Abstract
Wireworms, the larvae of click beetles (Coleoptera: Elateridae), are soil-dwelling insect pests inflicting major economic damage on many types of agricultural crops worldwide. The objective of this work was to identify the female-produced sex pheromones of the Pacific Coast wireworm, Limonius canus LeConte, and the sugarbeet wireworm, L. californicus (Mannerheim) (Coleoptera: Elateridae). Headspace volatiles from separate groups of female L. canus and L. californicus were collected on Porapak Q and analyzed by gas chromatography with electroantennographic detection (GC-EAD) and GC-mass spectrometry. GC-EAD recordings revealed strong responses from male L. canus and male L. californicus antennae to the same compound, which appeared below GC detection threshold. The structure of this candidate pheromone component was deduced from the results of micro-analytical treatments of extracts, retention index calculations on four GC columns, and by syntheses of more than 25 model compounds which were assessed for their GC retention characteristics and electrophysiological activity. The EAD-active compound was identified as (E)-4-ethyloct-4-enoic acid, which we name limoniic acid. In field experiments in British Columbia and Alberta, Canada, traps baited with synthetic limoniic acid captured large numbers of male Limonius click beetles, whereas unbaited control traps captured few. Compared to traps baited with the analogue, (E)-5-ethyloct-4-enoic acid, traps baited with limoniic acid captured 9-times more male L. californicus, and 6.5-times more male western field wireworms, L. infuscatus Motschulsky, but 2.3-times fewer male L. canus. Limoniic acid can now be developed for detection, monitoring and possibly control of L. californicus, L. infuscatus and L. canus populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wireworms, the larvae of click beetles (Coleoptera: Elateridae), are soil-dwelling insect pests inflicting major economic damage on many types of agricultural crops worldwide (Traugott et al. 2015). Several Limonius species are among the most important crop pests of cereals, potatoes, and other vegetables in North America. These species include L. californicus, L. canus, and L. infuscatus in the US Pacific Northwest (PNW) and British Columbia (Andrews et al. 2020; Milosavljević et al. 2016; Vernon and van Herk 2013), L. californicus in Alberta and Montana (Etzler et al. 2014; Morales-Rodriguez et al. 2014; van Herk and Vernon 2014), and L. agonus in Ontario and the northeastern USA (Vernon and van Herk 2013). The economic impact of these pest species is difficult to measure but yield reductions of up to 70% have been reported for cereal crops in the PNW (Reddy et al. 2014), and complete stand destruction of spring wheat, Triticum aestivum, has been observed in fields with high L. californicus populations in southern Alberta (van Herk et al. 2018a). Understanding the ecology of communication, primarily sexual communication, of elaterid beetles would help understand their movement within and across landscapes and facilitate the development of new pheromone-based tactics for detection, monitoring and possibly control of elaterid populations (Vernon and van Herk 2013).
Research on sex pheromones produced by female click beetles has focused on species of major economic concern in Europe (Tóth 2013). Sex pheromones, almost exclusively acetates and esters, have been identified in more than 30 elaterid species, primarily in the genera Agriotes, Elater and Melanotus (Tóth 2013). In comparison, the communication ecology of elaterids native to North America is not as well understood, and there are genera for which no sex pheromone has been identified or shown to be effective in the field.
Research on sex pheromones of Limonius spp. is incomplete and inconclusive. Ethanol extracts of female L. californicus elicited sexual behavior by males in laboratory bioassays (Lilly 1959), and paper chromatography of extracts coupled with observations of beetle behavior led to the hypothesis that the pheromone was a free fatty acid (Lehman 1932). This was later identified as pentanoic (valeric) acid (Jacobson et al. 1968) which caused attraction of males in the field, according to unpublished data by Lilly cited by Jacobson et al. (1968). More recent field testing of synthetic pentanoic acid as a lure for male L. canus in 2003 yielded no trap captures (B. Vernon, unpubl. data).
