We demonstrate that absolute measurements of the photoluminescence quantum yield of solutions can be made using an integrating sphere and a conventional fluorimeter. With this method the need for measurements against a luminescence standard is overcome. The sphere is mounted inside a commercial fluorimeter, which gives flexibility in excitation and emission wavelength ranges. A number of compounds have been investigated and the results are compared to literature values and data obtained using a comparative method.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Luminescence is the term used to describe the process by which and excited electronic state decays to a lower state by the emission radiation, mainly in the UV–visible-near infrared parts of the electromagnetic spectrum. Different terms can be used to describe the luminescence, depending on the mechanism of generating the emitting species, for example luminescence can obtained via triboluminescence (stress, mechanically induced), bio- and chemiluminescence (from a bio/chemical reaction), photoluminescence (PL, light-induced excitation) and electroluminescence (EL, results from the recombination of injected charges). Luminescence spectroscopy has become a very important tool and is used in many different applications [1, 2]. For instance, EL is the basis for many types of devices in display technologies [3] and PL is widely used as a probe or analytical tool in physics, chemistry, biology and medicine [1, 2]. Owing to the importance of the luminescence phenomena it is therefore vitally important to be able to make a careful analysis of the process itself. These aspects include spectral characteristics, excited-state dynamics and quantum efficiencies. Through photoexcitation, the latter is also referred to as the photo luminescence quantum yield (PLQY) and its measurement is the focus of this paper. An excited electronic state of a molecule in the condensed phases can return back to the ground state through a number of decay pathways. These include radiative (fluorescence and phosphorescence) and non-radiative processes such as structural and conformational relaxation, quenching (photochemical) and electronic energy transfer. The relative rates of these processes are very sensitive to both the structure of the molecules and their environment, and will therefore have an impact on the observed photophysical properties. Hence, the measurement of PLQYs is a very powerful tool to characterize the photophysics of electronic transitions and to investigate environmental impacts on a chromophore in the condensed phase.
The methods used to determine the PLQY can be divided into two different experimental approaches: the comparative [2, 4–6] and the absolute methods [7–10]. In the first approach, a known luminescence standard is used as a reference and the PLQY is accordingly obtained by a comparison of the luminescence spectra of samples of the unknown and standard with normalized absorptions. This is a widely used method, and is suitable for the study of weakly absorbing, isotropic samples such as dilute solutions. However, the method has some serious drawbacks. The first is the reliance on a luminescence “standard.” The chosen material should ideally have similar optical properties as the material under investigation, i.e. the absorption and luminescence characteristics. While there are a number of luminescence standards, which absorb in the UV–vis region, there are few materials available for longer wavelengths limiting the applicability of this method. But a much more serious problem arises if the emitting species have an anisotropic orientation or some form of long-range order. In this case the positioning and the orientation of the macroscopic sample can affect the outcome of the measurement. As a consequence, the use of a known luminescence standard is no longer possible and it is instead necessary to collect all of the emission from the sample, overcoming the orientational dependence. This can be achieved using an integrating sphere that collects all the emission (2π steradians) from a sample, allowing the determination of the PLQY by an absolute measurement [8–10].
In this method the PLQY is determined relative to the depletion of the excitation intensity (i.e. the absorption), hence relieving the need of a reference compound/material. This in itself is a huge simplification of the measurement of PLQYs. The absolute method has been the standard approach for measurements of thin films over the last decade [8–10]. We have also recently demonstrated that it is possible to use an integrating sphere in combination with a commercial fluorimeter [10]. We now take this method a step further and demonstrate that it is also possible to determine PLQY's of solutions, significantly simplifying the measurement. It removes the need for a standard, which makes the measurement tedious and prone to error, and in addition, it is now possible to determine the PLQY over a wide spectral range. The device we have developed allows measurements of both solid samples and solutions, and can be fitted in a commercial fluorimeter, permitting a broad range of excitation and emission wavelengths to be used. This method should give a facile method for the determination of PLQYs for the widespread scientific community.
