Abstract
A new anthracene-based fluorescent PET sensor 1 with a tridentate ionophore of amide/β-amino alcohol displays very good selectivity and sensitivity for Fe3+ (K a = 1.6 × 103 M−1) and Hg2+ (K a = 2.1 × 103 M−1) in CH3CN–H2O (3:7, v/v) with detection limit of 1 μM. More fluorescence enhancement was observed when 1 selectively detected Fe3+ or Hg2+ in CH3CN and its detection limit was up to 0.03 μM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Detection of cations is of great interest and importance in the fields of chemical, biological, medicinal, and environmental sciences [1–7]. Among several analytic methods for cations, fluorescent chemosensors offer distinct advantages of sensitivity, selectivity, response time, and local observation [1–7]. Fluorescent chemosensors for cations usually consist of two parts: ionophore and fluorophore. The ionophore is responsible for selective recognition of cations, and the fluorophore immediately convert the recognition events into optical signals by means of several types of photophysics mechanisms.
Photo-induced electron transfer (PET), one type of photophysics mechanisms used in fluorescent chemosensors, usually involves promotion of an electron in HOMO of the fluorophore to LUMO upon excitation, followed by PET from HOMO of donor in the ionophore to that of the fluorophore, causing fluorescence quenching of the fluorophore [1–7]. Upon binding with a cation, HOMO energy of the ionophore decreases and PET becomes thermodynamically unfavorable, so that fluorescence quenching is suppressed.
There are some known fluorescent PET sensors for selective recognition of cations, such as 2 for Ag+ [8, 9], 3 for K+ [10], 4 for Zn2+ [11], 5 for Zn2+ and Cu2+ [12, 13], 6 for Zn2+ and Cu2+ [14, 15], 7 for Ni2+ and Cu2+ [16–18], 8 for Ni2+ and Cu2+ [19], 9 for Hg2+ and Cu2+ [20], and 10 for Cu2+ and Hg2+ [21, 22]. It is clear that development of highly selective fluorescent chemosensors for various metal ions is still a challenge.

In this article, we use anthracene as a fluorophore and PET as a photophysics mechanism of the fluorophore. A tridentate ligand of amide/β-amino alcohol was designed as an ionophore for selective recognition of cations. Then we synthesized the fluorescent PET cation sensor 1 by U-4CR followed by N-methylation and reduction [23] (Scheme 1). The ionophore and fluorophore of 1 are close enough to communicate with each other, and its performance as a fluorescent PET cation sensor is shown in this article.
Experimental
General
All the reagents were obtained from commercial suppliers and used as received. Counter anions for all the metal ions are all chloride. All UV-visible absorption spectra were recorded on a Perkin Elmer Lamda 40 spectrophotometer and all fluorescence emission spectra were recorded on a Perkin Elmer LS45 fluorescence spectrophotometer.
Absorption titration experiments
Absorption spectra of 1 (1.8 × 10−5 M) with addition of 0, 0.6, 1.2, 1.6, 1.8 equiv. of FeCl3 or HgCl2 in CH3CN–H2O (3:7, v/v) were measured on a Perkin Elmer Lamda 40 spectrophotometer. The association constant was calculated from the plot of the absorbance ratio, A 0/(A − A 0), versus 1/[metal cation] according to Eq. 1 [24], where [C g] is the concentration of the added metal ion, ɛ M and ɛ c are molar extinction coefficients of the free 1 and a complex of 1/metal cation at a selected wavelength, and A 0 and A denotes the absorbance of free 1 and the solution after adding a metal cation at a selected wavelength.
Results and discussion
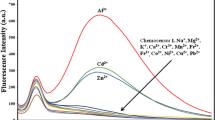
The fluoroionophoric properties of 1 were investigated by fluorescence measurements in the presence of various metal ions. The fluorescence spectrum of the free host 1 was measured in CH3CN–H2O (3:7, v/v, 7.3 × 10−7 M, λ ex = 368 nm) and it showed very weak fluorescence, indicating that fluorescence of the anthracene group in 1 is quenched by intramolecular photo-induced electron transfer from the lone pair of electrons of the nitrogen to the adjacent anthracene group. Upon interaction of 1 with 20 equiv. of alkali metals (Na+, K+), alkaline earth metals (Mg2+, Ca2+), transition metals (Mn2+, Co2+, Ni2+, Zn2+, Ag+), or Pb2+, the fluorescence was affected very little (Fig. 1). Similarly, fluorescence of 1 was increased a little by addition of 20 equiv. of Cu2+. However, Fe3+ or Hg2+ made a significant fluorescence enhancement of 1 in CH3CN–H2O (3:7, v/v) and their detection limit can be up to 1 μM.
