Abstract
This is the first report on the preparation and utilization of a novel red-region fluorescent dye (tetracarboxy aluminum phthalocyanine) doped silica nanoparticles. In these nanoparticles, the tetracarboxy aluminum phthalocyanine molecules were covalently bound to silica matrix to protect the dye leaking from nanoparticles in bio-applications. The surface of the nanoparticles was modified by amino groups and easily bioconjugated with goat anti-human IgG antibody. By employing these nanoparticles as fluorescent probe, a sensitive fluoroimmunoassay method has been developed for the determination of trace level of human IgG. The calibration graph for human IgG was linear over the range of 0–500 ng mL−1 with a detection limit of 1.6 ng mL−1. Compared with the corresponding system using free AlC4Pc as a probe for determining human IgG, the sensitivity of the proposed system was notably increased. The method was applied to the analysis of human IgG in human sera with satisfactory results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Application of small organic dyes as fluorescent labeling of biological materials is very widespread; they play an important role in the life science including diagnostics and biological imaging [1]. However, some organic fluorophores suffer from the poor photostability and brightness, and most of them possess fluorescence excitation and emission in the relatively short wavelength region as well as with a narrow Stokes’ shift, which can involve problems of light scattering, and their fluorescence emission can be compromised with the background fluorescence from the sample matrix [2, 3]. So, in recent years, many researchers’ interests focus on how to prepare new style fluorescent probes for biochemical applications [4, 5].
Among these, the dye-doped silica nanoparticles have opened a promising field towards the development of luminescent biolabels. Many studies on this aspect were reported [6–10], particularly since 1992 van Blaaderen and co-workers [11] described the first work of incorporating dyes or fluorophores through covalent bonds into colloidal silica spheres, which can greatly decreased the leakage from the silica matrix. The well-developed conjugation chemistry and biological compatibility of silica make dye-doped nanoparticles an attractive material platform for a diverse range of applications, especially in imaging applications in living biological systems [9, 10]. However, to the best of our knowledge, most of the reports concentrated on the preparation and application of the rhodamine, fluorescein or europium types of fluorescent nanoparticles. While the encapsulation of dyes with fluorescent bands in the red or near-infrared region, for example, the cyanine and phthalocyanine compounds, has not attract much attention yet.
In this work, using water-in-oil (W/O) reverse microemulsion method and co-hydrolysis sol-gel technique [11–14], tetracarboxy aluminum phthalocyanine (denoted as AlC4Pc, a red-region fluorescence dye, Fig. 1), was encapsulated in silica nanoparticles through covalent binding. AlC4Pc firstly reacted with 3-aminopropyltriethoxysilane (APTEOS) in the presence of ethyl-[3-(dimethylamino)propyl]carbodiimide hydrochloride (EDC) to form the AlC4Pc silylanization precursor. Then, by controlling the cohydrolysis of AlC4Pc silylanization precursor, tetraethyl orthosilicate (TEOS) and APTEOS in W/O microemulsion, the surface amino-functionalized AlC4Pc silica nanoparticles (NH2-AlC4Pc-SiO2 Nps) were formed. Since AlC4Pc were covalently linked to the silica matrix, no dye leaking was observed and the fluorescence of these nanoparticles was highly stable during the bio-applications. Moreover, the background fluorescence and the scattered light induced by bio-molecules or matrix can be effectively avoided by using red-region excitation and emission (617/690 nm), because most bio-molecules or matrix have their principal absorption and fluorescence emission in the region below 600 nm [15]. Employing these nanoparticles as fluorescent probes, a sensitive fluoroimmunoassay method has been developed for the determination of trace level of human IgG. It was found that the sensitivity of the proposed system for determining human IgG was notably increased compared with the corresponding system using free AlC4Pc as a probe. Meanwhile, we think, besides as probe to determine trace level biological molecules, these NH2-AlC4Pc-SiO2 Nps also can be applied to the field of biomedical imaging as a promising fluorescence label in the future, which will greatly extend the application range of red region dye and provide new path for biological label.
