Abstract
Vector control and personal protection against anthropophilic mosquitoes mainly rely on the use of insecticides and repellents. The search for mosquito-attractive semiochemicals has been the subject of intense studies for decades, and new compounds or odor blends are regularly proposed as lures for odor-baited traps. We present a comprehensive and up-to-date review of all the studies that have evaluated the attractiveness of volatiles to mosquitoes, including individual chemical compounds, synthetic blends of compounds, or natural host or plant odors. A total of 388 studies were analysed, and our survey highlights the existence of 105 attractants (77 volatile compounds, 17 organism odors, and 11 synthetic blends) that have been proved effective in attracting one or several mosquito species. The exhaustive list of these attractants is presented in various tables, while the most common mosquito attractants - for which effective attractiveness has been demonstrated in numerous studies – are discussed throughout the text. The increasing knowledge on compounds attractive to mosquitoes may now serve as the basis for complementary vector control strategies, such as those involving lure-and-kill traps, or the development of mass trapping. This review also points out the necessity of further improving the search for new volatile attractants, such as new compound blends in specific ratios, considering that mosquito attraction to odors may vary over the life of the mosquito or among species. Finally, the use of mosquito attractants will undoubtedly have an increasingly important role to play in future integrated vector management programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mosquitoes are responsible for the transmission of widespread and sometimes deadly infectious diseases, including malaria, chikungunya, dengue, and Zika. A great number of research projects have focused on methods to control vector-borne diseases by preventing people from being bitten by mosquitoes, such as the use of insecticides or repellents (Dye-Braumuller et al. 2020). For example, insecticide-treated nets (ITNs) and indoor residual spraying (IRS) of insecticides have been shown to limit vector-human contacts, although insecticide resistance threatens these strategies (Liu 2015). Repellents have been used so far mainly for personal protection against biting vectors. The use of repellents has also proved effective in reducing disease transmission by blocking contact between anthropophilic blood-sucking mosquitoes and human hosts (Debboun and Strickman 2013). The development of new bio-sourced mosquito repellents opens perspectives for more eco-friendly disease control (Grison et al. 2020). In parallel, novel promising strategies identify the potential of spatial repellents for disease prevention at the community level (Norris and Coats 2017).
In addition to the use of biocides, vector control and personal protection against anthropophilic mosquitoes may also rely on the use of trapping methods, in which target mosquitoes are attracted to and caught in specifically designed odor-baited traps (Achee et al. 2019). For example, the recent rapid expansion of the invasive mosquito Aedes albopictus, which has colonized every continent and many countries in the past 30 years (Bonizzoni et al. 2013; Paupy et al. 2009), raised an emerging risk for public health (Ae. albopictus is the main vector of several viruses). This mosquito is also an important source of nuisance for urban populations. This context has led to an increasing number of studies devoted to the development of new trapping techniques against this species, including attempts to use mosquito-attractive lures to control it (Akhoundi et al. 2018; Roiz et al. 2016). Moreover, improving the efficacy of trapping methods has several scientific and practical purposes. It would support local survey of mosquito species and their population abundance, surveillance of invasive mosquito species, monitoring of vector-borne pathogens, and prediction of disease epidemics (Akhoundi et al. 2018; Englbrecht et al. 2015). Mosquito attractants may thus play a key role in future programs of integrated vector management for the control and surveillance of mosquitoes. The use of attractants may help reduce the use of insecticides in coming years (Mafra-Neto and Dekker 2019; Mweresa et al. 2020).
The efficacy of trapping methods depends upon both the trapping device and the use of effective mosquito attractants. Many trapping devices (types of traps) are now available and have been already evaluated in numerous comparative studies for different mosquito species (Bazin and Williams 2018; Kline 2006; Thornton et al. 2016). Regarding mosquito-attractive compounds, many studies have already investigated how mosquitoes locate and choose their host, or are guided towards plants, and have reported numerous chemical compounds that are attractive to mosquitoes. As host location in blood-sucking insects is known to be mostly mediated by olfactory cues (Syed 2015; Takken and Knols 1999; Takken and Knols 2010), the key role of vertebrate host odors has been particularly investigated in anthropophilic mosquitoes that transmit pathogens to humans (e.g. Anopheles: Meijerink et al. 2000; Takken and Knols 1999; Culex: Syed and Leal 2009; Aedes: Dekker et al. 2005; Logan et al. 2008. Together with attraction to host odors, adult mosquitoes are guided by olfaction towards many other volatile compounds, at all stages of their biological cycle: mating, oviposition, or sugar-feeding on plants (Bentley and Day 1989; Foster and Takken 2004a; Mozūraitis et al. 2020; Nyasembe and Torto 2014; Vaníčková et al. 2017). Consequently, many volatile compounds, blends of compounds, or natural odor extracts are already used today for the trapping of different mosquito species (Wooding et al. 2020). However, the use of attractants for mosquito surveillance and control remains empirical and the precise conditions of efficacy are so far undefined.
Classifying a compound as a “mosquito attractant” requires a proper verification of the compound’s properties and its attractive effects. Particularly, some of the following points must be addressed when evaluating the attractiveness of a compound: (i) its specificity concerning different mosquito species and non-target insects, (ii) its specificity with regard to specific physiological stage and sex (immature stages, sugar-feeding adults, host-seeking females, breeding site location), (iii) the relative contribution of the compound to the attractiveness when presented individually or in combination with other compounds, (iv) the effect of the dose on the valence of the tested compound, and the possible existence and activity of isomeric forms of the compound.
Although mosquito-attractant volatiles have been the object of intensive study for more than a century, and have been the subject of several reviews (Mwingira et al. 2020; Smallegange and Takken 2010; Sukumaran 2016; Takken 1991; Takken and Knols 1999; Wooding et al. 2020), new attractive molecules or odor blends are regularly investigated as mosquito lures, and particular developments have been proposed over the last decade. A first complete list of attractive compounds was established by Smallegange and Takken (2010), for the three main mosquito species of importance to human health, Aedes aegypti, Anopheles gambiae, and Culex quinquefasciatus. More recently, Wooding et al. (2020) proposed an updated list of the main semiochemicals mediating different stages of adult mosquito life, mating, oviposition, host-seeking, and sugar-feeding, in 11 mosquito species.
The present review aims to substantially supplement these data by providing a comprehensive list of all the compounds, blends of molecules, or natural odors that have been successfully evaluated for mosquito attractiveness. In this paper, the term “attractant” is used in the sense defined by Dethier et al. (1960), although other definitions have been proposed afterwards (Plimmer et al. 1982), as “a chemical which causes insects to make oriented movements towards its source”. We considered in this review all compounds that were demonstrated to be attractive to mosquitoes in the light of laboratory behavioural assays (such as in olfactometer or wind tunnel) or under field conditions (by using diverse trapping methods). The goal of this review is to gather for each attractant a list of studies, methods used, compounds tested in combination, and effects on different mosquito species. We used a chemical approach, in which the classification was based on the name of compounds (instead of listing the compounds per mosquito species). This gives a simple view of the attractive effect of each compound/odor and the range of its biological activity and allows easy comparison of its effects on different mosquito species, including those less frequently studied. Presenting the effects by type of chemical, rather than by mosquito species, allows a quick census of all the studies performed with a particular compound on any mosquito species.
We first propose, in section 1, a brief historical view of the discovery of mosquito attractants throughout the last 100 years. In section 2, we detail the methods used in constructing the list of attractants. Section 3 presents the full list of known mosquito attractants. Then, in section 4, we propose future directions in the study of mosquito attractants.
History of the Discovery of Mosquito Attractants
At the beginning of the 20th century, odors were not considered to play any role in mosquito attraction. Temperature was first imagined as the main attractive cue for these insects: mosquitoes were held to detect the presence of living warm-blooded animals by their attraction to the « warm air rising from hot surfaces » (Howlett 1910). This author verified the effect of heat by placing adult mosquitoes in a gauze bag close to a tube full of hot water, and reported movements and aggregation of Aedes (Stegomyia) scutellaris mosquitoes that proved the role of the hot air. Regarding the role of smell, he concluded that « blood and sweat have no apparent influence ». Other authors also proposed that heat was attractive to mosquitoes, such as Crumb (1922) . This author also recorded an effect of breath on mosquito activation but excluded carbon dioxide as a possible attractant. In the same year, Rudolfs (1922) was the first to hypothesize that « CO2 produced by breathing is the initial attractive agent for the mosquitoes ». He also suggested that other compounds such as ammonia, benzoic acid and some amino acids might be attractive to Aedes species. Laboratory tests using cow or pig blood showed that blood enhanced mosquito attraction (Burgess and Brown 1957; Reuter 1936; Van Thiel and Weurman 1947). Among the constituents of blood likely responsible for this effect, it was long thought that blood is attractive because it contains CO2 « transpired through the skin from the cutaneous capillaries » (Burgess and Brown 1957; Van Thiel 1937). However, excretion of carbon dioxide through the skin was later considered as too low to play a role in this way, and human breath sources of CO2 were then found to play a major role in mosquito attraction (Brouwer 1960; Gillies 1980). Carbon dioxide was then rapidly proved to be highly effective for catching mosquitoes during field trapping campaigns (Brown 1951; Headlee 1934; Huffaker 1942; Huffaker and Back 1943; Reeves 1951; Van Thiel and Weurman 1947).
Haddow (1942) was the first to test human odors in behavioural assays, and found that the presence of unwashed children within huts allowed catching many more mosquitoes compared to huts with washed children inside. Such preferences were found for An. gambiae, An. funestus, An. pharoensis, Taeniorhynchus africanus and T. uniformis, and similar results were obtained when children were replaced by clothes they had worn. Haddow suggested that the « attractant powers of the various constituents of sweat » be further explored. A few years later, Willies (1947) used an experimental dual-port olfactometer to show that mosquito species such as Ae. aegypti and An. quadrimaculatus were strongly attracted to the odor from a human arm. At the same period, similar experiments conducted by Parker (1948) , Ribbands (1949) , Muirhead-Thomson (1951a, b), Thompson and Brown (1955) , and later by Mayer and James (1969) and Price et al. (1979) , confirmed the primary importance of human odors for mosquito attraction. Nonetheless, few authors still considered skin odors (Wright 1962, 1975) or human sweat (Brown 1966) to have an obvious role in mosquito attraction.
The use of human odor extracts as bait for mosquito traps was developed only in the 1990s.
Following observations that feet are the preferred biting sites for the main human malaria vectors in Africa, An. gambiae and An. arabiensis (De Jong and Knols 1995; Dekker and Takken 1998), foot odors have been suspected to be highly attractive to anthropophilic mosquitoes. Njiru et al. (2006) showed that traps baited with nylon socks worn by human subjects were very efficient for catching adult mosquitoes under field conditions. Other authors then successfully used traps baited with nylon socks to collect mosquitoes (Jawara et al. 2009; Qiu et al. 2006b; Schmied et al. 2008; Smallegange et al. 2010).
Before the first detailed analyses of the chemical composition of human body odors, and particularly skin and sweat volatiles, in the early 2000s (reviewed by Dormont et al. 2013), some authors investigated the possible role of isolated compounds already known to be produced by sweat. Among these molecules, the effect of lactic acid on mosquito behavior was investigated very early, by Rudlofs (1922). This author found a repellent effect of lactic acid, and further studies also found this compound to be either repellent or neutral to different mosquito species (Brown et al. 1951; Reuter 1936; Schaerffenberg and Kupka 1959; Skinner et al. 1968). The high concentrations tested in these studies may explain the contradiction between their results and those of other studies showing lactic acid to be an attractant at more relevant concentrations (Acree et al. 1968). Indeed, Miiller (1968) showed that the valence of L-lactic acid changes from repellence to attraction depending on concentration. The attractant effect of lactic acid was later confirmed by other laboratory bioassays (Dekker et al. 2002; Smallegange et al. 2005; Smith et al. 1970) and by physiological studies (Davis and Sokolove 1976). Lactic acid has been successfully used for baited-traps in the field, though often in combination with other attractants (Hoel et al. 2007; Jawara et al. 2011; Kline et al. 1990a).
Ammonia is another molecule targeted by early investigations of possible attractant. Both Rudolfs (1922) and Reuter (1936) conducted tests with ammonia on Aedes and Anopheles species, but found this compound little (or not at all) attractive. Later, Brown (1951) and Rössler (1961) tested ammonia for the attraction of Ae. aegypti, but also concluded that this compound did not affect mosquitoes. Geier et al (1999a) were the first to suspect synergistic effects with lactic acid, and demonstrated that ammonia was actually an attractive compound for Ae. aegypti when combined with lactic acid. Physiological activity of mosquito antennae to ammonia has subsequently been shown by Meijerink et al. (2001) , and ammonia has been added to the list of attractant components for baited-traps in the field (Jawara et al. 2011; Njiru et al. 2006).
Carboxylic acids were also early suspected to play a role in mosquito attraction. The effects of compounds such as benzoic, formic, propionic, butyric, and caproic acids were examined by Rudolfs (1922) and Reuter (1936), and later by Delong et al. (1949) and Brown et al. (1951). However, these studies did not show any attractant effect of these compounds on mosquitoes. In 1973, Carlson et al. were the first to show the attractant effect to Ae. aegypti of several 2C-5C carboxylic acids in an olfactometer. Later, several studies showed physiological and behavioral activities of diverse short- and medium-chain carboxylic acids for An. gambiae and Ae. aegypti (Cork and Park 1996; Knols et al. 1997; Meijerink and van Loon 1999; Pappenberger et al. 1996) and carboxylic acids have thus been used in baited-traps experiments (Jawara et al. 2011; Smallegange et al. 2009).
The mosquito-attractant effect of another compound, 1-octen-3-ol (hereafter “OCT”), now often used in baited-traps, was shown by Takken and Kline (1989) . The role of this compound as an insect attractant was first mentioned by Buttery and Kamm (1980), who found that a volatile compound from alfalfa (Medicago sativa), OCT, could be likely responsible for the attraction of the major pest, the alfalfa seed chalcid. Hall et al. (1984) discovered the attractiveness of OCT to tsetse flies by investigating the olfactory stimulants from cattle odors by electroantennography (EAG). Takken and Kline (1989) were the first to show the efficacy of OCT as a mosquito attractant, used individually in baited-traps or synergistically with CO2, and this compound is now often used in bait traps during field experiments (Cooper et al. 2004; Kline and Mann 1998; Rueda et al. 2001; Shone et al. 2003; Vythilingam et al. 1992).
Since the early 2000s, several new mosquito traps, developed either for scientific use or a commercial purpose, have been designed and tested under field conditions. Most of them rely on the use of synthetic olfactory lures, consisting of specific blends of volatile compounds shown to attract mosquitoes in previous studies. Several synthetic blends (see chapt. 4 for details) have been formulated and used as baits for mosquito-trapping devices. The lures usually consist of a mixture of a few compounds, including often lactic acid, ammonia, and carboxylic acids, e.g. the USDA blend (Bernier et al. 2001), the BG-lure (Geier et al. 2004a, b), the Ifakara blend (Okumu et al. 2010a), or the Mbita blend (Mukabana et al. 2012b).
Although the role of skin microorganisms in the production of body odors has long been known (Shelley et al. 1953), and the influence of microbiota on skin attractiveness to mosquitoes was early suggested by Schreck and James (1968), the effect of skin compounds of bacterial origin on mosquito behaviour has been only studied since the 1990’s. De Jong and Knols (1995) observed a significant decrease in the attractiveness of feet to mosquitoes when they were washed with an antibacterial agent. Later, the same team documented the responses of mosquitoes to diverse volatiles of bacterial origin extracted from Limburger cheese (Knols et al. 1997), while other authors investigated the possible role of skin microbiota present in human sweat on mosquito behaviour (Braks et al. 1999, 2000; Braks and Takken 1999). The influence of microorganisms on mosquito attraction is now clearly established (Verhulst et al. 2010a, 2009, 2011a, b).
Literature Search and Data Extraction
As mentioned above, we considered in this review all chemicals that were demonstrated to be attractive to mosquito species, based on laboratory behavioural assays or field trapping experiments. Studies that investigated compounds only through electrophysiology (electroantennography, single sensillum recording, calcium imaging, …) were excluded from this review. These methods examine antennal detection by mosquitoes but do not evaluate the effective attractiveness of a compound, which requires behavioural assays. We took into account only those electrophysiology experiments that aimed at understanding the underlying mechanisms of attraction by compounds shown to be active in behavioural tests.
We reviewed the different types of volatile chemical cues used by mosquitoes in various contexts of adult mosquito life, such as odors involved in sugar-feeding, host-finding cues, and odors guiding gravid females towards oviposition sites. Volatiles involved in mating, such as sex pheromones, have been well covered in recent detailed reviews (Pitts et al. 2014; Vaníčková et al. 2017; Wooding et al. 2020) and were not included in our study.
Our bibliographic database was elaborated by searching published articles containing the terms “mosquito” AND “attractant” OR “odor” OR “volatile” OR “host” OR “behaviour” OR “trap” OR “semiochemicals”. Literature searches were conducted using PubMed and Web of Science until June 2020. A total of 388 studies were included in this review, from which 316 studies experimentally evaluated the attractiveness of 105 compounds and odors (38 different mosquito species studied) (Tables 1 and 2), and 72 other studies tested or simply used synthetic attractive blends (Table 3).
As mentioned above, studies that employed only electrophysiological methods were not included in this review, nor were studies of pheromones and compounds identified to serve as chemical cues in mating. We also did not retain studies that only compared the efficiency of different trap types, in which trapping effectiveness was evaluated in the light of trap configuration (and without evaluating odor bait constituents).
For all included studies, we extracted the following data: names of the volatile compounds/odors and other compounds tested, names of target mosquito species, methods used to evaluate attractiveness, the effect of the compounds/odors on mosquito behaviour, and effects of combinations of compounds (including synergy). Data are synthesized in Tables 1 to 3.
Most Common Effective Attractants
“Mosquito attractants” in this review are divided into three main categories: (i) single volatile compounds that have been proved to be attractive in the absence of any other odorant source or in combination with other compounds; (ii) host or plant natural odors or odor extracts; (iii) blends of volatile compounds, such as synthetic blends and commercial lures. All the individual compounds, natural odors from living organisms, or synthetic blends that are attractive to adult mosquitoes are presented in Tables 1, 2, and 3, respectively. A total of 77 compounds, 17 organism odors, and 11 synthetic blends have been observed to attract mosquitoes.