MATERIALS AND METHODS
The solvents and compounds used in this study were of spectroscopic grade. The optical density OD of the solutions at the excitation wavelength was less 0.15 when recorded in a 1-cm pathlength cuvette. The fluorimeter used was a HORIBA Jobin-Yvon Fluorolog 3–22 Tau-3, but the device can be used with all HORIBA Jobin-Yvon spectrofluorimeters.
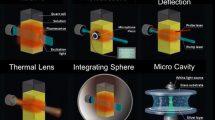
The device is based upon a Labsphere® optical Spectralon® integrating sphere (diameter of 100 mm), which provide a reflectance >99% over 400–1500 nm range (>95% within 250–2500 nm). The sphere accessories were made from Teflon® (rod and sample holders) or Spectralon® (baffle) (Fig. 1).
In Fig. 2, sample holders for thin films (left) and microcuvettes for solution measurements (right) is shown. The cuvette is a homemade cylindrical optical quartz cuvette with a diameter of 8 mm, and is equipped with a homemade Teflon® stopper.
It must be noted that a very high reflectance within the sphere is key to the success of this method. The high reflectances of Spectralon® and Teflon® materials mean that there is little fall of the signal intensity or changes in the spectrum profile, even after numerous reflections within the sphere.
A spectral correction curve for our sphere and sample holder, which is used to obtain corrected spectra was determined in two ways. The first measured the response function of a calibrated white light source (LS-1-CAL calibrated Tungsten Halogen light source from Ocean Optics Inc.) that was allowed to enter the excitation port of the integrating sphere. The ratio of the calibrated vs. measured spectrum gives us response function of the system. In the second method a correction curve was created from the spectra of 10 fluorescence standards (see Table I) measured as dilute solutions within the sphere [11]. The two correction curves obtained by these two methods matched well, and show that the device does not induce any major perturbation above 400 nm.
The PLQY is determined using a method based upon that originally developed by de Mello et al. [9]. In this approach the PLQY (Φ PL) is given by
with
In these equations, E in(λ) and E out(λ) are the integrated luminescence as a result of direct excitation of the film (sample IN, see Fig. 3) and secondary excitation (sample OUT), respectively. The latter emission is due to reflected excitation light from sphere walls hitting the sample [8–10]. X empty(λ) is the Au: Kindly check whether the running title is OK. integrated excitation profile with the empty sphere. α is the film absorptance (Absorptance, α, is the fraction of light absorbed, equal to one minus the transmittance (IUPAC definition), which is obtained by measuring the integrated excitation bands, i.e. the emission signal measured across the excitation wavelength (±5 nm), for two positions of the sample as follows: X in(λ) is the integrated excitation when the sample lies directly in the excitation path and X out(λ) is the integrated excitation when the excitation light first hits the sphere wall as previously explained.
In the “IN” configuration (Fig. 3), absorption of the incident beam is due to direct and secondary excitation, for the same reason the emission collected via the exit port is due to both these excitation. The secondary excitation and fluorescence values are obtained by recording the same spectra in the “OUT” configuration, where no direct light hits the sample.
So as to record the emission spectrum from the sample that is representative of that obtained over the full 2π steradian solid angle, with no privileged solid angle, a deflector, or baffle (see Fig. 3), is placed in front of the exit port. This effectively prevents light passing directly from the sample to the detector. Therefore, there is no need to correct for the refractive index of the solvent, as opposed to the comparative method. For each emission measurement, the spectrum was compared with “usual” fluorescence spectrum (diluted solution in a 1-cm square cuvette), so as to correct for any possible reabsorption effect [9].