Experimental
Materials
AlC4Pc was synthesized and purified according to the literature [16]. Tetraethoxysilane (TEOS), 3-aminopropyltriethoxysilane (APTEOS) and Triton X-100 were purchased from Aldrich Chemical Co. Inc. N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC) and Sephadex G-50 were purchased from Sigma. Human IgG (denoted by IgG) and goat anti-human IgG polyclonal antibody (denoted by IgG antibody) were kindly offered by the Cancer Research Center of Xiamen University. Bovine serum albumin (BSA) was obtained from Sino-American Biotechnology Company (Shanghai, China). Glutaraldehyde (50%) and Tween-20 was purchased from Amresco Chemical Company (USA). A phosphate buffered saline (PBS) solution (pH 7.4) at a concentration of 0.01 mol L−1, a Tris-HCl buffer (pH 7.4, containing 0.05% Tween-20) at a concentration of 0.05 mol L−1 and a Na2CO3-NaHCO3 buffer solution (pH 9.0) at a concentration of 0.05 mol L−1 were used. AlC4Pc-labeled goat anti-human IgG polyclonal antibody (Ab-AlC4Pc) was synthesized in our laboratory. The 96-well plate was a product of Corning Glass Works (New York). All other chemicals were the guaranteed or analytic grade reagents commercially available. The distilled, de-ionized water was used throughout.
Apparatus
A Hitachi F-4500 spectrofluorimeter (Tokyo, Japan) equipped with a plotter unit and a 1-cm quartz cell was used for fluorescence measurements. The absorption spectrum was obtained on a Beckman DU-7400 UV/VIS diode array spectrophotometer (Fullerton, CA, USA). Transmission electron microscopy (TEM) images of the nanoparticles were obtained using a Hitachi 600 TEM. A TGL-16C supercentrifuge (Shanghai, China), and a SHZ-88 water-bath vibrator (Jiangsu, China) were used.
Synthesis of surface amino-functionalized AlC4Pc silica nanoparticles (NH2-AlC4Pc-SiO2 Nps)
The NH2-AlC4Pc-SiO2 Nps were synthesized according to a two-step process. AlC4Pc was first covalently bound to APTEOS by amide bond to form AlC4Pc silylanization precursor. Typically, 6.0 mg of AlC4Pc was added to anhydrous dimethylformamide (DMF) and stirred until the complete dissolution of AlC4Pc, then 12.0 mg of EDC was added with stirring for 30 min. Finally, 0.02 mL of APTEOS was added to the mixture and the reaction was stirred for 20 h at room temperature to form AlC4Pc silylanization precursor.
The NH2-AlC4Pc-SiO2 Nps were then synthesized by adding an appropriate amount of AlC4Pc silylanization precursor to a W/O microemulsion prepared by mixing 12.30 mL of cyclohexane, 1.00 mL of Triton X-100, 1.04 mL of n-hexanol and 0.30 mL of water. After stirring for 15 min, 0.22 mL net TEOS and 0.030 mL APTEOS were added into the mixture and the mixture was stirred vigorously for another 15 min to impel TEOS and APTEOS diffused into the nanoreactor. Then 0.3 mL of concentrated ammonia water was added to initiate silane hydrolysis and polymerization. After reacting for 5 h under ice bath, the materials were put in refrigerator and aged for 72 h to ensure completion of the condensation, the nanoparticles were isolated from the microemulsion by acetone, followed by centrifuging and washing with ethanol and water several times to remove any surfactant and unreacted materials. The nanoparticles were then dried in a vacuum for over 20 h at 30°C.