In this section, we present the most common effective attractants (see Fig. 1). These are volatile compounds or odors already well known for their efficacy in trapping adult mosquitoes, and they have been proved attractive in more than 70% of the studies that examined these compounds. Compounds for which fewer than 10 studies are currently available are not included in the text of this section. The section classifies the attractants in three groups with respect to the three tables, 1) attractive compounds, 2) attractive odors from organisms, and 3) synthetic blends designed to mimic host odors.
Attractive Compounds
Carbon dioxide
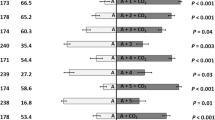
Carbon dioxide has long been considered to be the most important stimulus mediating attraction in host-seeking behaviour for mosquitoes (Gillies 1980) (Fig. 1). Expired breath from animals contains high levels of carbon dioxide (4% in breath vs 0.035% in atmospheric air) and thus acts for blood-feeding insects as a key signal associated with the presence of a vertebrate host.
Fluctuating levels of CO2, perceived as intermittent filamentous plumes of CO2 by mosquitoes, help females to orientate their flight towards the host source (Dekker and Carde 2011; Dekker et al. 2005; Geier et al. 1999b). However a simple exposure to CO2 also results in flight activation in many mosquito species (Gillies 1980), and detection of CO2 enhances attractiveness to several host-related olfactory cues, such as OCT (Vythilingam et al. 1992; Kline and Mann, 1998), human skin odors (Dekker et al. 2005; Webster et al. 2015), heat or lactic acid (McMeniman et al. 2014; van Breugel et al. 2015).
Carbon dioxide has been widely used for decades as a mosquito attractant (alone or in combination with other stimuli) in trapping devices and remains among the main olfactory cues associated with mosquito traps used for scientific and public-health purposes worldwide (Wooding et al. 2020).
The addition of a CO2-release system substantially increases trapping efficacy (CDC traps, Newhouse et al. 1966; Xue et al. 2008; MM-X traps, Njiru et al. 2006; BGS and MM-X traps, Schmied et al. 2008; Roiz et al., 2015). Different sources of CO2 have been evaluated to find a low-cost, widely applicable and safe CO2 source: pressurized gas cylinder, dry ice blocks or pellets (Newhouse et al. 1966; Oli et al. 2005), yeast-generated CO2 (Jerry et al. 2017; Saitoh et al. 2004; Smallegange et al. 2010), propane-derived product (Kline 2002), yeast-fermentation of molasses (Busula et al. 2015; Mweresa et al. 2014), and chemical reactions such as citric acid + sodium bicarbonate, or vinegar + sodium bicarbonate (Hoel et al. 2015). Yeast-generated CO2 is considered to be the best CO2 source for odor-baited traps in the field (Smallegange et al. 2010; Jerry et al 2017; Hoël et al. 2015).
More than 93% of the 122 studies that have tested CO2 attractiveness in the field or under behavioural assays showed a significant increase in mosquito catches or attraction (Table 1). Under field conditions, CO2 alone enhanced trapping efficacy in 96% of the studies, while adding CO2 to other attractant components resulted in increased mosquito catches for 84% of the studies. CO2 was found to be the most important synergist for many candidate attractants, such as ammonia (Hawaria et al. 2016; Njiru et al. 2006; Spitzen et al. 2008), lactic acid (Eiras and Jepson 1991; El-Sisi et al. 2019; Hoel et al. 2007; Spitzen et al. 2008; Verhulst et al. 2011a), linlool oxide (Nyasembe et al. 2015), 3-methyl-1-butanol (Menger et al. 2014b; van Loon et al. 2015), OCT (Hapairai et al. 2013; Kline et al. 1991b), or tetradecanoic acid (Van Loon et al. 2015).
Lactic Acid
The attractiveness of L-lactic acid (or 2-hydroxypropanoic acid, hereafter LA) was first reported following chemical analyses of the acidified fraction of forearm-washing extracts, and after exposure of Aedes aegypti females to this compound in an olfactometer. Humans and other vertebrates naturally produce the levorotatory isomer of lactic acid, L-lactic acid, while the presence of D-lactic acid in body fluids usually results from bacterial infections (Smith et al. 1986).
LA is a by-product of glycolysis in many animals, generated from pyruvic acid under anaerobic conditions in various tissues, such as muscles, brain, kidney, or red blood cells (Gladden 2004). Because LA has low volatility, body emanations of this compound are linked to its high concentration in sweat (Derbyshire et al. 2012). LA is majoritarily produced within eccrine-sweat glands, whose density on human skin surfaces is markedly higher than in all other mammals (Best et al. 2019; Montagna 1985). Comparing the amounts of LA emissions from the skin surface of humans and several other mammals, Dekker et al. (2005) found concentrations of LA at least five times higher in samples from humans than in those from other mammals. Consequently, the high levels of this compound released from the human body have been hypothesized to represent a specific human host-recognition cue for anthropophilic mosquitoes (Dekker et al. 2005; Steib et al. 2001). For example, addition of LA to non-attractive mammal odors dramatically increases their attractiveness to Ae. Aegypti (Steib et al. 2001). Moreover, LA may also emanate from human breath (Jackson et al. 2017; Marek et al. 2010). The respective parts of LA release from breath versus from sweaty skin remain unknown so that global LA emissions from a vertebrate cannot be concluded yet as a strict human-specific signal for anthropophilic mosquitoes (Dekker et al. 2002). In humans, variation among persons in the production of LA is suspected to play a key role in determining attractiveness to mosquitoes (Acree et al. 1968; Dekker et al. 2002; Smith et al. 1970).
LA has rarely been reported to be efficient alone, but rather acts in combination or synergistically with carbon dioxide or other compounds (Acree et al. 1968). For example, Ae aegypti has been observed to be attracted by LA alone (Geier and Boeckh 1999; Geier et al. 1996), whereas LA is attractive to An. gambiae only in combination with CO2 or ammonia (Dekker et al. 2002; Smallegange et al. 2005) and attractive to Ae. albopictus only when combined with OCT (Hoel et al. 2007). Among the 49 studies that evaluated the effect of LA on mosquito attractiveness in the laboratory or in the field, 42 (86%) reported an attractive effect or an increased number of mosquito catches when this compound was combined with other attractants (Table 1).
LA is a regular component of synthetic blends or lures that have been developed to increase mosquito trapping in the field (see Table 3). A repellent effect of LA for adult mosquitoes has been reported in a few studies (Brown et al. 1951; Rudolfs 1922; Shirai et al. 2001).
Ammonia
Ammonia was initially examined as a possible attractive compound by several authors (Rudolfs 1922; Reuter 1936; Brown et al. 1951), but no effect on mosquito orientation was found until Geier et al. (1999a) tested ammonia in combination with lactic acid, and found a strong additive effect. Although most later authors found ammonia to be active only when other compounds are present (synergy with lactic acid, tetradecanoic acid, or CO2), a few studies showed that ammonia presented alone can significantly attract mosquitoes, including Ae. aegypti (Mathew et al. 2013) and An. gambiae (Braks et al. 2001).
Ammonia is constantly produced in various tissues of the body of living organisms, mainly as a result of amino-acid catabolism (Walker 2014). The presence of ammonia in human body odors can be detected both in exhaled breath and skin emanations, but the respective parts of these emissions have not yet been compared in detail (Geier et al. 2002). The level of ammonia in sweat is strongly linked to the amounts of plasma ammonia (Czarnowski et al. 1992), while ammonia in breath may originate from different sources (Chen et al. 2014).
Ammonia has been successfully included in the composition of most of the modern synthetic lures used in field trap systems, such as in the “Synthetic blend” (Smallegange et al. 2005), the BG-Lure (Geier et al. 2004a,b), the Ifakara blend 1 (Okumu et al. 2010b), the Mtiba blend (Mukabana et al. 2012b), and the Mix-5 (Xie et al. 2019) (see Table 3).
1-octen-3-ol (OCT)
OCT, also known as the “mushroom alcohol”, is a natural compound typically produced and emitted by fungi. It derives from the enzymatic oxygenation of linoleic acid (Wurzenberger and Grosch 1984), and has been identified in the volatiles of many mushroom species (Dickschat 2017), but also in the flower scent of numerous plant species (Knudsen et al. 2006) and volatiles from bacteria (Davis et al. 2013). The presence of OCT in mammal body odors was first documented by Hall et al. (1984), who analyzed the volatiles emitted by oxen. OCT has been identified as a natural ligand of the bovine odorant-binding protein present in the respiratory and olfactory nasal mucosa of ruminants (Ramoni et al. 2001).
Although OCT is sometimes regarded as a component of human sweat or exhaled breath, its presence in human body odors remains to be demonstrated. While more than 90 studies have already investigated the composition of exhaled breath and human skin odors (see the reviews of Dormont et al. 2013, Lawal et al. 2017, and more recent papers), only three studies have detected the presence of 1-octen-3-ol in skin volatile extracts (Bernier et al. 2000; Cork and Park 1996; Gallagher et al. 2008).
The efficacy of OCT as an insect attractant was first reported by Hall et al. (1984) for tsestse flies. Takken and Kline (1989) demonstrated that OCT can also be used as a mosquito attractant, and found increased catches of host-seeking mosquitoes such as Aedes or Anopheles species, while Culex species were observed to be only weakly attracted to OCT (Kline et al. 1991a; Kline 2007). For example, OCT is particularly efficient in attracting Ae. Albopictus (Qualls and Mullen 2007; Roiz et al. 2016), whereas OCT-baited traps failed to catch C. quinquefasciatus (Majeed et al. 2016; Mboera et al. 2000c) and other Culex species (Essen et al. 1994; Kemme et al. 1993; Kline 2007). OCT may even have a repellent effect for some species, such as C. quinquefasciatus (Xu et al. 2015) and (at high doses) Ae. Albopictus (Guha et al. 2014). Interestingly, OCT and CO2 unequivocally act synergistically in field trapping experiments (Kline et al. 1991a, b; Kline and Mann 1998) whereas OCT is often found not to be attractive by itself (Kline et al. 1991a; Rueda et al. 2001; Russell 2004; Shone et al. 2003; Vythilingam et al. 1992). OCT has thus been widely used for the trapping of mosquitoes since the 1990’s, and 36 of the 48 studies (75%) that evaluated OCT efficacy in the field found a significant increase in mosquito catches (Table 1). Both (R)- and (S)- enantiomers of OCT were found effective in catching mosquitoes in the field, but (R)-1-octen-3-ol was more attractive than the isomeric mixture (Kline et al. 2007), confirming observations that olfactory receptor neurons are more sensitive to this enantiomer (Bohbot and Dickens 2009).
Surprisingly, this attractive compound is not included in the synthetic blends or commercial lures developed for the trapping of mosquitoes, except the TrapTech lure (Table 3). However, OCT is the principal component provided with a few commercial traps, such as the Dragonfly or MM-X traps (Kline 2006).
Fatty Acids
Carboxylic acids, and particularly fatty acids (FAs) (carboxylic acids carrying an aliphatic chain), have long been suspected to be attractive compounds for mosquitoes. Both short-chain (C2-C5) and medium-chain (C6-C11) FAs are commonly found in volatile emissions from human skin (Ara et al. 2006; Caroprese et al. 2009; Dormont et al. 2013). Volatile FAs in skin odors originate from the metabolism of glycerol, lactic acid, amino acids, and diverse skin lipids, under the action of various skin bacteria (James et al. 2004; James et al. 2013). Interestingly, the presence of volatile FAs emanating from skin host may serve as a chemical signature for host-seeking anthropophilic mosquitoes to locate a human host: other hosts such as non-human mammals or birds do not emit such volatile compounds (Nicolaides et al. 1968).
Rudolfs (1922) and Reuter (1936) were the first to imagine the possible effects of some C2-C6 FAs on mosquito behaviour. However, the first demonstration of FAs activity on adult mosquitoes was made much later, by Carlson et al. (1973), who screened the attractiveness of numerous acids for Ae. aegypti females. Many FAs have since been shown to attract diverse species of mosquitoes (Puri et al. 2006; Seenivasagan et al. 2014; Smallegange et al. 2009) (see Table 1), and have been successfully used, individually or in FAs blends, as attractive lures for field trapping (Jawara et al. 2011). For instance, several FAs have been incorporated in the formulation of widely used synthetic blends designed to mimic human odor in the field, such as ifakara blend (which includes C3 to C5 FAs), Mbita blend (which includes tetradecanoic acid), or BG-lure (in which hexanoic acid is the dominant compound) (see Table 3).
Natural Host Odors
Human Odors
The crucial role of human body odors was early suspected, following observations showing that some mosquito species are preferentially attracted to human hosts and rely on human odors to detect and select their host (Takken 1991). Because several mosquito species bite some parts of the body more frequently than others (De jong and Knols 1995), several body regions have been studied to evaluate their relative attractiveness to host-seeking mosquitoes. Hands and feet odorare among the most frequent body parts whose odors have been included in behavioural bioassays (see Table 2). Tests using armpit sweat extracts, other skin sweat extracts, or breath volatiles, have also been conducted to elucidate the effects of human body odors. Some studies have even tested the attractiveness to mosquitoes of odor of the entire human body, under laboratory conditions using olfactometer devices (Lacroix et al. 2005; Lefevre et al. 2009; Olanga et al. 2010) or under field conditions using diverse trapping methods (Table 2).
Most studies using human odors as stimuli to attract host-seeking mosquitoes have reported a strong and significant attractive effect, at least for mosquito species exhibiting a high degree of anthropophily: for both Ae. aegypti and An. gambiae, 100% of studies have found human odor samples very effective in attracting mosquitoes. Among odors emanating from various body parts, foot odors have been demonstrated to be highly attractive to several anthropophilic mosquitoes (De Jong and Knols 1995; Dekker et al. 1998; Lacey and Carde 2011). Following such observations, Njiru et al. (2006) demonstrated that traps baited with nylon socks worn by human subjects were very efficient in catching adult mosquitoes under field conditions. Other authors have also successfully used traps baited with nylon socks to collect mosquitoes (Jawara et al. 2009; Qiu et al. 2004; Schmied et al. 2008; Smallegange et al. 2010). Foot odors consist of numerous volatile compounds and particularly include various FAs (Ara et al. 2006; Caroprese et al. 2009) that have been shown to elicit strong olfactory responses of female mosquitoes (Meijerink and van Loon 1999; Smallegange et al. 2009).
Most of the synthetic blends used to enhance trapping efficiency in the field consist of mixtures mimicking human odors, and thus include components that have been chemically identified from volatile skin or sweat samples (Table 3). Such lures often involve compounds such as lactic acid, ammonia, aldehydes, and diverse FAs, all of which have been recorded in human skin odors and observed to elicit mosquito behavioural and electrophysiological responses.
Interestingly, differences in attractiveness among persons have long been known. For example, in several early studies, babies and children were observed to be less bitten than adults (Carnevale et al. 1976; Clyde and Shute 1958; Muirhead-Thomson 1951a; Thomas 1951), while pregnant women have been shown to attract more host-seeking mosquitoes than other women (Ansell et al. 2002; Himeidan et al. 2004; Lindsay et al. 2000). Several authors have also recorded clear variation in attractiveness to female mosquitoes among individuals that cannot be accounted for by such differences in age and reproductive state (Burkot 1988; Curtis 1986; Knols et al. 1995; Lindsay et al. 1993; Michael et al. 2001; Scott et al. 2006). Such heterogeneity in human-mosquito interactions is suspected to result from differences in human odor profiles (Bernier et al. 2002; Geier et al. 2002; Logan et al. 2008). However, chemical explanations for the differences in attractiveness among distinct human odor profiles remain to be established. Among the main components currently isolated in skin emanations, the ratio of two terpenes 6-methyl-5-hepten-2-one (“sulcatone”) and geranylacetone has been hypothesized to partly explain why some people are more attractive to mosquitoes than others (Leal et al. 2017; Logan et al. 2010). 6-methyl-5-hepten-2-one is even considered to be a key attractant that leads Ae. aegypti to preferentially bite human hosts (McBride et al. 2014). The relative proportions of aldehydes such as nonanal, decanal, or octanal, which are regularly found in skin volatile extracts (Dormont et al. 2013), have been also proposed to play a key role in the attraction of anthropophilic mosquitoes (Jacob et al. 2018; Leal et al. 2017; Owino et al. 2015; Syed and Leal 2009). Other authors have considered L-lactic acid to be a key compound in which inter-individual variation in levels emitted could explain the differential attractiveness that is frequently observed (Dekker et al. 2002). Mukabana et al. (2004) suggest that the breath of some individuals contains repellent compounds, perhaps explaining why some humans are bitten more than others.
However, why some persons attract more host-seeking mosquitoes than others is still not fully understood. Investigating this question requires a detailed examination of the chemical composition of human odors, but analysis of skin volatiles remains complex and is often subject to uncertainties in identification (Charpentier et al. 2012; Dormont et al. 2013).
More recently, the key role of commensal cutaneous microorganisms, i.e. skin microbiota, in human attractiveness to mosquitoes has been highlighted by some authors (Braks et al. 1999; Braks and Takken 1999; Busula et al. 2015; Takken and Verhulst 2017; Verhulst et al. 2010b). Human-associated microbiota living on the skin have been shown to strongly influence the production of skin volatiles, which contain both volatile compounds of microbial origin and bacteria-transformed compounds of sweat origin. Variation in the distribution of skin bacteria on the human body, which is mainly linked to the local abundance of skin glands of different types (Kearney et al. 1984), may explain the differences in body odor composition among body parts. For example, the strong odor emanating from feet (Ara et al. 2006; Caroprese et al. 2009), which is highly attractive to host-seeking mosquitoes (De Jong and Knols 1995; Dekker et al. 1998), is likely determined by the specific and unique microbiota populations associated with this body part (Adamczyk et al. 2020). At the same time, the varying composition of skin microbiota among human subjects (Byrd et al. 2018; Fierer et al. 2008; Grice et al. 2009) may likely contribute to the differences of human odors among subjects, and thus to the differences in human attractiveness to mosquitoes. To summarize, the human skin microbiota likely play a crucial role in the formation of human body odors, and thus in the chemical ecology of anthropophilic mosquitoes searching for a human host (Verhulst et al. 2010a, 2011a, b; 2010b).