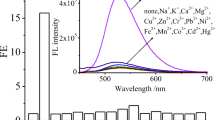
When the fluoroionophoric properties of 1 were investigated in CH3CN, the fluorescence enhancement was dramatically increased by 40-fold, and so is the selectivity of 1 for Hg2+ and Fe3+ (Fig. 2). Its detection limit for Hg2+ and Fe3+ can be up to 0.03 μM. On the other hand, the fluorescence enhancement of 1 by Cu2+ in CH3CN became insignificant. In comparison with other fluorescent PET sensors 2∼10 [8–22], 1 shows outstanding selectivity for Fe3+ and Hg2+ and excellent sensitivity (up to 0.03 μM in CH3CN), which is close to that of 2∼10.
Absorption spectrum of 1 was measured in CH3CN–H2O (3:7, v/v) and it showed characteristic absorption of anthracene at around 345∼395 nm. The absorption titration spectra of 1 with Fe3+ in CH3CN–H2O (3:7, v/v) are shown in Fig. 3. Isosbestic points found in the absorption titration spectra strongly suggest that the solution involves two-species equilibrium between free host 1 and one host-guest complex. Absorption changes at 345∼395 nm were quite significant in the absorption titration spectra and they were used to calculate the association constant (K a) between 1 and Fe3+. According to Eq. 1 [24], the plot of the absorbance ratio, A 0/(A − A 0), versus 1/[Fe3+] was generated and a straight line was obtained, indicating that the host–guest complex throughout the titration is 1:1 1/Fe3+ complex and the association constant (K a) is 1.6 × 103 M−1. Similar results were found with the UV-Vis titration of 1 with Hg2+. The straight line of the absorbance ratio, A 0/(A − A 0), versus 1/[Hg2+] indicates that the host–guest complex throughout the titration is 1:1 1/Hg2+ complex and the association constant (K a) is 2.1 × 103 M−1.
According to the fluorescence measurement results for the fluoroionophoric properties of 1, the tridentate ionophore of 1 selectively binds with Fe3+ and Hg2+. Once the host–guest complex is formed, the lone pair of electrons on the nitrogen of 1 is stabilized and it is thermodynamically unfavorable for them to do intramolecular photo-induced electron transfer to the adjacent anthracene group upon photoexcitation of 1, resulting in the significant fluorescence enhancement. To our knowledge, it is the first time to report that the tridentate ionophore of amide/β-amino alcohol does selective recognition of Fe3+ and Hg2+.
Conclusion
The tridentate ionophore of amide/β-amino alcohol in 1 selectively recognizes Fe3+ and Hg2+. The fluorophore of anthracene in 1 responses the recognition event of the ionophore by following PET mechanism. The sensitivity of 1 for Fe3+ and Hg2+ in CH3CN can be up to 0.03 μM.