Preparation of NH2-AlC4Pc-SiO2 Nps-labeled goat anti-human IgG polyclonal antibody
To form the IgG antibody conjugated fluorescent nanoparticles, the NH2-AlC4Pc-SiO2 Nps were reacted with goat anti-human IgG antibody by using glutaraldehyde as a coupling agent [17]. The reaction is illustrated in Fig. 2. Firstly, forming the glutaraldehyde activated NH2-AlC4Pc-SiO2 Nps. 10 mg of NH2-AlC4Pc-SiO2 Nps were dispersed into a phosphate buffered saline (PBS, pH 7.4) containing 2.5% glutaraldehyde for about 1 h, and then these nanoparticles were centrifuged and washed with the phosphate buffer and re-dissolved in the glutaraldehyde PBS solution. Secondly, 1.1 mg of goat anti-human IgG antibody was added to the glutaraldehyde PBS solution and incubated at 4°C for 12 h to perform the fluorescent NH2-AlC4Pc-SiO2-IgG antibody conjugates (Ab-NH2-AlC4Pc-SiO2 Nps). Afterward, the fluorescent Ab-NH2-AlC4Pc-SiO2 Nps were centrifuged and washed with PBS several times to remove excess antibody and unbound glutaraldehyde and kept at 4°C in PBS.
Preparation of AlC4Pc-labeled goat anti-human IgG antibody
AlC4Pc was bound to IgG antibody with EDC as a coupling reagent. 0.6 mg AlC4Pc was first mixed with 3.0 mg EDC and stirred at room temperature for 30 min, then 1.1 mg of IgG antibody was added and finally kept at 4°C for 6 h. The conjugate was passed through a 25 × 1.5 cm column packed with Sephadex G-50 by using PBS (pH 7.4) as the eluent. The concentrations of IgG antibody and AlC4Pc in AlC4Pc-IgG antibody (Ab-AlC4Pc) conjugate were determined by measuring the corresponding absorbance at 280 and 685 nm on the assumption that the absorbance of each constituent remained unchanged before and after labeling. From the calibration graphs for AlC4Pc and IgG antibody, the concentrations were calculated to be 1.8 × 10−5 mol L−1 and 2.9 × 10−6 mol L−1, respectively. The approximate molar ratio of AlC4Pc to IgG antibody was 6.
Fluoroimmunoassay
The immunoassay procedure for the determination of human IgG was shown in Fig. 3. 100 μl of goat anti-human IgG polyclonal antibody solution (1:10000 dilution of the original IgG antibody solution of 44 mg mL−1 with 0.05 mol L−1 sodium carbonate buffer, pH 9.0) was first coated onto the polystyrene surface of the 96-well plate at 4°C overnight. The plate was washed three times with Tris-HCl buffer (pH 7.4, containing 0.05% Tween-20) and treated with 100 μl of 0.01 mol L−1 phosphate buffered saline (PBS, pH 7.4) in the presence of 1% BSA at 37°C for 1 h to block the antibody-unoccupied binding sites; then the wells were washed three times with Tris–HCl buffer. A known amount of standard human IgG solution was added to react with the coated IgG antibody. When the IgG antibody has recognized IgG, 100 μl of Ab-NH2-AlC4Pc-SiO2 Nps solution or Ab-AlC4Pc solution were added and allowed to incubate for 2 h at 37°C. Then the plate was washed three times with certain amounts of Tris–HCl buffer and the concentration of IgG can be determined via the fluorescence intensity of the washing solution measured at 690 nm with the excitation at 617 nm. A calibration curve of fluorescence intensity vs. IgG concentration was obtained.