Cow Odors
Among odors from diverse animals that have been tested to attract mosquitoes, such as those of birdsodor (Spanoudis et al. 2020; Syed and Leal 2009), or of the mouse odor(Le Goff et al. 2017; McCall et al. 1996), volatile emissions from cows and their effects on mosquito attractiveness have been investigated by many authors (Table 2). Cow odors were found to be equally attractive to human odors for host-seeking adult females of anthropophilic species, such as Ae. aegypti (Majeed et al. 2016) and Ae. arabiensis (Abong’o et al. 2018; Duchemin et al. 2001). Adult females of Mansonia africana were observed to prefer cow odors to odors of several other mammalian odors (Bakker et al. 2020). Even for mosquito species known to prefer cow over man for blood meals, e.g. for the zoophilic A. quadriannulatus, the attractive effects of cow odors in host-seeking behaviour were found to be as great as that of CO2, suggesting that the dominant role of host odors relative to that of CO2 characterizes only anthropophilic mosquito species (Dekker and Takken 1998).
Plant Odors
Volatiles from plants represent another source of odors that have a great potential to serve as mosquito attractants. Mosquitoes may be guided towards plant-originated odors for two main reasons: when searching for sugar sources (usually floral nectar) (Foster 1995; Takken and Knols 1999) or oviposition sites (Afify and Galizia 2015; Mwingira et al. 2020; Wooding et al. 2020). In both cases, olfactory cues emanating from plants, e.g. floral volatiles or odors from grass-infused water, play a major role in attraction of adult mosquitoes.
Regarding sugar feeding, many species of mosquitoes are attracted by floral volatiles from diverse angiosperm species (Barredo and DeGennaro 2020; Nyasembe and Torto 2014) (and see Table 2). Whole flower extracts, as well as fruit odors, have been shown attractive to several mosquito species. Single volatile compounds emanating from floral or vegetative plant parts have been also shown to be behaviourally active as mosquito attractants, such as diverse terpenes, fatty acid derivatives, or even green leaf volatiles (Jhumur et al. 2007; Nyasembe and Torto 2014; Wooding et al. 2020). Interestingly, some terpenes, which are naturally not produced by mammals have recently been isolated in the odors of malaria-infected mammals and were hypothesized to explain the enhanced host attractiveness of infected individuals to mosquitoes (Emami et al. 2017; Kelly et al. 2015). Sugar feeding concerns Both males and females feed on sugar sources and identifying the attractants mediating this behaviour may be of high interest if new mosquito control strategies require trapping of males.
The selection of oviposition sites has been demonstrated to be mediated by several olfactory cues, such as specific pheromones, emanations from larvae, pupae, and eggs, and also compounds of bacterial or plant origin (Afify and Galizia 2015; Mwingira et al. 2020; Wooding et al. 2020). Emanations from grass or hay infusion have been shown to attract gravid females of several mosquito species. Electrophysiological and behavioural tests with various plant-produced volatile compounds, or molecules of bacterial origin from water containing fermenting leaves (Ponnusamy et al. 2008), revealed some molecules involved in oviposition site selection by female mosquitoes. For example, compounds such as 4-methylphenol, 4-ethylphenol, indole, phenol, or 3-methylindole are attractive to females searching for oviposition sites in Ae. aegypti (Baak-Baak et al. 2013), Ae. albopictus (Diaz-Santiz et al. 2020), Ae. triseriatus (Bentley et al. 1979) and C. quinquefasciatus (Beehler et al. 1994; Millar et al. 1992). In other cases, blends of terpenes were found to attract gravid females, such as in A. arabiensis (Wondwosen et al. 2016).
Synthetic Blends
The efficacy of mosquito traps primarily relies on the effectiveness of the odor bait released by the trapping device. Decades of research on mosquito attractants have highlighted some isolated chemical compounds that are very effective in luring and catching flying adult mosquitoes, as well as other compounds weakly attractive on their own, but that proved biologically very active in combination with other molecules. In the meantime, constant progress in the analysis of the chemical composition of human body odors has provided many other interesting compounds to be evaluated for their potential attractiveness to mosquitoes. Several authors have developed mixtures of synthetic compounds designed to mimic naturally-emitted odors of a human body. Such synthetic blends generally include volatile compounds identified to be specifically emitted by skin (e.g. free fatty acids), together with compounds found to occur in significantly higher levels in odors of humans compared to those of other animals (e.g. lactic acid).
A first candidate blend, including lactic acid, acetone, and dimethyl disulphide, and referred to as “USDA blend”, was proposed by Bernier et al. (2001) (Table 3). This first blend was found to be an attractive odor to Ae. aegypti as human odor in olfactometer bioassays (Williams et al. 2006a). Most of the synthetic blends subsequently developed to mimic human scent contain two main attractive components, lactic acid and ammonia, except Lurex, which only consists of lactic acid (Table 3). The blends mainly differ by the composition of carboxylic acids added to the mixture. Among the three most often used synthetic blends—BG-lure, ifakara blend, and Mbita blend—the first one remains the most widely-used lure chosen for odor-baited trapping studies on mosquitoes. To date, BG-lure has been included in 29 studies, while the ifakara and Mbita blends have been used in 10 and 12 studies, respectively. The BG-lure has proven effective in catching 17 different mosquito species, as well as other insect species (Ortiz et al. 2020). This artificial lure, which is presented as an « artificial human skin scent », consists of a blend of ammonia, lactic acid, and hexanoic acid in specific proportions (Geier et al. 2004a, b; Kröckel et al. 2006), and is currently proposed with the commercial Biogents® Sentinel mosquito trap (Biogents GmbH, Regensburg, Germany) as the “BG-Sweetscent”, in which synthetic compounds are contained within sachets instead of cartridges for the BG-lure.
Surprisingly, OCT, which has long been considered a promising mosquito attractant, is not a component of the synthetic attractive blends listed in Table 3, except for one, the TrapTech lure. OCT has often been tentatively used as an attractant lure in experimental field trapping, with contrasting results for different target mosquito species (Table 1). However, some authors reported OCT to be a strong and effective attractant for mosquitoes such as Ae. albopictus (Li et al. 2010; Qualls and Mullen 2007), Ae. taeniorhynchus (Kline et al. 1991b; Takken and Kline 1989), and A. darlingi (Vezenegho et al. 2014), and this compound is now successfully used in trapping diverse mosquito species over the world (Duffield et al. 2019; Evans et al. 2019; Ibáñez-Justicia et al. 2020; Ortega-Morales et al. 2019). The commercially available traps of Mosquito magnet® (Woodstream Corp., Lancaster, PA 17602) are proposed with OCT as the main attractant lure.
Perspectives
More knowledge about the compounds attractive to mosquitoes is undoubtedly needed to further improve the efficiency of baited-traps for mosquito surveillance and control. Identifying the most attractive compounds or blends of compounds, and characterizing the concentrations and ratios that result in the greatest activity, remains to be done for many mosquito species that are vectors of diseases.
Understanding how adult mosquitoes are attracted to volatile signals also requires improving our knowledge of mosquito olfaction. The molecular mechanisms of compound detection by odorant receptors in mosquitoes have been investigated in diverse mosquito species, and recent findings, such as those of Degennaro et al. (2013), McBride et al. (2014), Raji et al. (2019), and Duval et al. (2019), may have promising applications for the use of semiochemicals in vector control programs.
Recent findings showing that mosquitoes are more attracted to malaria-infected than to uninfected hosts may help us find new candidate attractant molecules. Indeed, enhanced attractiveness to adult mosquitoes linked to a change in host odors after an infection has been observed for several models, including humans (Lacroix et al. 2005; Robinson et al. 2018), birds (Cornet et al. 2013; Diez-Fernandez et al. 2020) and mice (De Moraes et al. 2014). Several volatile compounds associated with malaria infection have been identified (De Boer et al. 2017; De Moraes et al. 2018; Kelly et al. 2015; Robinson et al. 2018) and such investigations may facilitate the discovery of new mosquito-attractive compounds in the future. Another way to find new attractive compounds is to conduct studies on mosquitoes other than anthropophilic species: the great majority of studies listed in this review deal with mosquito species that bite humans. Research programs focusing on the chemical ecology of zoophilic mosquito species would probably help us identify interesting new chemical attractants.
Another key point in the use of attractive odors is the design of odor dispenser devices that will deliver volatile compounds under precise conditions. Delivery techniques should ensure constant release rates of attractants in the appropriate amounts (and for blends, in the appropriate proportions) and permit odor delivery over a long period.
Different delivery methods have been developed and used in odor-baited traps, such as low-density polyethylene (LDPE) sachets (Jawara et al. 2011; Torr et al. 2008), nylon strips (Mukabana et al. 2012a; Mweresa et al. 2015; Okumu et al. 2010a), and classic glass vials (Costantini et al. 2001). However, these dispensing devices have often been evaluated based on their efficiency in catching host-seeking mosquitoes, but have not been examined in terms of the chemical composition of the odor released and its stability over a prolonged period of use. Another promising method has been recently developed, which consists of putting synthetic compounds into a glass vial sealed with a polytetrafluoroethylene/rubber septum, in which is inserted a micro-capillary tube. Such a device ensures a regular amount of volatile emissions, and release rates can be calibrated by the length and the diameter of the capillary tube (Erb et al. 2015; Proffit et al. 2020).
Further investigations should also address more precisely the different contexts (adult life stages) in which adult mosquitoes are guided by attractive volatile cues. While it is now well known that distinct odor signals are used by mosquitoes searching for mates, sugar meals, hosts, or oviposition sites, a large majority of studies have focused on chemical signals mediating host-seeking behaviour (254 of the 316 studies, Tables 1 and 2). Most of the currently available commercial traps rely on odor blends used by flying mosquitoes looking for hosts, and 10 of the 11 synthetic blends listed in Table 3 are designed to attract host-seeking mosquitoes. However, a list of semiochemicals attractive to females searching for oviposition sites is now available (Table 1). Targeting gravid female adults searching for oviposition sites by using Autocidal Gravid Ovitraps (Mackay et al. 2013) under mass trapping programs may allow a consequent reduction of mosquito female populations (Barrera et al. 2014; Lega et al. 2020).
Reducing the density of mosquito local populations and reducing the survivorship of adult females, in particular in risk areas of virus transmission, by attracting adult females to odor-baited traps is now considered to be a valuable complementary vector control method. The use of lure-and-kill traps, or the recent development of mass trapping, have proved effective in durably decreasing vector populations in some cases (Degener et al. 2014; Johnson et al. 2017) and it is expected that the use of mosquito attractants will have an increasingly important role to play in integrated vector control strategies.
References
Abong’o B, Yu X, Donnelly MJ, Geier M, Gibson G, Gimnig J, ter Kuile F, Lobo NF, Ochomo E, Munga S, Ombok M, Samuels A, Torr SJ, Hawkes FM (2018) Host Decoy Trap (HDT) with cattle odour is highly effective for collection of exophagic malaria vectors. Parasites Vectors 11:1–11
Achee NL, Grieco JP, Vatandoost H et al (2019) Alternative strategies for mosquito-borne arbovirus control. PLoS Negl Trop Dis 13:e0006822
Acree F, Turner R, Gouck H, Beroza M, Smith N (1968) L-Lactic acid: a mosquito attractant isolated from humans. Science 161:1346–1347
Adamczyk K, Garncarczyk A, Antonczak P, Wcislo-Dziadecka D (2020) The foot microbiome. J Cosmet Dermatol 19:1039–1043
Afify A, Galizia CG (2015) Chemosensory cues for mosquito oviposition site selection. J Med Entomol 52:120–130
Akaratovic KI, Kiser JP, Gordon S, Abadam CF (2017) Evaluation of the Trapping Performance of Four Biogents AG Traps and Two Lures for the Surveillance of Aedes albopictus and Other Host-Seeking Mosquitoes. J Am Mosq Control Assoc 33:108–115
Akhoundi M, Jourdain F, Chandre F, Delaunay P, Roiz D (2018) Effectiveness of a field trap barrier system for controlling Aedes albopictus: a “removal trapping” strategy. Parasites Vectors 11:1–7
Allan S, Bernier UR, Kline D (2006) Attraction of mosquitoes to volatiles associated with blood. J Vector Ecol 31:71–79
Anderson JF, McKnight S, Ferrandino FJ (2012) Aedes japonicus japonicus and associated woodland species attracted to Centers for Disease Control and Prevention miniature light traps baited with carbon dioxide and the Traptech® mosquito lure. J Am Mosq Control Ass 28:184–191
Andreasen M, Birtles A, Curtis C, Wood R (2004) Enhanced blood feeding of Anopheles mosquitoes (Diptera: Culicidae) through membranes with applied host odour. Bull Entomol Res 94:291–295
Andrianjafy TM, Ramanandraibe VV, Andrianarijaona ET, Ramarosandratana NH, Ravaomanarivo LH, Mavingui P, Lemaire M (2020) Field assessment of 4-hydroxycoumarin as an attractant for anthropophilic Anopheles spp. vectors of malaria in Madagascar. Sci Rep 10:3048
Andrianjafy TM, Ravaomanarivo LH, Ramanandraibe VV, Rakotondramanga MF, Mavingui P, Lemaire M (2017) New bioassay to evaluate repellency and attractively of chemical products against adults mosquitoes Aedes albopictus and Culex quinquefasciatus. Ann Comm Med Practice 3:1020–1031
Andrianjafy TM, Ravaomanarivo LH, Ramanandraibe VV, Rakotondramanga MF, Mavingui P, Lemaire M (2018) Synthesis, bioassays and field evaluation of hydroxycoumarins and their alkyl derivatives as repellents or kairomones for Aedes albopictus Skuse (Diptera: Culicidae). J Chem Ecol 44:299–311
Ansell J, Hamilton K, Pinder M, Walraven G, Lindsay S (2002) Short-range attractiveness of pregnant women to Anopheles gambiae mosquitoes. Trans R Soc Trop Med Hyg 96:113–116
Ara K, Hama M, Akiba S et al (2006) Foot odor due to microbial metabolism and its control. Can J Microbiol 52:357–364
Asmare Y, Hill SR, Hopkins RJ, Tekie H, Ignell R (2017) The role of grass volatiles on oviposition site selection by Anopheles arabiensis and Anopheles coluzzii. Malar J 16:1–9
Baak-Baak CM, Rodríguez-Ramírez AD, García-Rejón JE, Ríos-Delgado S, Torres-Estrada JL (2013) Development and laboratory evaluation of chemically-based baited ovitrap for the monitoring of Aedes aegypti. J Vector Ecol 38:175–181
Bakker JW, Loy DE, Takken W, Hahn BH, Verhulst NO (2020) Attraction of mosquitoes to primate odours and implications for zoonotic Plasmodium transmission. Med Vet Entomol 34:17–26
Balestrino F, Schaffner F, Forgia D, Paslaru A, Torgerson PR, Mathis A, Veronesi E (2016) Field evaluation of baited traps for surveillance of Aedes japonicus japonicus in Switzerland. Med Vet Entomol 30:64–72
Bar-Zeev M, Maibach H, Khan A (1977) Studies on the attraction of Aedes aegypti (Diptera: Culicidae) to man. J Med Entomol 14:113–120
Barredo E, DeGennaro M (2020) Not just from blood: mosquito nutrient acquisition from nectar sources. Trends Parasitol 36:473–484
Barrera R, Amador M, Acevedo V, Caban B, Felix G, Mackay AJ (2014) Use of the CDC autocidal gravid ovitrap to control and prevent outbreaks of Aedes aegypti (Diptera: Culicidae). J Med Entomol 51:145–154