Working with L. canus, Lehman (1932) ran field experiments showing that hexanoic acid, and to a lesser extent, pentanoic and butanoic acids, attracted males. Onsager et al. (1968) reported that crude extracts of females contained a sex pheromone that attracted males and stimulated sexual activity both in laboratory and field experiments. Based on their chemical analyses, they further concluded that the sex pheromone was likely a carboxylic acid. Similarly, Butler et al. (1975) inferred from various chemical analyses that the sex pheromone of L. canus consisted of two components, one of which was hexanoic acid and the other possibly a “branched 10-carbon unsaturated acid”.
Here we report re-analyses and field testing of the major component of the sex pheromones produced by female L. canus and L. californicus, and the discovery that the same compound is a sex attractant for L. infuscatus.
Methods and Materials
Field Collection of Beetles
In April/May 2018, click beetles were collected using both sweep nets and Vernon Pitfall Traps (VPT) (van Herk et al. 2018b) at sites known to have high populations, in Kelowna, British Columbia (L. canus) and in Granum, Alberta (L. californicus). Beetles were separated by sex using both antennal length, which is notably shorter in females than males in both species, and careful extrusion of genitalia so as not to damage the beetles’ abdomen. Beetles were housed in groups and maintained at room temperature in the laboratory for up to two months, during which time a small cube of apple was provided for food and moisture.
Collection of Headspace Volatiles
From the end of April to mid-June 2018, volatiles emitted by separate groups of 20 female L. californicus and 15 female L. canus were collected. Live beetles were placed in a Pyrex® glass chamber (8 cm high × 8 cm diameter) fitted with a moist cotton wick (Richmond Dental, Charlotte, NC 28205, USA) as a source of water and walk-on substrate. A mechanical pump (Neptune Dyna-pump, Model 2 Dover, NJ 07801, USA) drew charcoal-filtered air at a flow rate of 0.5 L·min−1 for 24 h through the chamber and through a glass column (6 mm outer diameter × 150 mm) containing 200 mg of manufacturer-preconditioned Porapak-Q™ adsorbent (50–80 mesh; Waters Associates, Milford, MA 01757, USA). After collection, the Porapak Q trap was desorbed with pentane/ether (2 ml, 50:50) and concentrated to 100 μl for analyses.
Gas Chromatography with Electroantennographic Detection (GC-EAD) Analyses
Aliquots of Porapak-Q extracts were analyzed by GC-EAD, with procedures and equipment previously described (Gries et al. 2002). Briefly, the GC-EAD setup employed a Hewlett-Packard 5890 gas chromatograph (GC) fitted with one of four GC columns (DB-5, DB-210, DB-23, FFAP; all 30 m × 0.32 mm ID, film thickness 0.25 μm; Agilent J & W columns, Agilent Technologies Inc., Santa Clara, CA 95051, USA). Helium served as the carrier gas (35 cm · s−1) with the following temperature programs: 50 °C for 1 min, then 20 °C · min−1 to 220 °C (DB-210, DB-23) or 280 °C (DB-5); or 100 °C for 1 min, then 20 °C · min−1 to 180 °C held for 15 min (FFAP). The injector port and flame ionization detector (FID) were set to 260 °C and 280 °C, respectively. For each GC-EAD recording, an antenna was carefully removed from a male and suspended between two glass capillary electrodes (1.0 × 0.58 × 100 mm; A-M Systems, Carlsborg, WA 98324, USA) prepared to accommodate the antenna and filled with a saline solution (Staddon and Everton 1980).
GC-Mass Spectrometric (MS) Analyses
Headspace collections were analyzed by GC-MS using both a Varian Saturn 2000 Ion Trap GC-MS and a 5977 A MSD coupled to a 7890B GC (all Agilent Technologies Inc). Both instruments were operated in full-scan electron impact mode and fitted with a DB-5MS column (30 m × 0.25 mm ID; Agilent J&W GC), using helium as the carrier gas (35 cm · s−1). The injector port and ion trap were set at 250 °C and 200 °C, respectively, and the temperature program was set at 50 °C for 5 min, then 10 °C · min−1 to 280 °C and held for 10 min. To identify candidate sex pheromone components, their retention indices relative to retention times of n-alkanes (Van den Dool and Kratz 1963) and mass spectra were compared with those of authentic standards that were purchased or synthesized (see below).
