients are positive, the function can show a minimum and when some are positive and the others are negative, the critical point can be a saddle point with a relative maximum for the variables with a negative term and with a relative minimum for the one with a positive coefficient.
In this work according to quadratic coefficients (Table 5), a maximum value for PIF intensity was found for monuron, chlorotoluron, propanil, linuron and neburon, a minimum for diuron and a saddle point for monolinuron and isoproturon in MeOH. On the other hand, a maximum was obtained for monuron and monolinuron and a saddle point for chlorotoluron, isoproturon and propanil in AcN.
Nevertheless, it is necessary to verify these conclusions by applying a second approach. In this sense, the LaGrange criterion [25] is generally used to determine the nature of this critical point. In a second-order model for three variables, LaGrange criterion is based on the calculation of the Hessian determinant (Δ1, Δ2 and Δ3) of the response function. If the quadratic equations show only one critical point, four situations are possible. Information on the nature of the surface can be obtained from the values and signs of Hessian coefficients. When Δ2 = 0 there is no information obtained. If Δ1 < 0, Δ2 > 0, Δ3 < 0, it is indicated as a relative maximum. When Δ1 > 0, Δ2 > 0, Δ3 > 0 it is indicated as a relative minima. If none of the above situations are obtained, it represents a saddle point, i.e. the critical point of the surface response which presents maximum response for levels of some variables and, simultaneously, indicates minimum response for levels of other variables of the analytical system studied.
The values of Hessian coefficients obtained in this work for experiments using both MeOH and AcN as solvent are shown in Table 6. As can be seen, when using MeOH as organic solvent the nature of critical point obtained using both approaches are similar while for AcN a saddle point was obtained for all pesticides using the last criterion.
Finally, in order to obtain the coordinates of the critical point (A c ,B c ,C c ) for each pesticide the corresponding polynomial function were calculated by solving the followed equations systems: ΔY/ΔA=0 ; ΔY/ΔB=0 ; ΔY/ΔC=0.
The values of the three variables under study for the critical point for all pesticides are shown in Table 7. In addition, the correlation between predicted and experimental values obtained for PIF intensity at the maximum value demonstrated that the model is well fitted.
Effect of water percentage
In this study, the higher intensity of fluorescence was obtained when using MeOH as organic solvent (except for isoproturon and propanil) and varied with the percentage of water in binary mixtures for each pesticide (Table 7). In general, the PIF intensity increased with the percentage of water in the mixture reached a maximum value between 80 and 20%, according to the compound, except for isoproturon that a diminution in the intensity occurred when the percentage of water increased in the mixture. As an example, Fig. 2 shows the surface responses related with the percentage of MeOH as solvent versus UV irradiation time and versus pH, respectively for chlorotoluron.
Effect of UV irradiation
The PIF intensity of all pesticides increased with the UV irradiation time, reaching a maximum for monuron (30 min), monolinuron (15 min), while no well-defined maximum values were found for the other compounds. Therefore, a maximum time of 40 min was selected as compromise between sensitivity and time analysis. As an example, Fig. 3 shows the surface responses for monuron by plotting UV irradiation time versus percentage of MeOH and versus pH, respectively.
Effect of pH
Finally, the results of F-values (Table 3 and 4) showed that the pH gives the most statistically significant effect on the fluorescence intensity for monuron, linuron and monolinuron in MeOH. However, when using AcN as solvent the pH of the media have a significant effect on the PIF signal only for monuron. Fig. 4 shows the surface responses of linuron by plotting pH versus percentage of MeOH and versus UV irradiation time.
Effect of β-Cyclodextrin concentration
CDs are cyclic oligosaccharides formed by an α-(1,4) linkage of glucopyranose units. The most commonly used oligosaccharides are α, β and γ-cyclodextrines with six, seven or eight glucopyranose units, respectively, which results in inner cavity diameters of different size. Further more, these compounds possess a hydrophobic exterior, which makes them soluble in water and an interior cavity which is less polar than water. These properties enable CDs to incorporate guess molecules on the basis of size and hydrophobicity. Thus, it is well established that β-CD size-selectively complex phenylurea herbicides [26].