Batista EP, Costa EF, Silva AA (2014) Anopheles darlingi (Diptera: Culicidae) displays increased attractiveness to infected individuals with Plasmodium vivax gametocytes. Parasites Vectors 7:1–4
Batista EP, Ngowo H, Opiyo M et al (2018) Field evaluation of the BG-Malaria trap for monitoring malaria vectors in rural Tanzanian villages. PloS one 13:e0205358
Batista EP, Ngowo HS, Opiyo M, Shubis GK, Meza FC, Okumu FO, Eiras AE (2017) Semi-field assessment of the BG-Malaria trap for monitoring the African malaria vector, Anopheles arabiensis. PLoS One 12:e0186696
Bazin M, Williams CR (2018) Mosquito traps for urban surveillance: collection efficacy and potential for use by citizen scientists. J Vector Ecol 43:98–103
Beavers G, Hanafi H, Tetreault G (1998) Response of mosquitoes (Diptera: Culicidae) to carbon dioxide and octenol in Egypt. J Egyptian Soc Parasitol 28:303–312
Becker N, Zgomba M, Petric D, Ludwig M (1995) Comparison of carbon dioxide, octenol and a host-odour as mosquito attractants in the Upper Rhine Valley, Germany. Med Vet Entomol 9:377–380
Beehler J, Millar J, Mulla M (1993) Synergism between chemical attractants and visual cues influencing oviposition of the mosquito, Culex quinquefasciatus (Diptera: Culicidae). J Chem Ecol 19:635–644
Beehler J, Millar J, Mulla M (1994) Field evaluation of synthetic compounds mediating oviposition in Culex mosquitoes (Diptera: Culicidae). J Chem Ecol 20:281–291
Bentley MD, Day JF (1989) Chemical ecology and behavioral aspects of mosquito oviposition. Annu Rev Entomol 34:401–421
Bentley MD, McDaniel IN, Yatagai M, Lee H-P, Maynard R (1979) p-Cresol: an oviposition attractant of Aedes triseriatus. Environ Entomol 8:206–209
Bernier U, Kline D, Schreck C, Yost R, Barnard D (2002) Chemical analysis of human skin emanations: comparison of volatiles from humans that differ in attraction of Aedes aegypti (Diptera: Culicidae). J Am Mosq Control Assoc 18:186–195
Bernier UR, Kline DL, Allan SA, Barnard DR (2007) Laboratory comparison of Aedes aegypti attraction to human odors and to synthetic human odor compounds and blends. J Am Mosq Control Assoc 23:288–293
Bernier UR, Kline DL, Barnard DR, Posey KH, Booth MM, Yost RA (2001) Chemical composition that attract arthropods. US Patent No 6, 267, 953, Washington, DC
Bernier UR, Kline DL, Barnard DR, Schreck CE, Yost RA (2000) Analysis of human skin emanations by gas chromatography/mass spectrometry. 2. Identification of volatile compounds that are candidate attractants for the yellow fever mosquito (Aedes aegypti). Anal Chem 72:747–756
Bernier UR, Kline DL, Posey KH, Booth MM, Yost RA, Barnard DR (2003) Synergistic attraction of Aedes aegypti (L.) to binary blends of L-lactic acid and acetone, dichloromethane, or dimethyl disulfide. J Med Entomol 40:653–656
Best A, Lieberman DE, Kamilar JM (2019) Diversity and evolution of human eccrine sweat gland density. J Therm Biol 84:331–338
Bhalala HV, Smith JD, O'Dea BA, Arias JR (2010) The efficacy of the BG-Sentinel™ CO2 nozzle in collecting host-seeking mosquitoes in Fairfax County, Virginia. J Am Mosq Control Assoc 26:226–228
Blackwell A, Hansson B, Wadhams L, Pickett J (1993) A behavioural and electrophysiological study of ovi position cues for Culex quinquefasciatus. Physiol Entomol 18:343–348
Bohbot JD, Dickens JC (2009) Characterization of an enantioselective odorant receptor in the yellow fever mosquito Aedes aegypti. PLoS One 4:e7032
Bonizzoni M, Gasperi G, Chen X, James AA (2013) The invasive mosquito species Aedes albopictus: current knowledge and future perspectives. Trends Parasitol 29:460–468
Bosch OJ, Geier M, Boeckh J (2000) Contribution of fatty acids to olfactory host finding of female Aedes aegypti. Chem Senses 25:323–330
Bowen M (1992) Patterns of sugar feeding in diapausing and nondiapausing Culex pipiens (Diptera: Culicidae) females. J Med Entomol 29:843–849
Braks M, Anderson R, Knols B (1999) Infochemicals in mosquito host selection: human skin microflora and Plasmodium parasites. Parasitol Today 15:409–413
Braks M, Meijerink J, Takken W (2001) The response of the malaria mosquito, Anopheles gambiae, to two components of human sweat, ammonia and l-lactic acid, in an olfactometer. Physiol Entomol 26:142–148
Braks MA, Scholte EJ, Takken W, Dekker T (2000) Microbial growth enhances the attractiveness of human sweat for the malaria mosquito, Anopheles gambiae sensu stricto (Diptera: Culicidae). Chemoecology 10:129–134
Braks MA, Takken W (1999) Incubated human sweat but not fresh sweat attracts the malaria mosquito Anopheles gambiae sensu stricto. J Chem Ecol 25:663–672
Brouwer R (1960) Variations in human body odour as a cause of individual differences of attraction for malaria mosquitoes. Trop Geogr Med 12:186–192
Brown A (1951) Factors in the attractiveness of bodies for mosquitoes. Nature 167:202–202
Brown A, Carmichael A (1961) Lysine and alanine as mosquito attractants. J Econom Entomol 54:317–324
Brown A, Sarkaria D, Thompson R (1951) Studies on the responses of the female Aedes mosquito. Part I.—The search for attractant vapours. Bull Entomol Res 42:105–114
Brown AW (1966) The attraction of mosquitoes to hosts. JAMA 196:249–252
Burgess L, Brown A (1957) Studies on the Responses of the female Aedes Mosquito: Part VIII.—The attractiveness of beef blood to Aedes aegypti (L.). Bull Entomol Res 48:783–793
Burkett D, Lee WJ, Lee KW et al (2001) Light, carbon dioxide, and octenol-baited mosquito trap and host-seeking activity evaluations for mosquitoes in a malarious area of the Republic of Korea. J Am Mosq Control Assoc 17:196–205
Burkot T (1988) Non-random host selection by anopheline mosquitoes. Parasitol Today 4:156–162
Busula AO, Takken W, Loy DE, Hahn BH, Mukabana WR, Verhulst NO (2015) Mosquito host preferences affect their response to synthetic and natural odour blends. Malar J 14:133–142
Buttery RG, Kamm JA (1980) Volatile components of alfalfa: possible insect host plant attractants. J Agr Food Chem 28:978–981
Byrd AL, Belkaid Y, Segre JA (2018) The human skin microbiome. Nature Rev Microbiol 16:143
Canyon D, Hii J (1997) Efficacy of carbon dioxide, 1-octen-3-ol, and lactic acid in modified Fay-Prince traps as compared to man-landing catch of Aedes aegypti. J Am Mosq Control Assoc 13:66–70
Carlson DA, Smith N, Gouck HK, Godwin DR (1973) Yellowfever mosquitoes: compounds related to lactic acid that attract females. J Econom Entomol 66:329–331
Carnevale P, Frézil J, Bosseno M, Le Pont F, Lancien J, Organization WH (1976) Etude de l'agressivité d'Anopheles gambiae A en fonction de l'âge et du sexe des sujets humains. Bull World Health Organization 56:147
Caroprese A, Gabbanini S, Beltramini C, Lucchi E, Valgimigli L (2009) HS-SPME-GC-MS analysis of body odor to test the efficacy of foot deodorant formulations. Skin Res Technol 15:503–510
Chaiphongpachara T, Chitsawaeng C, Chansukh KK (2019) Comparison of the larvicidal and adult mosquito attractant efficacy between straw mushroom Volvariella volvacea extract and octenol (1-octen-3-ol) on mosquito vectors (Diptera: Culicidae). J Appl Pharm Sci 9:95–99
Chaiphongpachara T, Padidpoo O, Chansukh K, Sumruayphol S (2018) Efficacies of five edible mushroom extracts as odor baits for resting boxes to attract mosquito vectors: a field study in Samut Songkhram province, Thailand. Trop Biomed 35:653–663
Charpentier MJ, Barthes N, Proffit M, Bessière JM, Grison C (2012) Critical thinking in the chemical ecology of mammalian communication: roadmap for future studies. Funct Ecol 26:769–774
Chen W, Metsälä M, Vaittinen O, Halonen L (2014) The origin of mouth-exhaled ammonia. J Breath Res 8:036003
Chen Z, Kearney CM (2015) Nectar protein content and attractiveness to Aedes aegypti and Culex pipiens in plants with nectar/insect associations. Acta Trop 146:81–88
Choo YM, Xu P, Hwang JK et al (2018) Reverse chemical ecology approach for the identification of an oviposition attractant for Culex quinquefasciatus. Proc Natl Acad Sci USA 115:714–719
Cilek J, Ikediobi CO, Hallmon CF et al (2012) Evaluation of several novel alkynols, alkenols, and selected host odor blends as attractants to female Aedes albopictus and Culex quinquefasciatus. J Am Mosq Control Assoc 28:199–205
Clyde D, Shute G (1958) Selective feeding habits of anophelines amongst Africans of different ages. Am J Trop Med Hygiene 7:543–545
Codeço CT, Lima AWS, Araújo SC et al (2015) Surveillance of Aedes aegypti: comparison of house index with four alternative traps. PLoS Negl Trop Dis 9:e0003475
Cook J, Majeed S, Ignell R, Pickett J, Birkett M, Logan J (2011a) Enantiomeric selectivity in behavioural and electrophysiological responses of Aedes aegypti and Culex quinquefasciatus mosquitoes. Bull Entomol Res 101:541–550
Cook JI, Majeed S, Ignell R, Pickett JA, Birkett MA, Logan JG (2011b) Enantiomeric selectivity in behavioural and electrophysiological responses of Aedes aegypti and Culex quinquefasciatus mosquitoes. Bull Entomol Res 101:541–550
Cooper R, Frances S, Popat S, Waterson D (2004) The effectiveness of light, 1-octen-3-ol, and carbon dioxide as attractants for anopheline mosquitoes in Madang Province. Papua New Guinea. J Am Mosq Control Assoc 20:239–242
Cooperband M, Cardé R (2006) Orientation of Culex mosquitoes to carbon dioxide-baited traps: flight manoeuvres and trapping efficiency. Med Vet Entomol 20:11–26
Cooperband MF, McElfresh JS, Millar JG, Carde RT (2008) Attraction of female Culex quinquefasciatus Say (Diptera: Culicidae) to odors from chicken feces. J Insect Physiol 54:1184–1192
Cork A, Park K (1996) Identification of electrophysiologically-active compounds for the malaria mosquito, Anopheles gambiae, in human sweat extracts. Med Vet Entomol 10:269–276
Cornet S, Nicot A, Rivero A, Gandon S (2013) Malaria infection increases bird attractiveness to uninfected mosquitoes. Ecol Lett 16:323–329
Costantini C, Birkett MA, Gibson G et al (2001) Electroantennogram and behavioural responses of the malaria vector Anopheles gambiae to human-specific sweat components. Med Vet Entomol 15:259–266
Costantini C, Gibson G, Sagnon NF, Torre AD, Brady J, Coluzzi M (1996) Mosquito responses to carbon dioxide in a West African Sudan savanna village. Med Vet Entomol 10:220–227
Costantini C, Sagnon NF, della Torre A, Diallo M, Brady J, Gibson G, Coluzzi M (1998) Odor-mediated host preferences of West African mosquitoes, with particular reference to malaria vectors. Am J Trop Med Hygiene 58:56–63
Crepeau TN, Healy SP, Bartlett-Healy K, Unlu I, Farajollahi A, Fonseca DM (2013) Effects of Biogents Sentinel trap field placement on capture rates of adult Asian tiger mosquitoes. Aedes albopictus. PloS one 8:e60524
Cribellier A, van Erp JA, Hiscox A, Lankheet MJ, van Leeuwen JL, Spitzen J, Muijres FT (2018) Flight behaviour of malaria mosquitoes around odour-baited traps: capture and escape dynamics. Royal Soc Open Sci 5:180246
Crumb S (1922) A Mosquito Attractant. Science (Washington) 4:1426
Curtis C (1986) Fact and fiction in mosquito attraction and repulsion. Parasitol Today 2:316–318
Czarnowski D, Gorski J, Jóźwiuk J, Boroń-Kaczmarska A (1992) Plasma ammonia is the principal source of ammonia in sweat European. J Appl Physiol Occup Physiol 65:135–137
da Cruz Ferreira DA, Degener CM, de Almeida Marques-Toledo C, Bendati MM, Fetzer LO, Teixeira CP, Eiras ÁE (2017) Meteorological variables and mosquito monitoring are good predictors for infestation trends of Aedes aegypti, the vector of dengue, chikungunya and Zika. Parasites Vectors 10:78
da Silva Paixão K, de Castro Pereira I, Lopes Alves Bottini L, Eduardo Eiras Á (2015) Volatile semiochemical-conditioned attraction of the male yellow fever mosquito, Aedes aegypti, to human hosts. J Vector Ecol 40:1–6
Davis EE, Sokolove PG (1976) Lactic acid-sensitive receptors on the antennae of the mosquito, Aedes aegypti. J Comp Physiol A 105:43–54
Davis TS, Crippen TL, Hofstetter RW, Tomberlin JK (2013) Microbial volatile emissions as insect semiochemicals. J Chem Ecol 39:840–859
Daykin P, Kellogg F, Wright R (1965) Host-finding and repulsion of Aedes aegypti. Can Entomol 97:239–263
De Boer JG, Robinson A, Powers SJ et al (2017) Odours of Plasmodium falciparum-infected participants influence mosquito-host interactions. Sci Rep 7:1–9
De Jong R, Knols B (1995) Selection of biting sites on man by two malaria mosquito species. Experientia 51:80–84
De Long DM, Davidson RH, Peffly RL et al (1949) Insect behaviour : mosquito attraction and repellency. Final Summary Report, Project. Office of the Quartermaster-General, Washington, p 272
de Melo DPO, Scherrer LR, Eiras AE (2012) Dengue fever occurrence and vector detection by larval survey, ovitrap and MosquiTRAP: a space-time clusters analysis. PloS one 7:e42125
De Moraes CM, Stanczyk NM, Betz HS, Pulido H, Sim DG, Read AF, Mescher MC (2014) Malaria-induced changes in host odors enhance mosquito attraction. Proc Natl Acad Sci 111:11079–11084
De Moraes CM, Wanjiku C, Stanczyk NM et al (2018) Volatile biomarkers of symptomatic and asymptomatic malaria infection in humans. Proc Natl Acad Sci 115:5780–5785
Debboun M, Strickman D (2013) Insect repellents and associated personal protection for a reduction in human disease. Med Vet Entomol 27:1–9
Degener C, Eiras AE, Azara TMF et al (2014) Evaluation of the effectiveness of mass trapping with BG-sentinel traps for dengue vector control: a cluster randomized controlled trial in Manaus, Brazil. J Med Entomol 51:408–420
Degener CM, Geier M, Kline D et al (2019) Field trials to evaluate the effectiveness of the Biogents®-Sweetscent lure in combination with several commercial mosquito traps and to assess the effectiveness of the Biogents-Mosquitaire trap with and without carbon dioxide. J Am Mosq Control Assoc 35:32–39
DeGennaro M, McBride CS, Seeholzer L et al (2013) orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature 498:487–491
Dekker T, Carde RT (2011) Moment-to-moment flight manoeuvres of the female yellow fever mosquito (Aedes aegypti L.) in response to plumes of carbon dioxide and human skin odour. J Exp Biol 214:3480–3494
Dekker T, Geier M, Carde RT (2005) Carbon dioxide instantly sensitizes female yellow fever mosquitoes to human skin odours. J Exp Biol 208:2963–2972
Dekker T, Steib B, Cardé R, Geier M (2002) L-lactic acid: a human-signifying host cue for the anthropophilic mosquito Anopheles gambiae. Med Vet Entomol 16:91–98
Dekker T, Takken W (1998) Differential responses of mosquito sibling species Anopheles arabiensis and An. quadriannulatus to carbon dioxide, a man or a calf. Med Vet Entomol 12:136–140
Dekker T, Takken W, Knols BG, Bouman E, van de Laak S, de Bever A, Huisman PW (1998) Selection of biting sites on a human host by Anopheles gambiae ss, An. arabiensis and An. quadriannulatus. Entomol Exp Appl 87:295-300
Derbyshire PJ, Barr H, Davis F, Higson SP (2012) Lactate in human sweat: a critical review of research to the present day. J Physiol Sci 62:429–440
Dethier V, Browne BL, Smith CN (1960) The designation of chemicals in terms of the responses they elicit from insects. J Econom Entomol 53:134–136
Diaz-Santiz E, Rojas JC, Casas-Martinez M, Cruz-Lopez L, Malo EA (2020) Rat volatiles as an attractant source for the Asian tiger mosquito, Aedes albopictus. Sci Rep 10:5170
Dickschat JS (2017) Fungal volatiles–a survey from edible mushrooms to moulds. Nat Prod Rep 34:310–328
Diez-Fernandez A, Martinez-de la Puente J, Gangoso L, Lopez P, Soriguer R, Martin J, Figuerola J (2020) Mosquitoes are attracted by the odour of Plasmodium-infected birds. Int J Parasitol
Ding YM, Hu Y, Yu BT, Mo XC, Mo JC (2016) Laboratory evaluation of differential attraction of Culex pipiens pallens to fruit-based sugar baits. Acta Trop 163:20–25
Dormont L, Bessiere JM, Cohuet A (2013) Human skin volatiles: a review. J Chem Ecol 39:569–578
Duchemin JB, Tsy JMLP, Rabarison P, Roux J, Coluzzi M, Costantini C (2001) Zoophily of Anopheles arabiensis and An. gambiae in Madagascar demonstrated by odour-baited entry traps. Med Vet Entomol 15:50–57
Duffield GE, Acri DJ, George GF, Sheppard AD, Beebe NW, Ritchie SA, Burkot TR (2019) Diel flight activity of wild-caught Anopheles farauti (ss) and An. hinesorum malaria mosquitoes from northern Queensland, Australia. Parasites Vectors 12(48)
Duvall LB, Ramos-Espiritu L, Barsoum KE, Glickman JF, Vosshall LB (2019) Small-molecule agonists of Aedes aegypti neuropeptide Y receptor block mosquito biting. Cell 176:687–701
Dye-Braumuller K, Fredregill C, Debboun M (2020) Mosquito control. In: Mosquitoes, Communities, and Public Health in Texas. pp 249-278.