Finally, it must be noted that, all of the spectra were recorded with the same excitation and emission monochromator bandpass (slits opening). Because of the high intensity of the excitation profiles, these were recorded through a gauze neutral density (ND) filter(s), reducing the light intensity without changing the spectral profile. The ND value of the filter used was then taken into account in the calculation of the X empty(λ) value, giving this last equation:
with
Finally, the device allows the use of a wide range of sample holders, which are mounted on the threaded sample rod (see Fig. 2). For solid samples, Teflon® holders were made so as to hold the samples at an angle to the incident light beam, preventing the back reflection of radiation out of the sphere via the excitation port (see Fig. 4).
RESULTS AND DISCUSSION
The corrected emission spectrum of fluorescein in 0.01 M sodium hydroxide solution is illustrated in Fig. 5, (excitation at 470 nm). This is consistent with the known profile for this compound, verifying that the correction method for the device is reliable.
Table II presents the compounds, excitation wavelength and the PLQY obtained for the compounds studied, together with their emission range and comparative Φ values from the literature or determined by the comparative method.
The PLQY of fluorescein was determined to be 0.90 ± 0.05, which is in good agreement with the accepted literature value [2]. We also used Rhodamine 101 in acidic (HClaq) methanol and obtained a PLQY of ∼1.00±0.05, with excitation at 535 nm, which is also in good agreement with previously reported literature values [1, 6].
We then made a comparative test using the absolute and relative method. Three acetylenic compounds were investigated in chloroform. The results show good correlation between the two methods, and are within the range of uncertainty for the two different methods. It must be kept in mind that the absolute PLQY values are always subject to controversy, even for “standard” compounds and it is not uncommon to find different values reported for the same materials [1, 2]. This is another cause of errors when the comparative method is used to determine quantum yields.
We believe that the integrating sphere device reported here will provide a quick and simple means of measuring luminescence quantum yields. Considering the importance of photophysics, this will represent the future of determining PLQYs. Work is currently under way to improve the device to allow the study of other sample types.
REFERENCES
J. R. Lackowicz (1999). Principles of Fluorescence Spectroscopy, 2nd ed., Kluwer, New York.
J. N. Demas and G. A. Crosby (1971). J. Phys. Chem. 75, 991.
J. H. Burroughes, D. D. C. Bradley, A. R. Brown, R. N. Marks, K. Mackay, R. H. Friend, P. L. Burn, and A. B. Holmes (1990). Nature 347, 539.
G. Weber and F. W. J. Teale (1957). Trans. Faraday Soc. 53, 646.
A. T. R. Williams, S. A. Winefield, and J. N. Miller (1983). Analyst 108, 1067.
J. B. Birks (1970). Photophysics of Aromatic Molecules, Wiley, New York.
S. I. Vavilov (1924). Zeitung. der. Physik. 22, 266.
N. C. Greenham, I. D. W. Samuel, G. R. Hayes, R. T. Phillips, Y. A. R. Kessener, S. C. Moratti, A. B. Holmes, and R. H. Friend (1995) Chem. Phys. Lett. 241, 89.
J. C. de Mello, H. F. Wittmann, and R. H. Friend (1997). Adv. Mater. 9, 230.
L.-O. Pålsson and A. P. Monkman (2002). Adv. Mater. 14, 757.
J. A. Gardecki and M. Marconelli (1998). Appl. Spect. 52, 1179.
ACKNOWLEDGMENTS
We gratefully acknowledge the assistance of Mr J. Hodgson and Mr N. Holmes of the Department of Chemistry mechanical workshop. We also thank Miss Karen Findlay for assistance with some of measurements and Prof Todd B. Marder (Durham University) for providing some of the compounds. A.B., L.P., L.O.P., and A.P.M. thank OneNorth East and CENAMPS for funding through the Nanotechnology IUC Programme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Porrès, L., Holland, A., Pålsson, LO. et al. Absolute Measurements of Photoluminescence Quantum Yields of Solutions Using an Integrating Sphere. J Fluoresc 16, 267–273 (2006). https://doi.org/10.1007/s10895-005-0054-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-005-0054-8