The influence of β-CD on PIF intensity on the optimum variables obtained for each pesticide when using MeOH-water solvent was investigated, except for propanil and isoproturon, because for these two pesticides the optimum solvent was 100% of MeOH.
Different concentrations of β-CD, ranging from 10−3 M to 7 × 10−3 M, were tested. The results obtained are shown in Fig. 5. It can be seen that for all pesticides the PIF signal obtained using β-CD was lower than those obtained for optimum values without addition of β-CD, except for monolinuron which showed a maximum at 4 × 10−3M β-CD.
This behaviour indicates that the photolysis reaction is slowed down when phenylurea herbicides are included in β-CD complexes, except for monolinuron, which probably yields photoproducts taking part of inclusion complexes with β-CD. These results are in agreement with those obtained by Mahedero et al. [26], who found that the optimum UV irradiation time was dramatically increased when phenylurea herbicides were in the presence of β-CD. This also explains why phenylurea solutions containing β-CD and being irradiated at the optimum UV irradiation time obtained for aqueous solutions, yield no fluorescence.
Conclusion
The use of UV irradiation allows the sensitive determination of eight non-fluorescent phenylureas by fluorescence detection. Application of Doehlert design allowed the rapid optimization of the main variables such as organic solvent, UV irradiation time and pH, which affect the PIF signal using only 13 experimental measurements. The main goals obtained with this approach are the reduction in the experimental effort, time and cost regarding the use of traditional optimization approaches to obtain the optimum variables in order to obtain higher sensibility on the fluorescence signal. The results obtained will be taken into account for the optimization of a method for the determination of the target phenylureas using fluorescence as a detection system coupled with the chromatographic technique.
References
Leon Gonzalez ME, Townshend A (1991) Determination of organophosphorus and carbamate pesticide standards by liquid-chromatography with detection by inhibition of immobilized acetylcholinesterase. J Chromatogr 539:47–54
Gerecke AC, Tixier C, Bartels T, Schwarzenbach RP, Müller SR (2001) Determination of phenylurea herbicides in natural waters at concentrations below 1 ng l−1 using solid-phase extraction, derivatization, and solid-phase microextraction gas chromatography mass spectrometry. J Chromatogr A 930(1–2):9–19
Peña F, Cárdenas S, Gallego M, Valcácel M (2002) Analysis of phenylurea herbicides from plants by GC/MS. Talanta 56(4):727–734
Martin Esteban A, Fernández P, Stevenson D, Cámara C (1997) Mixed immunosorbent for selective on-line trace enrichment and liquid chromatography of phenylurea herbicides in environmental waters. Analyst 122(10):1113–1117
Pichon V, Bouzige M, Hennion MC (1998) New trends in environmental trace-analysis of organic pollutants: class-selective immunoextraction and clean-up in one step using immunosorbents. Anal Chim Acta 376(1):21–35
Lin HH, Sung YH, Huang SD (2003) Solid-phase microextraction coupled with high-performance liquid chromatography for the determination of phenylurea herbicides in aqueous samples. J Chromatogr A 1012(1):57–66
van der Heeft E, Dijkman E, Baumann RA, Hogendoorn EA (2000) Comparison of various liquid chromatographic methods involving UV and atmospheric pressure chemical ionization mass spectrometric detection for the efficient trace analysis of phenylurea herbicides in various types of water samples. J Chromatogr A 879(1):39–50