Edman JD (1979) Orientation of some Florida mosquitoes (Diptera: Culicidae) toward small vertebrates and carbon dioxide in the field. J Med Entomol 15:292–296
Eiras Á, Rose A, Geier M (2004) New tools for monitoring gravid females of the mosquitoes Aedes aegypti and Aedes albopictus (Diptera: Culicidae), vectors of dengue and other arboviral diseases International. J Med Entomol 293(51)
Eiras A, Santanna A (2001) Atraentes de Oviposição de Mosquitos. Patente; Privilégio e Inovação. n. PI0106701-0 Atraentes de Oviposição de Mosquitos. 20 de dez de:2001
Eiras AE, Jepson P (1994) Responses of female Aedes aegypti (Diptera: Culicidae) to host odours and convection currents using an olfactometer bioassay. Bull Entomol Res 84:207–211
Eiras AE, Jepson PC (1991) Host location by Aedes aegypti (Diptera: Culicidae): a wind tunnel study of chemical cues. Bull Entomol Res 81:151–160
Eiras ÁE, Resende MC (2009) Preliminary evaluation of the" Dengue-MI" technology for Aedes aegypti monitoring and control. Cadernos de Saúde Pública 25:S45–S58
El-Sisi AG, Mahmoud HI, Abdel-Hamid YM, Moselh WA, Taha RH (2019) Laboratory evaluation of some local components as attractants to the mosquito, Culex pipiens females. Egyptian Acad J Biol Sci E Med Entomol Parasitol 11:75–85
Emami SN, Lindberg BG, Hua S et al (2017) A key malaria metabolite modulates vector blood seeking, feeding, and susceptibility to infection. Science 355:1076–1080
Englbrecht C, Gordon S, Venturelli C, Rose A, Geier M (2015) Evaluation of BG-Sentinel trap as a management tool to reduce Aedes albopictus nuisance in an urban environment in Italy. J Am Mosq Control Assoc 31:16–25
Erb M, Veyrat N, Robert CA, Xu H, Frey M, Ton J, Turlings TC (2015) Indole is an essential herbivore-induced volatile priming signal in maize. Nature Comm 6:1–10
Essen PV, Kemme J, Ritchie S, Kay B (1994) Differential responses of Aedes and Culex mosquitoes to octenol or light in combination with carbon dioxide in Queensland, Australia. Med Vet Entomol 8:63–67
Evans MV, Hintz CW, Jones L, Shiau J, Solano N, Drake JM, Murdock CC (2019) Microclimate and larval habitat density predict adult Aedes albopictus abundance in urban areas. Am J Trop Med Hygiene 101:362–370
Farajollahi A, Kesavaraju B, Price DC, Williams GM, Healy SP, Gaugler R, Nelder MP (2009) Field efficacy of BG-Sentinel and industry-standard traps for Aedes albopictus (Diptera: Culicidae) and West Nile virus surveillance. J Med Entomol 46:919–925
Fávaro EA, Dibo MR, Mondini A et al (2006) Physiological state of Aedes (Stegomyia) aegypti mosquitoes captured with MosquiTRAPs™ in Mirassol, São Paulo, Brazil. J Vector Ecol 31:285–291
Fernandez-Grandon GM, Gezan SA, Armour JA, Pickett JA, Logan JG (2015) Heritability of attractiveness to mosquitoes. PLoS One 10:e0122716
Fierer N, Hamady M, Lauber CL, Knight R (2008) The influence of sex, handedness, and washing on the diversity of hand surface bacteria. Proc Natl Acad Sci 105:17994–17999
Foster WA (1995) Mosquito sugar feeding and reproductive energetics. Annu Rev Entomol 40:443–474
Foster W, Takken W (2004a) Nectar-related vs. human-related volatiles: behavioural response and choice by female and male Anopheles gambiae (Diptera: Culicidae) between emergence and first feeding. Bull Entomol Res 94:145–157
Foster WA, Takken W (2004b) Nectar-related vs. human-related volatiles: behavioural response and choice by female and male Anopheles gambiae (Diptera: Culicidae) between emergence and first feeding. Bull Entomol Res 94:145–157
Frei J, Krober T, Troccaz M, Starkenmann C, Guerin PM (2017) Behavioral response of the malaria mosquito, Anopheles gambiae, to human sweat inoculated with axilla bacteria and to volatiles composing human axillary odor. Chem Senses 42:121–131
Gallagher M, Wysocki CJ, Leyden JJ, Spielman A, Sun X, Preti G (2008) Analyses of volatile organic compounds from human skin. British J Dermatol 159:780–791
Ganesan K, Mendki MJ, Suryanarayana MV, Prakash S, Malhotra RC (2006) Studies of Aedes aegypti (Diptera: Culicidae) ovipositional responses to newly identified semiochemicals from conspecific eggs. Austral J Entomol 45:75–80
Geier M, Boeckh J (1999) A new Y-tube olfactometer for mosquitoes to measure the attractiveness of host odours. Entomol Exp Appl 92:9–19
Geier M, Bosch OJ, Boeckh J (1999a) Ammonia as an attractive component of host odour for the yellow fever mosquito, Aedes aegypti. Chem Senses 24:647–653
Geier M, Bosch OJ, Boeckh J (1999b) Influence of odour plume structure on upwind flight of mosquitoes towards hosts. J Exp Biol 202:1639–1648
Geier M, Bosh O, Steib B, Rose A, Boeckh J (2002) Odour-guides host finding mosquitoes: identification of new attractants on human skin. In: Proc Int Conf Urban pests, 2002. Citeseer, pp 37-46
Geier M, Rose A, Eiras A (2004a) Insektenfalle. Worldwide patent no. WO 04/054358 A2
Geier M, Rose A, Eiras Á (2004b) A new lure for host-seeking anthropophilic mosquitoes and a novel type of a simple, non-CO2 mosquito trap. Int J Med Microbiol 293:50
Geier M, Sass H, Boeckh J (1996) A search for components in human body odour that attract females of Aedes aegypti. In: Olfaction in mosquitoes-host interactions. Ciba Fundation Symposium. pp 132-148
Ghaninia M, Majeed S, Dekker T, Hill SR, Ignell R (2019) Hold your breath - Differential behavioral and sensory acuity of mosquitoes to acetone and carbon dioxide. PLoS One 14:e0226815
Gillies M (1980) The role of carbon dioxide in host-finding by mosquitoes (Diptera: Culicidae): a review. Bull Entomol Res 70:525–532
Gillies M, Wilkes T (1969) A comparison of the range of attraction of animal baits and of carbon dioxide for some West African mosquitoes. Bull Entomol Res 59:441–456
Gillies M, Wilkes T (1970) The range of attraction of single baits for some West African mosquitoes. Bull Entomol Res 60:225–235
Gjullin C (1961) Oviposition responses of Culex pipiens quinquefasciatus Say to waters treated with various chemicals. Mosq News 21:2
Gjullin C, Johnsen J, Plapp J (1965) The effect of odors released by various waters on the oviposition sites selected by two species of Culex. Mosq News 25:3
Gladden L (2004) Lactate metabolism: a new paradigm for the third millennium. J Physiol 558:5–30
Gonzalez PV, González Audino PA, Masuh HM (2014) Electrophysiological and behavioural response of Aedes albopictus to n-heinecosane, an ovipositional pheromone of Aedes aegypti. Entomol Exp Appl 151:191–197
Gouagna LC, Poueme RS, Dabiré KR, Ouédraogo JB, Fontenille D, Simard F (2010) Patterns of sugar feeding and host plant preferences in adult males of An. gambiae (Diptera: Culicidae). J Vector Ecol 35:267–276
Govella NJ, Maliti DF, Mlwale AT et al (2016) An improved mosquito electrocuting trap that safely reproduces epidemiologically relevant metrics of mosquito human-feeding behaviours as determined by human landing catch. Malar J 15:465
Grice EA, Kong HH, Conlan S et al (2009) Topographical and temporal diversity of the human skin microbiome. Science 324:1190–1192
Grison C, Carrasco D, Pelissier F, Moderc A (2020) Reflexion on bio-sourced mosquito repellents: nature, activity and preparation, Front Ecol Evol 8
Guha L, Seenivasagan T, Iqbal ST, Agrawal OP, Parashar BD (2014) Behavioral and electrophysiological responses of Aedes albopictus to certain acids and alcohols present in human skin emanations. Parasitol Res 113:3781–3787
Haddow A (1942) The mosquito fauna and climate of native huts at Kisumu, Kenya. Bull Entomol Res 33:91–142
Hall D, Beevor P, Cork A, Nesbitt BF, Vale G (1984) 1-Octen-3-ol. A potent olfactory stimulant and attractant for tsetse isolated from cattle odours. Int J Trop Insect Sci 5:335–339
Hao H, Sun J, Dai J (2012) Preliminary analysis of several attractants and spatial repellents for the mosquito, Aedes albopictus using an olfactometer. J Insect Sci 12:76
Hao H, Sun J, Dai J (2013) Dose-dependent behavioral response of the mosquito Aedes albopictus to floral odorous compounds. J Insect Sci 13:127
Hapairai LK, Joseph H, Sang MAC et al (2013) Field evaluation of selected traps and lures for monitoring the filarial and arbovirus vector, Aedes polynesiensis (Diptera: Culicidae), in French Polynesia. J Med Entomol 50:731–739
Hawaria D, Santiago D, Yewhalaw D (2016) Efficient attractants and simple odor-baited sticky trap for surveillance of Anopheles arabiensis Patton mosquito in Ethiopia. J Inf Develop Countries 10:82–89
Hawkes F, Young S, Gibson G (2012) Modification of spontaneous activity patterns in the malaria vector Anopheles gambiae sensu stricto when presented with host-associated stimuli. Physiol Entomol 37:233–240
Hawkes FM, Dabire RK, Sawadogo SP, Torr SJ, Gibson G (2017) Exploiting Anopheles responses to thermal, odour and visual stimuli to improve surveillance and control of malaria. Sci Rep 7:17283
Headlee TJ (1934) Mosquito work in New Jersey for the year 1933. Proc New Jers Mosq Exterm Assoc 21:8–37
Healy T, Copland M (1995) Activation of Anopheles gambiae mosquitoes by carbon dioxide and human breath. Med Vet Entomol 9:331–336
Healy T, Copland M (2000) Human sweat and 2-oxopentanoic acid elicit a landing response from Anopheles gambiae. Med Vet Entomol 14:195–200
Healy T, Copland M, Cork A, Przyborowska A, Halket J (2002) Landing responses of Anopheles gambiae elicited by oxocarboxylic acids. Med Vet Entomol 16:126–132
Healy T, Jepson P (1988) The location of floral nectar sources by mosquitoes: the long-range responses of Anopheles arabiensis Patton (Diptera: Culicidae) to Achillea millefolium flowers and isolated floral odour. Bull Entomol Res 78:651–657
Himeidan YE, Elbashir MI, Adam I (2004) Attractiveness of pregnant women to the malaria vector, Anopheles arabiensis, in Sudan. Ann Trop Med Parasitol 98:631–633
Hiscox A, Otieno B, Kibet A et al (2014) Development and optimization of the Suna trap as a tool for mosquito monitoring and control. Mal J 13:257
Hiwat H, Andriessen R, Md R, Koenraadt CJM, Takken W (2011) Carbon dioxide baited trap catches do not correlate with human landing collections of Anopheles aquasalis in Suriname. Memórias do Instituto Oswaldo Cruz 106:360–364
Hoel D, Kline D, Allan S, Grant A (2007) Evaluation of carbon dioxide, 1-octen-3-ol, and lactic acid as baits in mosquito magnet™ pro traps for Aedes albopictus in north central Florida. J Am Mosq Control Assoc 23:11–18
Hoel DF, Dunford JC, Kline DL et al (2015) A Comparison of carbon dioxide sources for mosquito capture in Centers for Disease Control and Prevention light traps on the Florida Gulf Coast. J Am Mosq Control Assoc 31:248–258
Hoel DF, Marika JA, Dunford JC, Irish SR, Geier M, Obermayr U, Wirtz RA (2014a) Optimizing collection of Anopheles gambiae s.s. (Diptera: Culicidae) in Biogents Sentinel traps. J Med Entomol 51:1268–1275
Hoel DF, Marika JA, Dunford JC, Irish SR, Geier M, Obermayr U, Wirtz RA (2014b) Optimizing collection of Anopheles gambiae ss (Diptera: Culicidae) in biogents sentinel traps. J Med Entomol 51:1268–1275
Homan T, Hiscox A, Mweresa CK et al (2016) The effect of mass mosquito trapping on malaria transmission and disease burden (SolarMal): a stepped-wedge cluster-randomised trial. TLancet 388:1193–1201
Howlett F (1910) The influence of temperature upon the biting of mosquitoes. Parasitology 3:479–484
Huffaker CB (1942) Developments in mosquito control: tests with carbon dioxide and light as attractants for mosquitoes, with species emphasis on the malaria mosquito, Anopheles quadrimaculatus. Mosq News 2:28–31
Huffaker CB, Back RC (1943) A study of methods of sampling mosquito populations. J Econom Entomol 36:561–569
Ibáñez-Justicia A, Smitz N, den Hartog W et al (2020) Detection of exotic mosquito species (Diptera: Culicidae) at international airports in Europe. Int J Environ Res Public Health 17:3450
Irish S, Batengana B, Eiras A, Cameron M (2015) Evaluation of the AtrAedes™ lure for collection of Culex quinquefasciatus in gravid traps. J Am Mosq Control Assoc 31:107–109
Irish SR, Chandre F, N'Guessan R (2008) Comparison of octenol-and BG Lure®-baited Biogents Sentinel traps and an encephalitis virus surveillance trap in Portland. J Am Mosq Control Assoc 24:393–398
Jackson TC, Zhang YV, Sime PJ, Phipps RP, Kottmann RM (2017) Development of an accurate and sensitive method for lactate analysis in exhaled breath condensate by LC MS/MS. J Chrom B 1061:468–473
Jacob JW, Tchouassi DP, Lagat ZO, Mathenge EM, Mweresa CK, Torto B (2018) Independent and interactive effect of plant-and mammalian-based odors on the response of the malaria vector, Anopheles gambiae. Acta trop 185:98–106
Jaleta KT, Hill SR, Birgersson G, Tekie H, Ignell R (2016) Chicken volatiles repel host-seeking malaria mosquitoes. Malaria J 15:1–9
James A, Casey J, Hyliands D, Mycock G (2004) Fatty acid metabolism by cutaneous bacteria and its role in axillary malodour. World J Microbiol Biotechn 20:787–793
James AG, Austin CJ, Cox DS, Taylor D, Calvert R (2013) Microbiological and biochemical origins of human axillary odour. FEMS Microbiol Ecol 83:527–540
Jawara M, Awolola TS, Pinder M, Jeffries D, Smallegange RC, Takken W, Conway DJ (2011) Field testing of different chemical combinations as odour baits for trapping wild mosquitoes in The Gambia. PLoS One 6:e19676
Jawara M, Smallegange RC, Jeffries D et al (2009) Optimizing odor-baited trap methods for collecting mosquitoes during the malaria season in The Gambia. PLoS One 4:e8167
Jerry DCT, Mohammed T, Mohammed A (2017) Yeast-generated CO2 : A convenient source of carbon dioxide for mosquito trapping using the BG-Sentinel® traps. Asian Pac J Trop Biomed 7:896–900
Jhumur US, Dötterl S, Jürgens A (2006) Naive and conditioned responses of Culex pipiens pipiens biotype molestus (Diptera: Culicidae) to flower odors. J Med Entomol 43:1164–1170
Jhumur US, Dötterl S, Jürgens A (2007) Electrophysiological and behavioural responses of mosquitoes to volatiles of Silene otites (Caryophyllaceae). Arthropod-Plant Interact 1:245–254
Johnson BJ, Ritchie SA, Fonseca DM (2017) The state of the art of lethal oviposition trap-based mass interventions for arboviral control. Insects 8:5
Kawada H, Honda S, Takagi M (2007) Comparative laboratory study on the reaction of Aedes aegypti and Aedes albopictus to different attractive cues in a mosquito trap. J Med Entomol 44:427–432
Kearney J, Harnby D, Gowland G, Holland K (1984) The follicular distribution and abundance of resident bacteria on human skin. Microbiol 130:797–801
Kelly M, Su C-Y, Schaber C, Crowley JR, Hsu F-F, Carlson JR, Odom AR (2015) Malaria parasites produce volatile mosquito attractants. MBio 6:2
Kemibala EE, Mafra-Neto A, Dekker T et al (2020) A zooprophylaxis strategy using L-lactic acid (Abate) to divert host-seeking malaria vectors from human host to treated non-host animals. Malar J 19:52
Kemme J, Van Essen P, Ritchie S, Kay B (1993) Response of mosquitoes to carbon dioxide and 1-octen-3-ol in southeast Queensland. Aus J Am Mosq Control Assoc 9:431–435
Khan A, Maibach HI, Strauss WG (1969) Gross variations in the response to man among yellow-fever mosquito populations in the laboratory. J Econom Entomol 62:96–98
Kitau J, Pates H, Rwegoshora TR et al (2010) The effect of Mosquito Magnet® Liberty Plus trap on the human mosquito biting rate under semi-field conditions. J Am Mosq Control Assoc 26:287–294
Kline D, Allan S, Bernier U, Welch C (2007) Evaluation of the enantiomers of 1-octen-3-ol and 1-octyn-3-ol as attractants for mosquitoes associated with a freshwater swamp in Florida. USA. Med Vet Entomol 21:323–331
Kline D, Dame D, Meisch M (1991a) Evaluation of 1-octen-3-ol and carbon dioxide as attractants for mosquitoes associated with irrigated rice fields in Arkansas. J Am Mosq Control Assoc 7:165–169
Kline D, Takken W, Wood J, Carlson D (1990a) Field studies on the potential of butanone, carbon dioxide, honey extract, l-octen-3-ol, L-lactic acid and phenols as attractants for mosquitoes. Med Vet Entomol 4:383–391
Kline D, Wood J, Cornell J (1991b) Interactive effects of l-octen-3-ol and carbon dioxide on mosquito (Diptera: Culicidae) surveillance and control. J Med Entomol 28:254–258
Kline D, Wood J, Morris C (1990b) Evaluation of 1-octen-3-ol as an attractant for Coquillettidia perturbans, Mansonia spp. and Culex spp. associated with phosphate mining operations. J Am Mosq Control Assoc 6:605–611
Kline DL (1999) Comparison of two American biophysics mosquito traps: the professional and a new counterflow geometry trap. J Am Mosq Control Assoc - Mosq News 15:276–282