Results and discussion
Synthesis of surface amino-functionalized AlC4Pc silica nanoparticles (NH2-AlC4Pc-SiO2 Nps)
In the presence of EDC, APTEOS can be linked with AlC4Pc via the formation of amido bond to obtain the AlC4Pc-functionalized silane precursor that then was co-hydrolyzed with TEOS and APTEOS in the nanoreactor of reverse microemulsion system to form monodisperse fluorescent nanoparticles. In the synthesis of AlC4Pc-APTEOS conjugate, we used an excess amount of APTEOS, and the reason was to be sure that all AlC4Pc molecules have been reacted. By the co-hydrolysis and co-polymerization of AlC4Pc-functionalized silane precursor, APTEOS and TEOS in the reverse microemulsion system, a coating of primary amine groups on the nanoparticle surface can be directly obtained to enable covalent conjugation of IgG antibody. Measurements by TEM show that the nanoparticles were spherical and uniform in size, about 60 nm in diameter (Fig. 4).
Further experiments also indicated that the nanoparticles size can be tailored from 50 to 100 nm through controlling the synthesis parameters, such as the molar ratio of water to Triton X-100, the molar ratio of water to TEOS, the reaction time, the aging time and the amount of n-hexanol et al.
Spectral characteristics
The electronic absorption spectra for the pure AlC4Pc and NH2-AlC4Pc-SiO2 Nps were measured in 0.01 mol L−1 of PBS solution (pH 7.4). As shown in Fig. 5, AlC4Pc has two absorption bands, a Soret band and a Q band, with the maximum wavelengths located at about 350 and 685 nm (curve B), respectively. Compared with free AlC4Pc, the absorption wavelengths of the fluorescent NH2-AlC4Pc-SiO2 Nps remains almost unchanged (curve A), however, the absorption intensity changed. The absorption of pure SiO2 (curve C) in the short wavelength region may affect that of the fluorescent nanoparticles. Furthermore, the thickness of SiO2 shell in fluorescent nanoparticles have an important impact on the absorption spectra of fluorescent nanoparticles, it was found that when the amounts of TEOS increased to a certain degree, the absorption feature of AlC4Pc will be blurred.
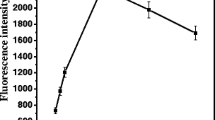
The fluorescence spectra for the free AlC4Pc and NH2-AlC4Pc-SiO2 Nps were exhibited in Fig. 6. It can be seen that the emission spectra of free AlC4Pc (insert) and NH2-AlC4Pc-SiO2 Nps we used in the fluoroimmunoassay experiments (B) had the same profile in aqueous solution. We also found that the fluorescence intensities of the NH2-AlC4Pc-SiO2 Nps solution passed through a maximum with the increasing AlC4Pc loading (A–D). At the same time, the emission maximum of the fluorescent nanoparticles was continuously red-shifted from 690 nm to 695 nm. Because of the π–π interactions and hydrophobic characteristics, AlC4Pc easily form aggregates in aqueous medium, the decrease of fluorescence intensity might due to form the dimers and other aggregates. The silica network could hold off certain AlC4Pc, however, with the increasing amount of AlC4Pc doped into the silica nanoparticles, the interaction between AlC4Pc molecules might still exist. Taking the above reasons into consideration, we chose NH2-AlC4Pc-SiO2 Nps with the loading amount of 100 mg/g which displayed an emission maximum at 690 nm with an excitation maximum at 617 nm as label in further experiments.
Stability
Our experiments indicated that no AlC4Pc molecules was washed out from the nanoparticles, either with hot water or hot ethanol for several times. In the period of our experiments, the nanoparticles was stored in PBS buffer for three months, no AlC4Pc molecules leaking was observed. It suggested that the anchored AlC4Pc molecules are adequately stable in the silica matrix.
In order to investigate the photostability of NH2-AlC4Pc-SiO2 Nps when they are exposed to an aqueous environment for biological applications, the NH2-AlC4Pc-SiO2 Nps were excited with a 150 W xenon lamp in a spectrofluorometer (excitation slit for 5 nm and emission slit was 10 nm). After continuous excitation for 1 h, the emission intensity of NH2-AlC4Pc-SiO2 Nps remained almost unchanged. This result suggested that the fluorescence of AlC4Pc was not affected by the silica network and it is adequate photostable under our experimental conditions.
