Abstract
Corrosion of modified 9Cr–1Mo steel (P91) and Indian Reduced Activation Ferritic Martensitic Steel (IN-RAFMS) was studied in static lead–17 atom% lithium eutectic for 355 h at 773 K. Exposure to molten Pb–17Li led to loss of weight in P91 material whereas IN-RAFMS gained weight which was possibly due to longer incubation period and post-exposure surface oxidation. Depletion of Fe and Cr occurred from a thin surface layer in both P91 and IN-RAFMS although higher oxygen buildup on the surface of IN-RAFMS justified its weight gain.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The lead–lithium cooled ceramic breeder (LLCB) blanket concept proposed by India for testing at ITER; uses lead–17 atom% lithium (Pb–17Li) eutectic as multiplier, breeder, and coolant for the CB zones [1]. A serious problem arising from the use of Pb–17Li is the corrosion of the containment material and its deleterious effect on the mechanical properties [2]. Thus, the choice of the best suited material becomes critical and includes consideration of its thermo-mechanical properties, capability to withstand radiation and compatibility with the coolant. Considering the mechanical integrity and radiation stability aspects; ferritic–martensitic (FM) steels containing low Ni and 7–10 % Cr and more recently, the reduced activation varieties of FM steels have been considered as potential candidates for fusion reactor applications [3]. The Indian variety of Reduced Activation Ferritic Martensitic Steel (IN-RAFMS) has been derived from its surrogate material, modified 9Cr–1Mo steel (P91) by replacing the radioactive elements like Mo and Nb by W and Ta respectively [4]. The high temperature corrosion compatibility of such materials with Pb–17Li over a specific time interval will be an important parameter in qualifying such steels within the operating band of fusion reactors like ITER [5]. In this regard, the compatibility of P91 and IN-RAFMS was studied in molten Pb–17Li at 773 K for duration of 355 h and the results have been reported here.
Experimental
Figure 1 shows the schematic of two static corrosion test capsules specifically developed for high temperature corrosion testing of metallic samples in liquid metals and alloys for a considerable duration of time. Each capsule consists of a one end closed SS316L cylinder (12 mm I.D. × a suitable length) for containing the liquid metal/alloy. The two capsules are rigidly mounted on a structural frame consisting of a base plate, s pipe support and a support plate. Rectangular shaped metallic samples of 2 mm thickness having a hole of 4 mm diameter drilled on one side, are initially prepared by wire EDM cutting facility and later finished by metallographic grinding, diamond polishing and ultrasonic cleaning. The samples are mounted on a molybdenum strip (Sample Holder) with the help of molybdenum pins through the drilled holes. Afterwards, the sample assembly is inserted into the test capsule and it is filled with solid Pb–17Li chunks of required quantity so as to ascertain complete dipping of the samples in the molten eutectic. Later the top flange is welded to the capsule and a positive pressure of high purity argon gas is maintained inside it. The capsule assembly is heated by coil heaters having 3 kW capacity and temperature of the capsules are monitored by K-type thermocouples. In the present experiment, two such capsules viz. Tube A and Tube B; having four samples each of IN-RAFMS and P91 respectively, were maintained at 773 K for 355 h continuously.
After the experiment was over, the samples were separated from molten Pb–17Li under inert gas atmosphere and later cleaned off the adherent Pb–17Li with a mixture of acetic acid, acetone and hydrogen peroxide in the ratio 1:1:1. The weight loss of the samples on exposure was measured to obtain the rate of weight change. Thereafter, the cross section of the samples were analyzed through optical microscopy and scanning electron microscope coupled with energy dispersive spectrometry (SEM–EDS) for analysis of micro-structural and composition changes due to Pb–17Li exposure.
Results and Discussion
The P91 samples showed an average weight loss of 0.9 mg after exposure to Pb–17Li for 355 h as indicated in Table 1. This could be extrapolated to a corrosion rate of 2.53 µg/h for P91 in static Pb–17Li. If the dimensions of an individual P91 sample are taken into account, this weight loss would lead to a thickness reduction rate of 0.026 µm/year as shown in Table 2. It is expected that this corrosion rate will significantly increase in an actual fusion reactor system, due to the presence of flowing Pb–17Li which would enhance its corrosive capability. Moreover, the existence of high intensity magnetic field would create a change in the velocity profile of the flowing Pb–17Li thereby affecting it’s interaction with the structural material [6]. On the other hand, no weight loss was observed for the IN-RAFMS material. Instead, gain in weight was noted at an average rate of 45.07 µg/h (Table 1). Such weight gains may be due to immediate surface oxidation post exposure and longer incubation periods (> 500 h) as have been previously reported for various other RAFM steels [7, 8]. In case of surface oxidation, the weight gain of one of the IN-RAFMS sample will correspond to thickness increase at a rate of 0.0116 µm/h as shown in Table 2.