Kline DL (2002) Evaluation of various models of propane-powered mosquito traps. J Vector Ecol 27:1–7
Kline DL (2006) Traps and trapping techniques for adult mosquito control. J Am Mosq Control Assoc 22:490–496
Kline DL (2007) Semiochemicals, traps/targets and mass trapping technology for mosquito management. J Am Mosq Control Assoc 23:241–251
Kline DL, Bernier UR, Hogsette JA (2012) Efficacy of three attractant blends tested in combination with carbon dioxide against natural populations of mosquitoes and biting flies at the Lower Suwannee Wildlife Refuge. J Am Mosq Control Assoc 28:123–127
Kline DL, Mann MO (1998) Evaluation of butanone, carbon dioxide, and 1-octen-3-ol as attractants for mosquitoes associated with north central Florida bay and cypress swamps. J Am Mosq Control Assoc 14:289–297
Klun JA, Kramer M, Debboun M (2013) Four simple stimuli that induce host-seeking and blood-feeding behaviors in two mosquito species, with a clue to DEET's mode of action. J Vector Ecol 38:143–153
Knols B, De Jong R (1996) Limburger cheese as an attractant for the malaria mosquito Anopheles gambiae ss. Parasitol Today 12:159–161
Knols BG, de Jong R, Takken W (1995) Differential attractiveness of isolated humans to mosquitoes in Tanzania. Trans Roy Soc Trop Med Hygiene 89:604–606
Knols BG, van Loon JJA, Cork A et al (1997) Behavioural and electrophysiological responses of the female malaria mosquito Anopheles gambiae (Diptera: Culicidae) to Limburger cheese volatiles. Bull Entomol Res 87:151–159
Knudsen JT, Eriksson R, Gershenzon J, Ståhl B (2006) Diversity and distribution of floral scent. Bot Rev 72(1)
Kramer WL, Mulla MS (1979) Oviposition attractants and repellents of mosquitoes: oviposition responses of Culex mosquitoes to organic infusions. Environ Entomol 8:1111–1117
Kröckel U, Rose A, Eiras ÁE, Geier M (2006) New tools for surveillance of adult yellow fever mosquitoes: comparison of trap catches with human landing rates in an urban environment. J Am Mosq Control Assoc 22:229–238
Kusakabe Y, Ikeshoji T (1990) Comparative attractancy of physical and chemical stimuli to aedine mosquitoes. Med Entomol Zool 41:219–225
Kweka EJ, Mahande AM (2009) Comparative evaluation of four mosquitoes sampling methods in rice irrigation schemes of lower Moshi northern Tanzania. Malaria J 8:1–5
Kweka EJ, Mwang'onde BJ, Kimaro E, Msangi S, Massenga CP, Mahande AM (2009) A resting box for outdoor sampling of adult Anopheles arabiensis in rice irrigation schemes of lower Moshi, northern Tanzania. Malaria J 8:1–6
Laarman J (1958) The host-seeking behaviour of anopheline mosquitoes. Trop Geog Med 10:293–305
Lacey ES, Carde RT (2011) Activation, orientation and landing of female Culex quinquefasciatus in response to carbon dioxide and odour from human feet: 3-D flight analysis in a wind tunnel. Med Vet Entomol 25:94–103
Lacey ES, Carde RT (2012) Location of and landing on a source of human body odour by female Culex quinquefasciatus in still and moving air. Physiol Entomol 37:153–159
Lacroix R, Mukabana WR, Gouagna LC, Koella JC (2005) Malaria infection increases attractiveness of humans to mosquitoes. PLoS Biol 3:e298
Lahondere C, Vinauger C, Okubo RP, Wolff GH, Chan JK, Akbari OS, Riffell JA (2020) The olfactory basis of orchid pollination by mosquitoes. Proc Natl Acad Sci USA 117:708–716
Lawal O, Ahmed WM, Nijsen TM, Goodacre R, Fowler SJ (2017) Exhaled breath analysis: a review of ‘breath-taking’methods for off-line analysis. Metabolomics 13:110
Le Goff G, Damiens D, Ruttee AH et al (2017) Comparison of efficiency of BG-Sentinel traps baited with mice, mouse-litter, and CO2 lures for field sampling of male and female Aedes albopictus mosquitoes. Insects 8
Leal HM, Hwang JK, Tan K, Leal WS (2017) Attraction of Culex mosquitoes to aldehydes from human emanations. Sci Rep 7:1–10
Lefevre T, Gouagna LC, Dabire KR, Elguero E, Fontenille D, Costantini C, Thomas F (2009) Evolutionary lability of odour-mediated host preference by the malaria vector Anopheles gambiae. Trop Med Int Health 14:228–236
Lega J, Brown H, Barrera R (2020) A 70% reduction in mosquito populations does not require removal of 70% of mosquitoes. J Med Entomol 57:1668–1670
Li CX, Dong YD, Zhang XL et al (2010) Evaluation of octenol and Lurex as baits in Mosquito Magnet Pro traps to collect vector mosquitoes in China. J Am Mosq Control Assoc 26:449–451
Lima JBP, Galardo AKR, Bastos LS, Lima AWS, Rosa-Freitas MG (2017) MosqTent: An individual portable protective double-chamber mosquito trap for anthropophilic mosquitoes. PLoS Negl Trop Dis 11:e0005245
Lindh JM, Okal MN, Herrera-Varela M, Borg-Karlson AK, Torto B, Lindsay SW, Fillinger U (2015) Discovery of an oviposition attractant for gravid malaria vectors of the Anopheles gambiae species complex. Malar J 14:119
Lindsay S, Adiamah J, Miller J, Pleass R, Armstrong J (1993) Variation in attractiveness of human subjects to malaria mosquitoes (Diptera: Culicidae) in The Gambia. J Med Entomol 30:368–373
Lindsay S, Ansell J, Selman C, Cox V, Hamilton K, Walraven G (2000) Effect of pregnancy on exposure to malaria mosquitoes. Lancet 355:1972
Liu H, Dixon D, Bibbs CS, Xue R-D (2019) Autocidal gravid ovitrap incorporation with attractants for control of gravid and host-seeking Aedes aegypti (Diptera: Culicidae). J Med Entomol 56:576–578
Liu N (2015) Insecticide resistance in mosquitoes: impact, mechanisms, and research directions. Annu Rev Entomol 60:537–559
Logan JG, Birkett MA, Clark SJ et al (2008) Identification of human-derived volatile chemicals that interfere with attraction of Aedes aegypti mosquitoes. J Chem Ecol 34:308
Logan JG, Stanczyk NM, Hassanali A et al (2010) Arm-in-cage testing of natural human-derived mosquito repellents. Malar J 9:239
Lorenz LM, Keane A, Moore JD et al (2013) Taxis assays measure directional movement of mosquitoes to olfactory cues. Parasites Vectors 6:131
Lothrop HD, Wheeler SS, Fang Y, Reisen WK (2012) Use of scented sugar bait stations to track mosquito-borne arbovirus transmission in California. J Med Entomol 49:1466–1472
Maciel-de-Freitas R, Eiras ÁE, Lourenço-de-Oliveira R (2006) Field evaluation of effectiveness of the BG-Sentinel, a new trap for capturing adult Aedes aegypti (Diptera: Culicidae). Mem Inst Oswaldo Cruz 101:321–325
Maciel-de-Freitas R, Peres RC, Alves F, Brandolini MB (2008) Mosquito traps designed to capture Aedes aegypti (Diptera: Culicidae) females: preliminary comparison of Adultrap, MosquiTRAP and backpack aspirator efficiency in a dengue-endemic area of Brazil. Mem Inst Oswaldo Cruz 103:602–605
Maciel-de-Freitas R, Lourenço-de-Oliveira R (2011) Does targeting key-containers effectively reduce Aedes aegypti population density? Trop Med Int Health 16:965–973
Mackay AJ, Amador M, Barrera R (2013) An improved autocidal gravid ovitrap for the control and surveillance of Aedes aegypti. Parasites Vectors 6:225
Mafra-Neto A, Dekker T (2019) Novel odor-based strategies for integrated management of vectors of disease. Curr Opin Insect Sci 34:105–111
Maibach HI, Skinner W, Strauss WG, Khan A (1966) Factors that attract and repel mosquitoes in human skin. JAMA 196:263–266
Majeed S, Hill SR, Birgersson G, Ignell R (2016) Detection and perception of generic host volatiles by mosquitoes modulate host preference: context dependence of (R)-1-octen-3-ol. R Soc Open Sci 3:160467
Majeed S, Hill SR, Dekker T, Ignell R (2017) Detection and perception of generic host volatiles by mosquitoes: responses to CO2 constrains host-seeking behaviour. R Soc Open Sci 4:170189
Majeed S, Hill SR, Ignell R (2014) Impact of elevated CO2 background levels on the host-seeking behaviour of Aedes aegypti. J Exp Biol 217:598–604
Marek E, Volke J, Hawener I, Platen P, Mückenhoff K, Marek W (2010) Measurements of lactate in exhaled breath condensate at rest and after maximal exercise in young and healthy subjects. J Breath Res 4:017105
Mathew N, Ayyanar E, Shanmugavelu S, Muthuswamy K (2013) Mosquito attractant blends to trap host seeking Aedes aegypti. Parasitol Res 112:1305–1312
Matowo NS, Koekemoer LL, Moore SJ, Mmbando AS, Mapua SA, Coetzee M, Okumu FO (2016) Combining synthetic human odours and low-cost electrocuting grids to attract and kill outdoor-biting mosquitoes: field and semi-field evaluation of an improved mosquito landing box. PloS one 11:e0145653
Mauer DJ, Rowley WA (1999) Attraction of Culex pipiens pipiens (Diptera: Culicidae) to flower volatiles. J Med Entomol 36:503–507
Mayer M, James J (1969) Attraction of Aedes aegypti (L.): responses to human arms, carbon dioxide, and air currents in a new type of olfactometer. Bull Entomol Res 58:629–642
Mboera L, Takken W (1997) Carbon dioxide chemotropism in mosquitoes (Diptera: Culicidae) and its potential in vector surveillance and management programmes. Med Vet Entomol 7:355–368
Mboera L, Takken W (1999) Odour-mediated host preference of Culex quinquefasciatus in Tanzania. Entomol Exp Appl 92:83–88
Mboera L, Takken W, Mdira K, Chuwa G, Pickett J (2000a) Oviposition and behavioral responses of Culex quinquefasciatus to skatole and synthetic oviposition pheromone in Tanzania. J Chem Ecol 26:1193–1203
Mboera L, Takken W, Mdira K, Pickett J (2000b) Sampling gravid Culex quinquefasciatus (Diptera: Culicidae) in Tanzania with traps baited with synthetic oviposition pheromone and grass infusions. J Med Entomol 37:172–176
Mboera L, Takken W, Sambu E (2000c) The response of Culex quinquefasciatus (Diptera: Culicidae) to traps baited with carbon dioxide, 1-octen-3-ol, acetone, butyric acid and human foot odour in Tanzania. Bull Entomol Res 90:155–159
Mburu MM, Mweresa CK, Omusula P, Hiscox A, Takken W, Mukabana WR (2017) 2-Butanone as a carbon dioxide mimic in attractant blends for the Afrotropical malaria mosquitoes Anopheles gambiae and Anopheles funestus. Malar J 16:351
McBride CS, Baier F, Omondi AB et al (2014) Evolution of mosquito preference for humans linked to an odorant receptor. Nature 515:222–227
McCall P, Harding G, Roberts J, Auty B (1996) Attraction and trapping of Aedes aegypti (Diptera: Culicidae) with host odors in the laboratory. J Med Entomol 33:177–179
McIver SB, McElligott PE (1989) Effects of release rates on the range of attraction of carbon dioxide to some southwestern Ontario mosquito species. J Am Mosq Control Assoc 5:6–9
Mclver SB (1968) Host preferences and discrimination by the mosquitoes Aedes aegypti and Culex tarsalis (Diptera: Culicidae). J Med Entomol 5:422–428
McMeniman CJ, Corfas RA, Matthews BJ, Ritchie SA, Vosshall LB (2014) Multimodal integration of carbon dioxide and other sensory cues drives mosquito attraction to humans. Cell 156:1060–1071
McPhatter L, Gerry AC (2017) Effect of CO2 concentration on mosquito collection rate using odor-baited suction traps. J Vector Ecol 42:44–50
Meeraus WH, Armistead JS, Arias JR (2008) Field comparison of novel and gold standard traps for collecting Aedes albopictus in northern Virginia. J Am Mosq Control Assoc 24:244–248
Meijerink J, Braks MAH, Brack AA et al (2000) Identification of olfactory stimulants for Anopheles gambiae from human sweat samples. J Chem Ecol 26:1367–1382
Meijerink J, Braks M, Van Loon J (2001) Olfactory receptors on the antennae of the malaria mosquito Anopheles gambiae are sensitive to ammonia and other sweat-borne components. J Insect Physiol 47:455–464
Meijerink J, van Loon JJ (1999) Sensitivities of antennal olfactory neurons of the malaria mosquito, Anopheles gambiae, to carboxylic acids. J Insect Physiol 45:365–373
Melo N, Wolff GH, Costa-da-Silva AL et al (2020) Geosmin Attracts Aedes aegypti Mosquitoes to Oviposition Sites. Curr Biol 30:127–134
Menger DJ, Omusula P, Holdinga M et al (2015) Field evaluation of a push-pull system to reduce malaria transmission. PLoS One 10:e0123415
Menger DJ, Otieno B, de Rijk M, Mukabana WR, van Loon JJ, Takken W (2014a) A push-pull system to reduce house entry of malaria mosquitoes. Malar J 13:119
Menger DJ, van Loon JJ, Takken W (2014b) Assessing the efficacy of candidate mosquito repellents against the background of an attractive source that mimics a human host. Med Vet Entomol 28:407–413
Mer G, Birnbaum D, Aioub A, Bachi R (1947) The attraction of mosquitoes by human beings. Statistical analysis of data. Parasitol 38:1–9
Meza FC, Roberts JM, Sobhy IS, Okumu FO, Tripet F, Bruce TJA (2020) Behavioural and electrophysiological responses of female Anopheles gambiae mosquitoes to volatiles from a Mango bait. J Chem Ecol 46:387–396
Michael E, Ramaiah KD, Hoti SL et al (2001) Quantifying mosquito biting patterns on humans by DNA fingerprinting of bloodmeals. Am J trop Med Hygiene 65:722–728
Michalet S, Minard G, Chevalier W et al (2019) Identification of human skin bacteria attractive to the Asian Tiger mosquito. Environ Microbiol 21:4662–4674
Millar JG, Chaney JD, Mulla MS (1992) Identification of oviposition attractants for Culex quinquefasciatus from fermented Bermuda grass infusions. J Am Mosq Control Assoc 8:11–17
Montagna W (1985) The anatomy of sweat glands. J Human Evol 14:3–22
Mozūraitis R, Hajkazemian M, Zawada JW et al (2020) Male swarming aggregation pheromones increase female attraction and mating success among multiple African malaria vector mosquito species. Nature Ecol Evol 4:1395–1401
Muirhead-Thomson R (1951a) Distribution of anopheline mosquito bites among different age groups. British Med J 1:1114
Muirhead-Thomson RC (1951b) Mosquito behaviour in relation to malaria transmission and control in the tropics. Arnold, London
Mukabana WR, Mweresa CK, Omusula P, Orindi BO, Smallegange RC, van Loon JJ, Takken W (2012a) Evaluation of low density polyethylene and nylon for delivery of synthetic mosquito attractants. Parasites Vectors 5:202
Mukabana WR, Mweresa CK, Otieno B, Omusula P, Smallegange RC, Van Loon JJ, Takken W (2012b) A novel synthetic odorant blend for trapping of malaria and other African mosquito species. J Chem Ecol 38:235–244
Mukabana WR, Takken W, Killeen GF, Knols BG (2004) Allomonal effect of breath contributes to differential attractiveness of humans to the African malaria vector Anopheles gambiae. Malar J 3(1)
Mullens BA, Gerry AC (1998) Comparison of bait cattle and carbon dioxide-baited suction traps for collecting Culicoides variipennis sonorensis (Diptera: Ceratopogonidae) and Culex quinquefasciatus Diptera: Culicidae. J Med Entomol 35:245–250
Müller GC, Beier JC, Traore SF et al (2010) Field experiments of Anopheles gambiae attraction to local fruits/seedpods and flowering plants in Mali to optimize strategies for malaria vector control in Africa using attractive toxic sugar bait methods. Malar J 9:262
Müller W (1968) Die Distanz-und Kontakt-Orientierung der Stechmücken (Aedes aegypti) (Wirtsfindung, Stechverhalten und Blutmahlzeit). Zeit Ver Physiol 58:241–303
Murphy MW, Dunton RF, Perich MJ, Rowley WA (2001) Attraction of Anopheles (Diptera: culicidae) to volatile chemicals in Western Kenya. J Med Entomol 38:242–244
Mweresa CK, Mukabana W, van Loon J, Dicke M, Takken W (2020) Use of semiochemicals for surveillance and control of hematophagous insects. Chemoecology 30:1–10
Mweresa CK, Mukabana WR, Omusula P, Otieno B, Van Loon JJ, Takken W (2016) Enhancing attraction of African malaria vectors to a synthetic odor blend. J Chem Ecol 42:508–516
Mweresa CK, Omusula P, Otieno B, Van Loon JJ, Takken W, Mukabana WR (2014) Molasses as a source of carbon dioxide for attracting the malaria mosquitoes Anopheles gambiae and Anopheles funestus. Malar J 13:160
Mweresa CK, Otieno B, Omusula P et al (2015) Understanding the long-lasting attraction of malaria mosquitoes to odor baits. PLoS One 10:e0121533
Mwingira V, Mboera LE, Dicke M, Takken W (2020) Exploiting the chemical ecology of mosquito oviposition behavior in mosquito surveillance and control: a review. J Vector Ecol 45:155–179
Newhouse VF, Chamberlain R, Johnston J, Sudia WD (1966) Use of dry ice to increase mosquito catches of the CDC miniature light trap. Mosq News 26:30–35
Nicolaides N, Fu HC, Rice GR (1968) The skin surface lipids of man compared with those of eighteen species of animals. J Inv Dermatol 51:83–89
Nikbakhtzadeh MR, Terbot JW, Otienoburu PE, Foster WA (2014) Olfactory basis of floral preference of the malaria vector Anopheles gambiae (Diptera: Culicidae) among common African plants. J Vector Ecol 39:372–383