Covalent immobilization of IgG antibody to NH2-AlC4Pc-SiO2 Nps
Figure 2 illustrated the procedure for the immobilization goat anti-human IgG antibody onto the NH2-AlC4Pc-SiO2 Nps by using the glutaradehyde conjugation method. The IgG antibody modified NH2-AlC4Pc-SiO2 Nps (Ab-NH2-AlC4Pc-SiO2 Nps) conjugates still maintain monodisperse, which could be verified by TEM measurements, to be suited to determine IgG directly.
In the control experiments, we synthesized AlC4Pc-IgG antibody (Ab-AlC4Pc) conjugates to evaluate the two labels’ sensitivity. The fluorescence intensity of Ab-NH2-AlC4Pc-SiO2 Nps compared with that of Ab-AlC4Pc at the same concentration of 100 ng ml−1 of antibody. As can be seen from Fig. 7, the fluorescence of the Ab-NH2-AlC4Pc-SiO2 Nps conjugates was about 5-fold stronger than that of Ab-AlC4Pc conjugates. Hence using fluorescent nanoparticles to label IgG antibody would provide a sufficient sensitivity in fluoroimmunoassay.
Calibration graph for human IgG
We prepared the Ab-NH2-AlC4Pc-SiO2 Nps to evaluate the usefulness of the fluorescent nanoparticles for fluoro-immunoassay. A good linear relationship was observed between the fluorescence intensity and the human IgG concentration in the range of 0.0–500 ng mL−1. The result is shown in Fig. 8a. The limit of detection (LOD = 3σ/S) for human IgG was 1.6 ng mL−1 calculated from the standard deviation of blank (n = 9). In order to make a direct comparison between Ab-NH2-AlC4Pc-SiO2 Nps and Ab-AlC4Pc, the assay using the Ab-AlC4Pc conjugates is also conducted (Fig. 8b) and the LOD of 6.2 ng mL−1, less sensitive than using Ab-NH2-AlC4Pc-SiO2 Nps. The results indicate that the sensitivity of immunoassay can be improved by using the Ab-NH2-AlC4Pc-SiO2 as a new fluorescence label.
Fluoroimmunoassay of human IgG in human blood sera
The human IgG levels in healthy human sera were determined by the proposed assay. The results obtained were summarized in Table 1 and coincident with the human IgG levels in normal human sera (10–14 mg mL−1) [18]. The relative standard deviation within a batch of determinations was below 7% (n = 6). The possibility of using the proposed method for the analysis of real samples was tested by determining the recovery of known amounts of IgG added to the sample. The results in Table 1 show that the proposed method is feasible in practice.
Conclusions
In this work, a novel class of fluorescent silica nanoparticles encapsulating red-region fluorescent dye of AlC4Pc through covalent bond have been prepared and characterized for the first time. The fluorescent nanoparticles in solution are high fluorescence, monodisperse, photostable, and supposed to be suitable for specific labeling of biological macromolecules. In addition, the longer excitation and emission wavelength of AlC4Pc will effectively decrease the interference of background fluorescence when the method was applied to complex biological assay. Here, human IgG was used as a model compound and fluorescence immunoassays as reference technique to demonstrate the applicability of fluorescent nanoparticles in biochemical analysis. The sensitivity of the proposed system for determining human IgG was notably increased compared with the counterpart using free AlC4Pc as a label. We think that the covalent binding of a fluorescent dye to silica nanoparticles described in this study not only is a powerful approach to extend the application range of red region dye, but also provides new path for biological label. It was anticipated that this kind of nanoparticles would exhibit a wide range of applications in biological imaging systems. Work along these aspects is now in progress in our laboratory.