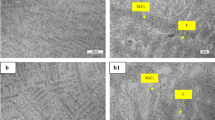
Figure 2 show the optical micrographs of the cross section of the P91 sample before and after exposure to Pb–17Li at 773 K. As compared to the unexposed surface in Fig. 2a, the exposed surface in Fig. 2b showed preferential attack along grain boundaries. Moreover, the grain size of P91 material was found to have almost doubled from 5.6 µm in the unexposed sample (Fig. 2c) to 10.6 µm (Fig. 2d) in the sample exposed to Pb–17Li. This is possibly because of the long term thermal ageing effect at 773 K. Figure 2e, f shows the martensite laths of the unexposed and exposed P91 material respectively. It could be observed that both the size and density of these laths had significantly increased after exposure to Pb–17Li for 355 h.
Figure 3 show the optical micrographs of the cross section of the IN-RAFMS sample before and after exposure to Pb–17Li at 773 K. The unexposed surface of IN-RAFMS is shown in Fig. 3a. As compared to the P91 material, preferential grain boundary attack was not observed on the surface of the IN-RAFMS material exposed to Pb–17Li as shown in Fig. 3b. Moreover, the grain size of IN-RAFMS became more uniform after exposure (Fig. 3d) as compared to the uneven grain size distribution noted in the unexposed matrix of IN-RAFMS in Fig. 3c. It could be observed that the martensitic lath size of unexposed IN-RAFMS material (Fig. 3e) was larger than that of P91 (Fig. 2e). Nevertheless, the lath size of IN-RAFMS decreased after exposure to Pb–17Li at 773 K (Fig. 3f) although an increase in lath density as noted in the case of P91, was observed in IN-RAFMS too.
Figure 4a, b show the SEM images of the cross section of the P91 and IN-RAFMS samples after exposure to Pb–17Li. No affected layer was found on the Pb–17Li facing surface of both the exposed samples. However, when EDS line scans of Fe, Cr and O were taken across the sample boundaries; it was observed that the concentrations of Fe and Cr decreased slightly from their matrix compositions at the exposed surface of both the samples. As shown in Fig. 4a, the oxygen concentration remained almost constant over the cross section of the P91 sample. The source of this oxygen could be from the metallographic grinding and polishing procedure followed during sample preparation. On the other hand, the oxygen content was found to increase significantly at Pb–17Li exposed surface of the IN-RAFMS sample as shown in Fig. 4b. This indicated that IN-RAFMS material exposed to Pb–17Li is prone to post exposure surface oxidation which also explains the reason behind its weight gain. Nevertheless, the results gave a priliminary evidence of the beter corrosion compatibility of IN- RAFMS towards molten Pb–17Li at 773 K, as compared to P91.
Conclusion
-
1.
The corrosion compatibility of P91 and IN-RAFMS material was tested in molten lead–17 atom% lithium eutectic for 355 h at 773 K in static condition.
-
2.
Weight loss occurred at a rate of 2.53 µg/h for P91 material whereas IN-RAFMS gained weight at a rate of 45 µg/h. The weight gain in IN-RAFMS was possibly due to a higher incubation period and post-exposure surface oxidation.
-
3.
Corrosion of both P91 and IN-RAFMS in Pb–17Li at 773 K led to depletion of Fe and Cr from the exposed surface. Grain boundary attack and grain size increase was prominent in P91. The size of martensitic laths increased in P91 whereas decreased in the case of IN-RAFMS.
References
E.R. Kumar, C. Danani, I. Sandeep, C. Chakrapani, N.R. Pragash, V. Chaudhari, C. Rotti, P.M. Raole, J. Alphonsa, S.P. Deshpande, Fusion Eng. Des. 83, 1169–1172 (2008)
P. Chakraborty, P.K. Pradhan, R.K. Fotedar, N. Krishnamurthy, J. Mater. Res. Technol. (2013). doi:10.1016/j.jmrt.2013.04.001
N. Baluc, D.S. Gelles, S. Jitsukawa, A. Kimura, R.L. Klueh, G.R. Odette et al., J. Nucl. Mater. 367–370, 33–41 (2007)
B. Raj, K.B.S. Rao, A.K. Bhaduri, Fusion Eng. Des. 85, 1460–1468 (2010)
H. Glasbrenner, J. Konys, Z. Vob, J. Nucl. Mater. 281, 225–230 (2000)
E. Platacis, A. Ziks, A. Poznjak, F. Muktepavela, A. Shisko, S. Sarada, P. Chakraborty, K. Sanjay, M. Vrushank, R. Fotedar, E.K. Rajendra, A.K. Suri, Magnetohydrodynamics 48(2), 343–350 (2012)
Y. Chena, Q. Huanga, S. Gaoa, Z. Zhua, X. Ling, Y. Songa, Y. Chenb, W. Wanga, Fusion Eng. Des. 85, 1909–1912 (2010)
H. Glasbrenner, J. Konys, H.D. Rohrig, K. Stein-Fechner, Z. Vosset, J. Nucl. Mater. 283–287, 1332–1335 (2000)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chakraborty, P., Kain, V., Pradhan, P.K. et al. Corrosion of Modified 9Cr–1Mo Steel and Indian RAFMS in Static Pb–17Li at 773 K. J Fusion Energ 34, 293–297 (2015). https://doi.org/10.1007/s10894-014-9800-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10894-014-9800-8