References
Valeur B, Leray I (2000) Design principles of fluorescent molecular sensors for cation recognition. Coord Chem Rev 205:3
de Silva AP, Fox DB, Huxley AJM, Moody TS (2000) Combining luminescence, coordination and electron transfer for signaling purposes. Coord Chem Rev 205:41
Prodi L, Bolletta F, Montalti M, Zaccheroni N (2000) Luminescent chemosensors for transition metal ions. Coord Chem Rev 205:59
Fabbrizzi L, Licchelli M, Rabaioli G, Taglietti A (2000) The design of luminescent sensors for anions and ionisable analytes. Coord Chem Rev 205:85
Robertson A, Shinkai S (2000) Cooperative binding in selective sensors catalysts and actuators. Coord Chem Rev 205:157
Keefe MH, Benkstein KD, Hupp JT (2000) Luminescent sensor molecules based on coordinated metals: a review of recent developments. Coord Chem Rev 205:201
de Silva AP, Gunaratne HQN, Gunnlaugsson T, Huxley AJM, McCoy CP, Rademacher JT, Rice TE (1997) Signaling recognition events with fluorescent sensors and switches. Chem Rev 97:1515
Konopelski JP, Kotzyba-Hilbert F, Lehn J-M, Desvergne J-P, Fages F, Castellan A, Bouas-Laurent H (1985) Synthesis, cation binding, and photophysical properties of macrobicyclic anthraceno-cryptands. J Chem Soc Chem Commun 7:433
Fages F, Desvergne J-P, Bouas-Laurent H, Marsau P, Lehn J-P, Kotzyba-Hilbert F, Albrecht-Gary AM, Al Joubbeh M (1989) Anthraceno-cryptands: a new class of cation-complexing macrobicyclic fluorophores. J Am Chem Soc 111:8672
de Silva AP, de Silva SA (1986) Fluorescent signaling crown ethers: ‘switching on’ of fluorescence by alkali metal ion recognition and binding in situ. J Chem Soc Chem Commun 23:1709
Akkaya EU, Huston ME, Czarnik AW (1990) Chelation-enchanced fluorescence of anthrylazamacrocycle conjugate probes in aqueous solution. J Am Chem Soc 112:3590
Sclafani JA, Maranto MT, Sisk TM, Van Arman SA (1996) An aqueous ratiometric fluorescence probe for Zn(II). Tetrahedron Lett 13:2193
Fabbrizzi L, Licchelli M, Pallavicini P, Taglietti A (1996) A zinc(II)-driven intramolecular photoinduced electron transfer. Inorg Chem 35:1733
Nanjappan P, Czarnik AW (1987) Metal ion catalyzed reactions of acrylonitrile, acrylamide, and ethyl acrylate by way of their Diels–Alder cycloadducts. J Am Chem Soc 109:1826
Huston ME, Haider KW, Czarnik AW (1988) Chelation enchanced fluorescence in 9,10-bis[[(2-(dimethylamino)ethyl)methylamino]methyl]anthracene. J Am Chem Soc 110:4460
Fabbrizzi L, Licchelli M, Pallavicini P, Perotti A, Sacchi D (1994) An anthracene-based fluorescent sensor for transition metal ions. Angew Chem Int Ed Engl 33:1975
Kramer R (1998) Fluorescent chemosensors for Cu2+ ions: fast, selective, and highly sensitive. Angew Chem Int Ed Engl 37:772
Fabbrizzi L, Lichelli M, Pallavicini P, Perotti A, Taglietti A, Sacchi D (1996) In situ 13C solid-state NMR and ex situ GC-MS analysis of the products of t-butyl alcohol dehydration on H-ZSM-5 zeolite catalyst. Chem Eur J 2:167
Fabbrizzi L, Licchelli M, Pallavicini P, Perotti A, Taglietti A, Sacchi D (1996) Fluorescent sensors for transition metals based on electron-transfer and energy-transfer mechanisms. Chem Eur J 2:75
Cha NR, Kim MY, Kim YH, Choe J-I, Chang S-K (2002) New Hg2+-selective fluoroionophores derived from p-t-butylcalix[4]arene-azacrown ethers. J Chem Soc, Perkin Trans 2:1193
Ohler JYNE, Vance DH, Aumiller WD, Czarnik AW (1997) A fluorescent chemosensor signaling only Hg(II) and Cu(II) in water. Tetrahedron Lett 38:3845
Yoon J, Ohler NE, Vance DH, Aumiller WD, Czarnik AW (1996) In: Desvergne J-P, Czarnik AW (eds) Fluorescent chemosensors of ion and molecule recognition, NATO-ASI Series. Kluwer, Dordrecht
Chen F-L, Fu H-K, Liu C-C, Sung M, Sung K (2006) Synthesis of novel chiral b-amino alcohols and diamino alcohols from products of Ugi 3-component reaction. ARKIVOC xii:91
Chou PT, Wu GR, Wei CY, Cheng CC, Chang CP, Hung FT (2000) Excited-state amine-imine double proton transfer in 7-azaindoline. J Phys Chem B 104:7818
Acknowledgments
Financial support from the National Science Council of Taiwan (NSC 95-2113-M-006-008) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sung, K., Fu, HK. & Hong, SH. A Fe3+/Hg2+-Selective Anthracene-Based Fluorescent PET Sensor with Tridentate Ionophore of Amide/β-Amino Alcohol. J Fluoresc 17, 383–389 (2007). https://doi.org/10.1007/s10895-007-0207-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-007-0207-z