References
Lakowicz JR (1999) Principles of fluorescence spectroscopy, 2nd ed. Kluwer Academic/Plenum Publishers, New York
Trau D, Yang W, Seydack M, Caruso F, Yu NT, Renneberg R (2002) Nanoencapsulated microcrystalline particles for superamplified biochemical assays. Anal Chem 74(21):5480–5486
Aguilar-Caballos MP, Gomez-Hens A, Perez-Bendito D (1999) Pesticide determination by stopped-flow fluoroimmunoassay using cresyl violet as label. Anal Chim Acta 381(2–3):147–154
Parak WJ, Gerion D, Pellegrino T, Zanchet D, Micheel C, Williams SC, Boudreau R, Le Gros MA, Larabell CA, Alivisatos AP (2003) Biological applications of colloidal nanocrystals. Nanotechnology 14:R15–R27
Wang F, Tan WB, Zhang Y, Fan XP, Wang MQ (2006) Luminescent nanomaterials for biological labelling. Nanotechnology 17:R1–R13
Verhaegh NAM, van Blaaderen A (1994) Dispersions of rhodamine-labeled silica spheres: synthesis, characterization, and fluorescence confocal scanning laser microscopy. Langmuir 10:1427–1438
Yang HH, Qu HY, Lin P, Li SH, Ding MT, Xu JG (2003) Nanometer fluorescent hybrid silica particle as ultrasensitive and photostable biological labels. Analyst 128:462–466
Hai XD, Tan MQ, Wang GL, Ye ZQ, Yuan JL, Matsumoto K (2004) Preparation and a time-resolved fluoroimmunoassay application of new europium fluorescent nanoparticles. Anal Sci 20:245–246
Santra S, Yang HS, Dutta D, Stanley JT, Holloway PH, Tan WH, Moudgil BM, Mericle RA (2004) TAT conjugated, FITC doped silica nanoparticles for bioimaging applications. Chem Commun, pp 2810–2811
Ow H, Larson DR, Srivastava M, Baird BA, Webb WW, Wiesner U (2005) Bright and stable core-shell fluorescent silica nanoparticles. Nano Lett 5(1):113–117
van Blaaderen A, Vrij A (1992) Synthesis and characterization of colloidal dispersions of fluorescent, monodisperse silica spheres. Langmuir 8:2921–2931
Arriagada FJ, Osseo-Asare K (1999) Controlled hydrolysis of tetraethoxysilane in a nonionic water-in-oil microemulsion: a statistical model of silica nucleation. Colloid Surf A-Physicochem Eng Asp 154(3):311–326
Arriagada FJ, Osseo-Asare K (1999) Synthesis of nanosize silica in a nonionic water-in-oil microemulsion: effects of the water/surfactant molar ratio and ammonia concentration. J Colloid Interface Sci 211(2):210–220
Yi DK, Selvan ST, Lee SS, Papaefthymiou GC, Kundaliya D, Ying JY (2005) Silica-coated nanocomposites of magnetic nanoparticles and quantum dots. J Am Chem Soc 127(14):4990–4991
Lakowicz JR (1999) Principles of Fluorescence spectroscopy, Chap 16, 2nd ed. Kluwer Academic/Plenum Publishers, New York
Chen FP, Xu DY (1990) Synthesis of water soluble phthalocyanines. Chin J Org Chem 10(6):550–555
Harlow E, Lane DP (1988) Antibodies: a laboratory manual, Chap 13. Cold Spring Harbor Laboratory, New York
Lin YX (1980) Immunology foundation (Chinese). Science and Technology Press of Fujian, Fuzhou, p 30
Acknowledgement
We gratefully acknowledge Mr. Chang-gong Zhang for providing Human IgG and goat anti-human IgG polyclonal antibody. This work was supported by the Science and Technology Innovation Project of Xiamen City (3502Z20022005) in China.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chen, XL., Zou, JL., Zhao, TT. et al. Preparation and Fluoroimmunoassay Application of New Red-Region Fluorescent Silica Nanoparticles. J Fluoresc 17, 235–241 (2007). https://doi.org/10.1007/s10895-007-0162-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-007-0162-8