Njiru BN, Mukabana WR, Takken W, Knols BG (2006) Trapping of the malaria vector Anopheles gambiae with odour-baited MM-X traps in semi-field conditions in western Kenya. Malar J 5:39
Norris EJ, Coats JR (2017) Current and future Repellent technologies: the potential of spatial repellents and their place in mosquito-borne disease control. Int J Environ Res Public Health 14
Nyasembe VO, Tchouassi DP, Mbogo CM, Sole CL, Pirk C, Torto B (2015) Linalool oxide: generalist plant based lure for mosquito disease vectors. Parasites Vectors 8:1–8
Nyasembe VO, Teal PE, Mukabana WR, Tumlinson JH, Torto B (2012) Behavioural response of the malaria vector Anopheles gambiae to host plant volatiles and synthetic blends. Parasites Vectors 5:234
Nyasembe VO, Torto B (2014) Volatile phytochemicals as mosquito semiochemicals. Phytochem Lett 8:196–201
Obenauer PJ, Abdel-Dayem MS, Stoops CA et al (2013) Field responses of Anopheles gambiae complex (Diptera: Culicidae) in Liberia using yeast-generated carbon dioxide and synthetic lure-baited light traps. J Med Entomol 50:863–870
Okumu F, Biswaro L, Mbeleyela E, Killeen GF, Mukabana R, Moore SJ (2010a) Using nylon strips to dispense mosquito attractants for sampling the malaria vector Anopheles gambiae s. J Med Entomol 47:274–282
Okumu FO, Killeen GF, Ogoma S et al (2010b) Development and field evaluation of a synthetic mosquito lure that is more attractive than humans. PloS one 5:e8951
Olagbemiro TO, Birkett MA, Mordue AJ, Pickett JA (2004) Laboratory and field responses of the mosquito, Culex quinquefasciatus, to plant-derived Culex spp. oviposition pheromone and the oviposition cue skatole. J Chem Ecol 30:965–976
Olanga EA, Okal MN, Mbadi PA, Kokwaro ED, Mukabana WR (2010) Attraction of Anopheles gambiae to odour baits augmented with heat and moisture. Malar J 9(6)
Oli K, Jeffery J, Vythilingam I (2005) Research note: a comparative study of adult mosquito trapping using dry ice and yeast generated carbon dioxide. Trop Biomed 22:249–251
Omer S (1979) Responses of females of Anopheles arabiensis and Culex pipiens fatigans to air currents, carbon dioxide and human hands in a flight-tunnel. Entomol Exp Appl 26:142–151
Omolo M, Njiru B, Ndiege I, Musau R, Hassanali A (2013) Differential attractiveness of human foot odours to Anopheles gambiae Giles sensu stricto (Diptera: Culicidae) and variation in their chemical composition. Acta Trop 128:144–148
Omondi WP, Owino EA, Odongo D, Mwangangi JM, Torto B, Tchouassi DP (2019) Differential response to plant- and human-derived odorants in field surveillance of the dengue vector, Aedes aegypti. Acta Trop 200:105163
Omrani S-M, Vatandoost H, Oshaghi M-A, Rahimi A (2012) Upwind responses of Anopheles stephensi to carbon dioxide and L-lactic acid: an olfactometer study. East Mediter Health J 18:1134–1142
Omrani S-M, Vatandoost H, Oshaghi MA et al (2010) Fabrication of an olfactometer for mosquito behavioural studies. J Vector Borne Dis 47:17–25
Ortega-Morales AI, Méndez-López R, Garza-Hernández JA et al (2019) The mosquitoes (Diptera: Culicidae) of Tabasco, Mexico. J Vector Ecol 44:57–67
Ortiz DG, Borges DA, Trinca LA, Eunice A, Gordon U, Geier M, Pinto MC (2020) Comparison of BG-Lure and BG-Sweetscents attractants for field sampling of phlebotomine sand flies. Acta Trop 202:105224
Otienoburu PE, Ebrahimi B, Phelan PL, Foster WA (2012) Analysis and optimization of a synthetic milkweed floral attractant for mosquitoes. J Chem Ecol 38:873–881
Otienoburu PE, Nikbakhtzadeh MR, Foster WA (2016) Orientation of Anopheles gambiae (Diptera: Culicidae) to plant-host volatiles in a novel diffusion-cage olfactometer. J Med Entomol 53:237–240
Owino EA, Sang R, Sole CL, Pirk C, Mbogo C, Torto B (2014) Field evaluation of natural human odours and the biogent-synthetic lure in trapping Aedes aegypti, vector of dengue and chikungunya viruses in Kenya. Parasites Vectors 7:451
Owino EA, Sang R, Sole CL, Pirk C, Mbogo C, Torto B (2015) An improved odor bait for monitoring populations of Aedes aegypti-vectors of dengue and chikungunya viruses in Kenya. Parasites Vectors 8:253
Paiva MHS, Barbosa RMR, Santos SA, Silva NM, Paula MB, Ayres CFJ, Leal WS (2019) An unsettling explanation for the failure of skatole-baited ovitraps to capture Culex mosquitoes. Insect Sci 26:873–880
Pappenberger B, Geier M, Boeckh J (1996) Responses of antennal olfactory receptors in the yellow fever mosquito Aedes aegypti to human body odours. In: Bock GR, Cardew G (eds) Olfaction in mosquito-host interactions, Ciba Foundation Symposia. John Wiley and Sons, New York, pp 254–266
Parker AH (1948) Stimuli involved in the attraction of Aedes aegypti, L., to man. Bull Entomol Res 39:387–397
Paskewitz S, Irwin P, Konwinski N, Larson S (2018) Impact of consumption of bananas on attraction of Anopheles stephensi to humans. Insects 9:129
Pates H, Takken W, Stuke K, Curtis C (2001) Differential behaviour of Anopheles gambiae sensu stricto (Diptera: Culicidae) to human and cow odours in the laboratory. Bull Entomol Res 91:289–296
Paula AR, Silva LE, Ribeiro A, Butt TM, Silva CP, Samuels RI (2018) Improving the delivery and efficiency of fungus-impregnated cloths for control of adult Aedes aegypti using a synthetic attractive lure. Parasites Vectors 11:1–9
Paupy C, Delatte H, Bagny L, Corbel V, Fontenille D (2009) Aedes albopictus, an arbovirus vector: from the darkness to the light. Microb Infect 11:1177–1185
Peach DA, Gries R, Young N et al (2019a) Attraction of Female Aedes aegypti (L.) to Aphid Honeydew. Insects 10(43)
Peach DA, Gries R, Zhai H, Young N, Gries G (2019b) Multimodal floral cues guide mosquitoes to tansy inflorescences. Sci Rep 9:1–10
Pezzin A, Sy V, Puggioli A, Veronesi R, Carrieri M, Maccagnani B, Bellini R (2016) Comparative study on the effectiveness of different mosquito traps in arbovirus surveillance with a focus on WNV detection. Acta Trop 153:93–100
Philippe-Janon JC, van den Hurk AF, Francis DP, Shivas MA, Jansen CC (2015) Field comparison of cyclopentanone versus carbon dioxide as an attractant for adult mosquitoes in Southeast Queensland, Australia. J Med Entomol 52:483–490
Pitts RJ, Mozūraitis R, Gauvin-Bialecki A, Lempérière G (2014) The roles of kairomones, synomones and pheromones in the chemically-mediated behaviour of male mosquitoes. Acta Trop 132:S26–S34
Plimmer J, Inscoe M, McGovern T (1982) Insect attractants. Annu Rev Pharmacol Toxicol 22:297–320
Pombi M, Jacobs F, Verhulst NO, Caputo B, della Torre A, Takken W (2014) Field evaluation of a novel synthetic odour blend and of the synergistic role of carbon dioxide for sampling host-seeking Aedes albopictus adults in Rome, Italy. Parasites Vectors 7:580
Ponlawat A, Khongtak P, Jaichapor B, Pongsiri A, Evans BP (2017) Field evaluation of two commercial mosquito traps baited with different attractants and colored lights for malaria vector surveillance in Thailand. Parasites Vectors 10:378
Ponnusamy L, Xu N, Böröczky K, Wesson DM, Ayyash LA, Schal C, Apperson CS (2010) Oviposition responses of the mosquitoes Aedes aegypti and Aedes albopictus to experimental plant infusions in laboratory bioassays. J Chem Ecol 36:709–719
Ponnusamy L, Xu N, Nojima S, Wesson DM, Schal C, Apperson CS (2008) Identification of bacteria and bacteria-associated chemical cues that mediate oviposition site preferences by Aedes aegypti. Proc Natl Acad Sci 105:9262–9267
Price GD, Smith N, Carlson DA (1979) The attraction of female mosquitoes (Anopheles quadrimaculatus Say) to stored human emanations in conjunction with adjusted levels of relative humidity, temperature, and carbon dioxide. J Chem Ecol 5:383–395
Proffit M, Lapeyre B, Buatois B et al (2020) Chemical signal is in the blend: bases of plant-pollinator encounter in a highly specialized interaction. Sci Rep 10:1–11
Puri SN, Mendki MJ, Sukumaran D, Ganesan K, Prakash S, Sekhar K (2006) Electroantennogram and behavioral responses of Culex quinquefasciatus (Diptera: Culicidae) females to chemicals found in human skin emanations. J Med Entomol 43:207–213
Qiu Y, Smallegange R, Van Loon J, Takken W (2011) Behavioural responses of Anopheles gambiae sensu stricto to components of human breath, sweat and urine depend on mixture composition and concentration. Med Vet Entomol 25:247–255
Qiu Y, Smallegange R, Van Loon J, Ter Braak C, Takken W (2006a) Interindividual variation in the attractiveness of human odours to the malaria mosquito Anopheles gambiae ss. Med Vet Entomol 20:280–287
Qiu YT, Smallegange RC, Smid HM et al (2004) GC-EAG analysis of human odours that attract the malaria mosquito Anopheles gambiae sensu stricto. Proc Netherlands Entomol Soc Meeting 15:59–64
Qiu YT, Smallegange RC, Van Loon JJ, Ter Braak CJ, Takken W (2006b) Interindividual variation in the attractiveness of human odours to the malaria mosquito Anopheles gambiae s. Med Vet Entomol 20:280–287
Qualls WA, Mullen GR (2007) Evaluation of the Mosquito Magnet ProTM trap with and without 1-octen-3-ol for collecting Aedes albopictus and other urban mosquitoes. J Am Mosq Control Assoc 23:131–136
Raji JI, Melo N, Castillo JS, Gonzalez S, Saldana V, Stensmyr MC, DeGennaro M (2019) Aedes aegypti mosquitoes detect acidic volatiles found in human odor using the IR8a pathway. Curr Biol 29:1253–1262
Ramoni R, Vincent F, Grolli S et al (2001) The insect attractant 1-octen-3-ol is the natural ligand of bovine odorant-binding protein. J Biol Chem 276:7150–7155
Reeves W (1951) Field studies on carbon dioxide as a possible host simulant to mosquitoes. Proc Soc Exp Biol Med 77:64–66
Reeves W (1953) Quantitative field studies on a carbon dioxide chemotropism of mosquitoes. Am J Trop Med Hygiene 2:325–331
Reiter P, Colon M (1991) Enhancement of the CDC ovitrap with hay infusions for daily monitoring of Aedes aegypti populations. J Am Mosq Control Assoc 7:52–55
Resende MC, TMFd Á, Costa IO, Heringer LC, MRd A, Acebal JL, Eiras ÁE (2012) Field optimisation of MosquiTRAP sampling for monitoring Aedes aegypti Linnaeus (Diptera: Culicidae). Mem Inst Oswaldo Cruz 107:294–302
Resende MC, IMd S, Eiras ÁE (2010) Avaliação da operacionalidade da armadilha MosquiTRAP no monitoramento de Aedes aegypti. Epidemiologia e Serviços de Saúde 19:329–338
Reuter J (1936) Oriënteerend onderzoek naar de oorzaak van het gedrag van Anopheles maculipennis Meigen bij de voedselkeuze, . Luctor et Emergo, Dissertation. University of Leiden, Leiden 118pp
Ribbands C (1949) Studies on the attractiveness of human populations to anophelines. Bull Entomol Res 40:227–238
Robinson A, Busula AO, Voets MA et al (2018) Plasmodium-associated changes in human odor attract mosquitoes. Proc Natl Acad Sci 115:E4209–E4218
Rochlin I, Kawalkowski M, Ninivaggi DV (2016) Comparison of Mosquito Magnet and Biogents Sentinel traps for operational surveillance of container-inhabiting Aedes (Diptera: Culicidae) species. J Med Entomol 53:454–459
Roiz D, Duperier S, Roussel M, Boussès P, Fontenille D, Simard F, Paupy C (2016) Trapping the Tiger: efficacy of the novel BG-Sentinel 2 with several attractants and carbon dioxide for collecting Aedes albopictus (Diptera: Culicidae) in Southern France. J Med Entomol 53:460–465
Roque RA, Eiras ÁE (2008) Calibration and evaluation of field cage for oviposition study with Aedes (Stegomyia) aegypti female (L.)(Diptera: Culicidae). Neotrop Entomol 37:478–485
Rose A, Kröckel U, Bergbauer R, Geier M, Eiras ÁE (2006) Der BG-sentinel, eine neuartige stechmückenfalle für forschung und überwachung. Mitt Dtsch Ges Allg Angew Entomol 15:345–348
Rössler ME (1961) Ernährungsphysiologische Untersuchungen an Scarabaeidenlarven (Oryctes nasicornis L., Melolontha melolontha L.). J Insect Physiol 6:62–80
Rubio-Palis Y (1996) Evaluation of light traps combined with carbon dioxide and 1-octen-3-ol to collect anophelines in Venezuela. J Am Mosq Control Assoc 12(91)
Rudolfs W (1922) Chemotropism of mosquitoes. Bull New Jersey Agric Exp Stn 367:4–23
Rueda LM, Harrison BA, Brown JS, Whitt PB, Harrison RL, Gardner RC (2001) Evaluation of 1-octen-3-ol, carbon dioxide, and light as attractants for mosquitoes associated with two distinct habitats in North Carolina. J Am Mosq Control Assoc -Mosq News 17:61–66
Russell RC (2004) The relative attractiveness of carbon dioxide and octenol in CDC-and EVS-type light traps for sampling the mosquitoes Aedes aegypti (L.), Aedes polynesiensis Marks, and Culex quinquefasciatus Say in Moorea, French Polynesia. J Vector Ecol 29:309
Saitoh Y, Hattori J, Chinone S, Nihei N, Tsuda Y, Kurahashi H, Kobayashi M (2004) Yeast-generated CO2 as a convenient source of carbon dioxide for adult mosquito sampling. J Am Mosq Control Assoc 20:261–264
Saratha R, Mathew N (2016) Development of a mosquito attractant blend of small molecules against host-seeking Aedes aegypti. Parasitol Res 115:1529–1536
Schaerffenberg B, Kupka E (1959) Der attractive factor des blutes fur blutsagende insekten. Naturwiss 46:457–458
Schmied WH, Takken W, Killeen GF, Knols BG, Smallegange RC (2008) Evaluation of two counterflow traps for testing behaviour-mediating compounds for the malaria vector Anopheles gambiae s.s. under semi-field conditions in Tanzania. Malar J 7:230
Schoelitsz B, Mwingira V, Mboera LEG et al (2020) Chemical mediation of oviposition by Anopheles mosquitoes: a push-pull system driven by volatiles associated with larval stages. J Chem Ecol 46:397–409
Schreck C, James J (1968) Broth cultures of bacteria that attract female mosquitoes. Mosq News 28
Schreck C, Kline D, Carlson D (1990) Mosquito attraction to substances from the skin of different humans. J Am Mosq Control Assoc 6:406–410
Schreck C, Smith N, Carlson D, Price G, Haile D, Godwin D (1982) A material isolated from human hands that attracts female mosquitoes. J Chem Ecol 8:429–438
Scott-Fiorenzano JM, Fulcher AP, Seeger KE et al (2017) Evaluations of dual attractant toxic sugar baits for surveillance and control of Aedes aegypti and Aedes albopictus in Florida. Parasites Vectors 10:9
Scott JJ, Crans SC, Crans WJ (2001) Use of an infusion-baited gravid trap to collect adult Ochlerotatus japonicus. J Am Mosq Control Assoc 17:142
Scott TW, Githeko AK, Fleisher A, Harrington LC, Yan G (2006) DNA profiling of human blood in anophelines from lowland and highland sites in western Kenya. Am J Trop Med Hygiene 75:231–237
Seenivasagan T, Guha L, Parashar B, Agrawal O, Sukumaran D (2014) Olfaction in Asian tiger mosquito Aedes albopictus: flight orientation response to certain saturated carboxylic acids in human skin emanations. Parasitol res 113:1927–1932
Seenivasagan T, Sharma KR, Prakash S (2012) Electroantennogram, flight orientation and oviposition responses of Anopheles stephensi and Aedes aegypti to a fatty acid ester-propyl octadecanoate. Acta Trop 124:54–61
Seenivasagan T, Sharma KR, Sekhar K, Ganesan K, Prakash S, Vijayaraghavan R (2009) Electroantennogram, flight orientation, and oviposition responses of Aedes aegypti to the oviposition pheromone n-heneicosane. Parasitol Res 104:827–833
Sharma KR, Seenivasagan T, Rao A, Ganesan K, Agarwal O, Malhotra R, Prakash S (2008) Oviposition responses of Aedes aegypti and Aedes albopictus to certain fatty acid esters. Parasitol Res 103:1065–1073
Sharma KR, Seenivasagan T, Rao A, Ganesan K, Agrawal O, Prakash S (2009) Mediation of oviposition responses in the malaria mosquito Anopheles stephensi Liston by certain fatty acid esters. Parasitol Res 104:281–286
Shelley WB, Hurley HJ, Nichols AC (1953) Axillary odor: experimental study of the role of bacteria, apocrine sweat, and deodorants. Am Arch Dermatol Syphilol 68:430–446
Shirai Y, Kamimura K, Seki T, Morohashi M (2001) L-lactic acid as a mosquito (Diptera: Culicidae) repellent on human and mouse skin. J Med Entomol 38:51–54
Shone SM, Ferrao PN, Lesser CR, Glass GE, Norris DE (2003) Evaluation of carbon dioxide -and 1-octen-3-ol- baited Centers for Disease Control Fay–Prince traps to collect Aedes albopictus. J Am Mosq Control Assoc 19:445
Silva IM, Eiras ÁE, Kline DL, Bernier UR (2005) Laboratory evaluation of mosquito traps baited with a synthetic human odor blend to capture Aedes aegypti. J Am Mosq Control Assoc 21:229–233
Sivakumar R, Jebanesan A, Govindarajan M, Rajasekar P (2011) Oviposition attractancy of dodecanoic, hexadecanoic and tetradecanoic acids against Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae). Eur Rev Med Pharmacol Sci 15:1172–1175
Skinner W, Tong H, Johnson H, Maibach H, Skidmore D (1968) Human sweat components—attractancy and repellency to mosquitoes. Experientia 24:679–680