Hogenboom AC, Hoffman MP, Jolly DA, Niessen WMA, Brinkman UAT (2000) On-line dual-precolumn-based trace enrichment for the determination of polar and acidic microcontaminants in river water by liquid chromatography with diode-array UV and tandem mass spectrometric detection. J Chromatogr A 885(1–2):377–388
Aaron JJ (1993) Molecular luminescence spectroscopy: methods and applications. Wiley, New York
Vilchez JL, El-Khattabi R, Blanc R, Navalon A (1998) Photochemical-fluorimetric method for the determination of the insecticide imidacloprid in water samples. Anal Chim Acta 371(2–3):247–253
Coly A, Aaron JJ (1988) Fluorimetric analysis of pesticides: methods, recent developments and applications. Talanta 46(5):815–843
de la Peña AM, Mahedero MC, Bautista Sánchez A (2002) High-performance liquid chromatographic determination of phenylureas by photochemically induced fluorescence detection. J Chromatogr A 950(1–2):287–291
Eremin SA, Laassis B, Aaron JJ (1996) Photochemical-fluorimetric method for the determination of total chlorophenoxyacid herbicides, Talanta 43(3):295–301
Gil García MD, Martínez Galera M, López López T, Martínez Vidal JL, Mahedero MC, Salinas F (2001) Photochemical-spectrofluorimetric method for the determination of benzoylurea insecticides: applications in river water samples and in technical formulations. Talanta 53(5):915–925
Vilchez JL, Valencia MC, Navalón A, Molinero-Morales B, Capitán-Vallvey LF (2001) Flow injection analysis of the insecticide imidacloprid in water samples with photochemically induced fluorescence detection. Anal Chim Acta 439(2):299–305
Doehlert DH (1970) Uniform shell designs, Royal Stat Soc Ser C Appl Stat 19(3):231–238
Araujo PW, Brereton RG (1996) Experimental design. 2. Optimization, Trends Anal Chem 15(2):63–70
Hu Y, Massart DL (1989) Uniform shell designs for optimization in reversed-phase liquid chromatography. J Chromatogr 485:311–323
Ferreira SLC, dos Santos WNL, Quintella CM, Neto BB, Bosque Sendra JM (2004) Doehlert matrix: a chemometric tool for analytical chemistry—review. Talanta 63(4):1061–1067
dos Santos WNL, Santos CMC, Ferreira SLC (2003) Application of three-variables Doehlert matrix for optimisation of an on-line pre-concentration system for zinc determination in natural water samples by flame atomic absorption spectrometry. Microchem J 75(3):211–221
García Campaña AM, Cuadros Rodríguez L, Lupiañez González A, Alés Barrero F, Román Ceba M (1997) Sequential response surface methodology for multioptimization in analytical chemistry with three-variable Doehlert designs. Anal Chim Acta 348(1–3):237–246
Korn MGA, dos Santos WPC, Korn M, Ferreira SLC (2005) Optimisation of focused-microwave assisted digestion procedure for Kjeldahl nitrogen determination in bean samples by factorial design and Doehlert design. Talanta 65(3):710–715
Santelli RE, Bezerra MA, de SantaAna OD, Cassella RJ, Ferreira SLC (2006) Multivariate technique for optimization of digestion procedure by focussed microwave system for determination of Mn, Zn and Fe in food samples using FAAS. Talanta 68(4):1083–1088
Box GEP, Hunter WG, Hunter JS (1978) Statistics for experimenters. Wiley, New York
Valderrama MJ (1989) Métodos matemáticos aplicados a las Ciencias Experimentales, Pirâmide, Madrid
Mahedero MC, Muñoz de la Peña A, Bautista A, Aaron JJ (2002) J Incl Phenom Macrocl Chem 42:61–70
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gil-García, M.D., Martínez-Galera, M., Parrilla-Vázquez, P. et al. Application of the Doehlert Design to Optimize the Signal Obtained in Photochemically Induced Fluorescence for the Determination of Eight Phenylureas. J Fluoresc 18, 365–373 (2008). https://doi.org/10.1007/s10895-007-0276-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-007-0276-z