Smallegange R, Geier M, Takken W (2002) Behavioural responses of Anopheles gambiae to ammonia, lactic acid and a fatty acid in a y-tube olfactometer. Proc Exp Appl Entomol 13:147–152
Smallegange R, Qiu YT, Galimard A, Posthumus M, Van Beek T, van Loon J, Takken W (2003) Why humans are attractive to malaria mosquitoes. Entomol Bericht 63:50-53
Smallegange RC, Bukovinszkine-Kiss G, Otieno B, Mbadi PA, Takken W, Mukabana WR, Van Loon JJ (2012) Identification of candidate volatiles that affect the behavioural response of the malaria mosquito Anopheles gambiae sensu stricto to an active kairomone blend: laboratory and semi-field assays. Physiol Entomol 37:60–71
Smallegange RC, Qiu YT, Bukovinszkine-Kiss G, Van Loon JJ, Takken W (2009) The effect of aliphatic carboxylic acids on olfaction-based host-seeking of the malaria mosquito Anopheles gambiae sensu stricto. J Chem Ecol 35:933–943
Smallegange RC, Qiu YT, van Loon JJ, Takken W (2005) Synergism between ammonia, lactic acid and carboxylic acids as kairomones in the host-seeking behaviour of the malaria mosquito Anopheles gambiae sensu stricto (Diptera: Culicidae). Chem Senses 30:145–152
Smallegange RC, Schmied WH, van Roey KJ, Verhulst NO, Spitzen J, Mukabana WR, Takken W (2010) Sugar-fermenting yeast as an organic source of carbon dioxide to attract the malaria mosquito Anopheles gambiae. Malar J 9:292
Smallegange RC, Takken W (2010) Host-seeking behaviour of mosquitoes: responses to olfactory stimuli in the laboratory. In: Olfaction in vector-host interactions. vol 2. Wageningen Academic Publishers, pp 143-180
Smallegange RC, van Gemert GJ, van de Vegte-Bolmer M, Gezan S, Takken W, Sauerwein RW, Logan JG (2013) Malaria infected mosquitoes express enhanced attraction to human odor. PLoS One 8:e63602
Smart M, Brown A (1956) Studies on the responses of the female Aedes mosquito. Part VII.—The effect of skin temperature, hue and moisture on the attractiveness of the human hand. Bull Entomol Res 47:89–100
Smith CN, Smith N, Gouck HK et al (1970) L-lactic acid as a factor in the attraction of Aedes aegypti (Diptera: Culicidae) to human hosts. Ann Entomol Soc Am 63:760–770
Smith SM, Eng RH, Buccini F (1986) Use of D-lactic acid measurements in the diagnosis of bacterial infections. J Infect Dis 154:658–664
Snetselaar J, Andriessen R, Suer RA, Osinga AJ, Knols BG, Farenhorst M (2014) Development and evaluation of a novel contamination device that targets multiple life-stages of Aedes aegypti. Parasites Vectors 7:200
Spanoudis CG, Andreadis SS, Bray DP, Savopoulou-Soultani M, Ignell R (2020) Behavioural response of the house mosquitoes Culex quinquefasciatus and Culex pipiens molestus to avian odours and its reliance on carbon dioxide. Med Vet Entomol 34:129–137
Spitzen J, Smallegange RC, Takken W (2008) Effect of human odours and positioning of CO2 release point on trap catches of the malaria mosquito Anopheles gambiae sensu stricto in an olfactometer. Physiol Entomol 33:116–122
Steib BM, Geier M, Boeckh J (2001) The effect of lactic acid on odour-related host preference of yellow fever mosquitoes. Chem Senses 26:523–528
Sukumaran D (2016) A review on use of attractants and traps for host seeking Aedes aegypti mosquitoes. Indian J Nat Prod Resources 7:207–214
Sukumaran D, Ponmariappan S, Sharma AK, Jha HK, Wasu YH, Sharma AK (2016) Application of biogenic carbon dioxide produced by yeast with different carbon sources for attraction of mosquitoes towards adult mosquito traps. Parasitol Res 115:1453–1462
Suman DS (2019) Evaluation of enhanced oviposition attractant formulations against Aedes and Culex vector mosquitoes in urban and semi-urban areas. Parasitol Res 118:743–750
Sumba LA, Guda TO, Deng AL, Hassanali A, Beier JC, Knols BG (2004) Mediation of oviposition site selection in the African malaria mosquito Anopheles gambiae (Diptera: Culicidae) by semiochemicals of microbial origin International. J Trop Insect Sci 24:260–265
Syed Z (2015) Chemical ecology and olfaction in arthropod vectors of diseases. Curr Opin Insect Sci 10:83–89
Syed Z, Leal WS (2009) Acute olfactory response of Culex mosquitoes to a human-and bird-derived attractant. Proc Natl Acad Sci 106:18803–18808
Takken W (1991) The role of olfaction in host-seeking of mosquitoes: a review. Int J Trop Insect Sci 12:287–295
Takken W, Dekker T, Wijnholds Y (1997) Odor-mediated flight behavior of Anopheles gambiae giles sensus stricto and An. stephensi liston in response to CO2, acetone, and 1-octen-3-ol (Diptera: Culicidae). J Insect Behav 10:395–407
Takken W, Kline D (1989) Carbon dioxide and 1-octen-3-ol as mosquito attractants. J Am Mosq Control Assoc 5:311–316
Takken W, Knols BG (1999) Odor-mediated behavior of Afrotropical malaria mosquitoes. Annu Rev Entomol 44:131–157
Takken W, Knols BG (2010) Olfaction in vector-host interactions, vol 2. Wageningen Academic Pub, The Netherlands
Takken W, Verhulst NO (2017) Chemical signaling in mosquito-host interactions: the role of human skin microbiota. Curr Opin Insect Sci 20:68–74
Tangena J-AA, Thammavong P, Hiscox A, Lindsay SW, Brey PT (2015) The human-baited double net trap: an alternative to human landing catches for collecting outdoor biting mosquitoes in Lao PDR. PLoS One 10:e0138735
Tauxe GM, MacWilliam D, Boyle SM, Guda T, Ray A (2013) Targeting a dual detector of skin and CO2 to modify mosquito host seeking. Cell 155:1365–1379
Tchouassi DP, Sang R, Sole CL, Bastos AD, Teal PE, Borgemeister C, Torto B (2013) Common host-derived chemicals increase catches of disease-transmitting mosquitoes and can improve early warning systems for Rift Valley fever virus. PLoS Negl Trop Dis 7:e2007
Thomas T (1951) Biting activity of Anopheles gambiae. British Med J 2:1402
Thompson R, Brown A (1955) The attractiveness of human sweat to mosquitoes and the role of carbon dioxide. Mosq News 15:80–84
Thornton JH, Batengana BM, Eiras AE, Irish SR (2016) Evaluation of collection methods for Culex quinquefasciatus, Aedes aegypti, and Aedes simpsoni in northeastern Tanzania. J Vector Ecol 41:265–270
Tian J, Mao J, Yu B, Fouad H, Ga'al H, Mao G, Mo J (2018) Laboratory and field evaluation of multiple compound attractants to Culex pipiens pallens. J Med Entomol 55:787–794
Torr S, Della Torre A, Calzetta M, Costantini C, Vale G (2008) Towards a fuller understanding of mosquito behaviour: use of electrocuting grids to compare the odour-orientated responses of Anopheles arabiensis and An. quadriannulatus in the field. Med Vet Entomol 22:93–108
Torres-Estrada JL, Meza-Alvarez RA, Cibrián-Tovar J, Rodríguez-Lopez MH, Arredondo-jimenez JI, Cruz-López L, Rojas-Leon JC (2005) Vegetation-derived cues for the selection of oviposition substrates by Anopheles albimanus under laboratory conditions. J Am Mosq Control Assoc 21:344–349
Trexler JD, Apperson CS, Gemeno C, Perich MJ, Carlson D, Schal C (2003) Field and laboratory evaluations of potential oviposition attractants for Aedes albopictus (Diptera: Culicidae). J Am Mosq Control Assoc 19:228–235
Trexler JD, Apperson CS, Schal C (1998) Laboratory and field evaluations of oviposition responses of Aedes albopictus and Aedes triseriatus (Diptera: Culicidae) to oak leaf infusions. J Med Entomol 35:967–976
Tseng W-H, Hsiao W-C, Juan D, Chan C-H, Hsiao W-S, Ma H-Y, Lee H-Y (2019) Secondary freeform lens device design with stearic acid for a low-glare mosquito-trapping system with ultraviolet light-emitting diodes. Electronics 8:624
Turner SL, Li N, Guda T, Githure J, Carde RT, Ray A (2011) Ultra-prolonged activation of CO2-sensing neurons disorients mosquitoes. Nature 474:87–91
Ugwu FSO, Onwuzurike JC (2018) Palm wines as potent attractant to mosquitoes. Anim Res Int 15:3055–3064
Unlu I, Faraji A, Indelicato N, Rochlin I (2016) TrapTech R-octenol lure does not improve the capture rates of Aedes albopictus (Diptera: Culicidae) and other container-inhabiting species in Biogents Sentinel traps. J Med Entomol 53:982–985
Unlu I, Farajollahi A (2014) A multiyear surveillance for Aedes albopictus with Biogents Sentinel trap counts for males and species composition of other mosquito species. J Am Mosq Control Assoc 30:122–125
van Breugel F, Riffell J, Fairhall A, Dickinson MH (2015) Mosquitoes use vision to associate odor plumes with thermal targets. Curr Biol 25:2123–2129
van de Straat B, Hiscox A, Takken W, Burkot TR (2019) Evaluating synthetic odours and trap designs for monitoring Anopheles farauti in Queensland, Australia. Malar J 18:299
van Loon JJ, Smallegange RC, Bukovinszkiné-Kiss G et al (2015) Mosquito attraction: crucial role of carbon dioxide in formulation of a five-component blend of human-derived volatiles. J Chem Ecol 41:567–573
Van Thiel P, Weurman C (1947) L'attraction exercée sur Anopheles maculipennis atroparvus par l'acide carbonique dans l'appareil de choix II. Acta Leidensia 18:219–228
Van Thiel PH (1937) Quelles sont les excitations incitant l'" Anopheles maculipennis atroparvus" à visiter ou à piquer l'homme ou le bétail? Bull Soc Path Exot 30:193–209
Vaníčková L, Canale A, Benelli G (2017) Sexual chemoecology of mosquitoes (Diptera, Culicidae): current knowledge and implications for vector control programs. Parasitol Int 66:190–195
Vargo AM, Foster WA (1982) Responsiveness of female Aedes aegypti (Diptera: Culicidae) to flower extracts. J med Entomol 19:710–718
Venkatesh P, Sen A (2017) Laboratory evaluation of synthetic blends of L-(+)-lactic acid, ammonia, and ketones as potential attractants for Aedes aegypti. J Am Mosq Control Assoc 33:301–308
Verhulst NO, Andriessen R, Groenhagen U et al (2010a) Differential attraction of malaria mosquitoes to volatile blends produced by human skin bacteria. PLoS One 5:e15829
Verhulst NO, Bakker JW, Hiscox A (2015) Modification of the Suna trap for improved survival and quality of mosquitoes in support of epidemiological studies. J Am Mosq Control Assoc 31:223–232
Verhulst NO, Beijleveld H, Knols BG, Takken W, Schraa G, Bouwmeester HJ, Smallegange RC (2009) Cultured skin microbiota attracts malaria mosquitoes. Malar J 8:302
Verhulst NO, Beijleveld H, Qiu YT et al (2013) Relation between HLA genes, human skin volatiles and attractiveness of humans to malaria mosquitoes. Infect Genet Evol 18:87–93
Verhulst NO, Mbadi PA, Kiss GB, Mukabana WR, van Loon JJ, Takken W, Smallegange RC (2011a) Improvement of a synthetic lure for Anopheles gambiae using compounds produced by human skin microbiota. Malar J 10(28)
Verhulst NO, Qiu YT, Beijleveld H et al (2011b) Composition of human skin microbiota affects attractiveness to malaria mosquitoes. PloS one 6:e28991
Verhulst NO, Takken W, Dicke M, Schraa G, Smallegange RC (2010b) Chemical ecology of interactions between human skin microbiota and mosquitoes. FEMS Microbiol Ecol 74:1–9
Verhulst NO, Weldegergis BT, Menger D, Takken W (2016) Attractiveness of volatiles from different body parts to the malaria mosquito Anopheles coluzzii is affected by deodorant compounds. Sci Rep 6:27141
Vezenegho SB, Adde A, Gaborit P et al (2014) Mosquito magnet® liberty plus trap baited with octenol confirmed best candidate for Anopheles surveillance and proved promising in predicting risk of malaria transmission in French Guiana. Malar J 13:384
Vythilingam I, Lian CG, Thim CS (1992) Evaluation of carbon dioxide and 1-octen-3-ol as mosquito attractants. Southeast Asian J Trop Med Public Health 23:328–331
Wagner S, Guidi V, Torgerson PR, Mathis A, Schaffner F (2018) Diversity and seasonal abundances of mosquitoes at potential arboviral transmission sites in two different climate zones in Switzerland. Med Vet Entomol 32:175–185
Wagner S, Mathis A (2016) Laboratory colonisation of Aedes geniculatus. J Eur Mosq Control Assoc 34:1–4
Walker V (2014) Ammonia metabolism and hyperammonemic disorders. In: Advances in clinical chemistry, Elsevier, pp 73-150
Wang J-N, Hou J, Zhong JY et al (2020) Relationships between traditional larval indices and meteorological factors with the adult density of Aedes albopictus captured by BG-mosquito trap. Plos One 15:e0234555
Wang Z, Mo J, Zhang S (2006) Laboratory and field evaluations of potential human host odors for Aedes albopictus Skuse (Diptera: Culicidae). J Agric Urban Entomol 23:57–64
Webster B, Lacey ES, Cardé RT (2015) Waiting with bated breath: opportunistic orientation to human odor in the malaria mosquito, Anopheles gambiae, is modulated by minute changes in carbon dioxide concentration. J Chem Ecol 41:59–66
Williams CR, Bergbauer R, Geier M, Kline DL, Bernier UR, Russell RC, Ritchie SA (2006a) Laboratory and field assessment of some kairomone blends for host-seeking Aedes aegypti. J Am Mosq Control Assoc 22:641–647
Williams CR, Ritchie SA, Russell RC, Eiras AE, Kline DL, Geier M (2006b) Geographic variation in attraction to human odor compounds by Aedes aegypti mosquitoes (Diptera: Culicidae): a laboratory study. J Chem Ecol 32:1625
Willis ER (1947) The olfactory responses of female mosquitoes. J Econom Entomol 40:769–778
Wondwosen B, Birgersson G, Seyoum E et al (2016) Rice volatiles lure gravid malaria mosquitoes, Anopheles arabiensis. Sci Rep 6:1–8
Wondwosen B, Hill SR, Birgersson G, Seyoum E, Tekie H, Ignell R (2017) A(maize)ing attraction: gravid Anopheles arabiensis are attracted and oviposit in response to maize pollen odours. Malar J 16:39
Wooding M, Naude Y, Rohwer E, Bouwer M (2020) Controlling mosquitoes with semiochemicals: a review. Parasites Vectors 13:80
Wright R (1962) The attraction and repulsion of mosquitoes. Wld Rev Pest Contr 1:1–12
Wright R (1975) Why mosquito repellents repel. Sci Am 233:104–111
Wurzenberger M, Grosch W (1984) Stereochemistry of the cleavage of the 10-hydroperoxide isomer of linoleic acid to 1-octen-3-ol by a hydroperoxide lyase from mushrooms (Psalliota bispora). Biochim Bioph Acta (BBA )-Lipids Lipid Metabol 795:163–165
Xie L, Yang W, Liu H et al (2019) Enhancing attraction of the vector mosquito Aedes albopictus by using a novel synthetic odorant blend. Parasites Vectors 12:382
Xu P, Zhu F, Buss GK, Leal WS (2015) 1-octen-3-ol - the attractant that repels. F1000Research 4:156. 10.12688/f1000research.6646.1
Xue R-D, Doyle MA, Kline DL (2008) Field evaluation of CDC and Mosquito Magnet® X traps baited with dry ice, CO2 sachet, and octenol against mosquitoes. J Am Mosq Control Assoc 24:249–252
Xue RD, Qualls WA, Kline DL, Zhao T-Y (2010) Evaluation of Lurex 3™, octenol, and CO2 sachet as baits in Mosquito Magnet® Pro traps against floodwater mosquitoes. J Am Mosq Control Assoc 26:344–345
Yu B-T, Ding Y-M, Hu Y, Tian J-X, Song X-G, Li Z-G, Mo J-C (2019) Attraction of Culex pipiens pallens (Diptera: Culicidae) to floret volatiles and synthetic blends of its nectar host plant Abelia chinensis Rubiales: Caprifoliaceae. J Med Entomol 56:29–34
Yu B-T, Huang S-Q, Ding Y-M, Fouad H, Li H-J, Mo J-C (2017) Laboratory evaluation of differential attraction of Culex pipiens pallens to the volatiles of flowers, fruits, and seed pods. J Asia-Pacific Entomol 20:1372–1376
Yu BT, Ding YM, Mo JC (2015) Behavioural response of female Culex pipiens pallens to common host plant volatiles and synthetic blends. Parasites Vectors 8:598
Zohdy S, Derfus K, Andrianjafy MT, Wright PC, Gillespie TR (2015) Field evaluation of synthetic lure (3-methyl-1-butanol) when compared to non odor-baited control in capturing Anopheles mosquitoes in varying land-use sites in Madagascar. Parasites vectors 8:145
Acknowledgements
We are grateful to Doyle McKey, University of Montpellier and CEFE-CNRS, and Caroline David, University Paul Valéry Montpellier, for reviewing the manuscript and for useful discussion.
Availability of Data and Material
excel files and all references in pdf
Code Availability
Not applicable
Author information
Authors and Affiliations
Contributions
LD conceived of the idea for the review, and carried out the literature search and the analysis. LD drafted the first version of the manuscript, and all authors critically revised the work and contributed to the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable
Consent to Participate
Not applicable
Consent for Publication
Not applicable
Conflicts of Interest/Competing Interests
Not applicable
Rights and permissions
About this article
Cite this article
Dormont, L., Mulatier, M., Carrasco, D. et al. Mosquito Attractants. J Chem Ecol 47, 351–393 (2021). https://doi.org/10.1007/s10886-021-01261-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-021-01261-2