Micro-Analytical Reactions
To determine whether the candidate pheromone component was an unsaturated carboxylic acid, aliquots of extracts were hydrogenated using palladium (5 wt%) on barium sulfate and a flow of hydrogen through the sample. Further aliquots were treated with N,O-bis(trimethylsilyl)-trifluoroacetamide (BSTFA with 1% TMCS; Ceriliant® analytical standard; Supelco, Sigma-Aldrich, MO 63103, USA) to convert carboxylic acids in the extracts to trimethyl silyl (TMS) esters which not only chromatograph better than carboxylic acids but are also more readily detectable by the GC-MS due to their higher molecular weight and characteristic mass spectral fragmentation pattern.
Chemicals
Aliphatic carboxylic acids (pentanoic, hexanoic, heptanoic, octanoic, nonanoic, decanoic) were purchased (Sigma-Aldrich). Syntheses of all model components as well as (E)-4-ethyloct-4-enoic acid (Table 1) are detailed in the Supporting Information.
GC-EAD Analyses of Model Compounds
To compare the electrophysiological activity of nonanoic acids with a methyl branch at C2, C3, C4, C5, C6, C7 or C8, aliquots of separate solutions of each compound (1 μl; 5 ng) were injected consecutively at 0.4- to 0.5-min intervals into the GC fitted with a DB-5 column, split ratio 10:1, and the oven maintained isothermal at 120 °C. All methyl branched nonanoic acids were tested in each of three series of analyses, randomizing the order of injection in each series and using the straight-chain nonanoic acid as a reference compound. The same protocol was used for analysis of ethyl branched octanoic acids.
Field Trapping Experiments
Field experiments were carried out near Kelowna, British Columbia (BC), Canada, and near Granum, Alberta (AB), Canada. All experiments used a randomized complete block design. VPT pitfall traps (van Herk et al. 2018b; available from Intko Supply Ltd., Chilliwack, BC) were placed at 10-m intervals at ground level along the edge of an agricultural field in each location. Traps were baited with test chemicals pipetted onto 100% cotton pellets (size #0; Richmond Dental, Charlotte, NC 28205, USA) inside 1-ml LDPE containers (diameter: 8 mm, height: 32 mm; wall thickness: 0.98 mm; Kartell Labware, Noviglio, Italy) which were then suspended with lids open from the roof of traps. Captured beetles were collected one or multiple times after experiment initiation, as follows: BC 2019 = 2 times; Alberta 2019 = 6 times; BC 2020 = 2 times; Alberta 2020 = one time. At times of collections, treatment positions of traps were not changed and care was taken not to cross-contaminate traps with test stimuli.
Experiments 1 and 2 (N = 12 each; BC: 16 April – 03 May 2019; AB: 03 May – 10 June 2019) tested three treatments: (E)-5-ethyloct-4-enoic acid at 40 mg and 4 mg and an unbaited control. Experiments 3 and 4 (BC: N = 8; 11–25 April 2020; AB: N = 12; 03–10 May 2020) tested four treatments: (1) (E)-4-ethyloct-4-enoic acid (4 mg), (2) (E)-5-ethyloct-4-enoic acid (4 mg), (3) (E)-4-ethyloct-4-enoic acid (2 mg) and (E)-5-ethyloct-4-enoic acid (2 mg), and (4) an unbaited control.
Species Identification and Statistical Analyses of Field Experiments
For Experiments 2 and 4 run in Granum (AB), identification of 50 randomly-selected beetles taken per treatment from a randomly selected replicate indicated a pure population of L. californicus in both 2019 and 2020. For Experiments 1 and 3 run in Kelowna (BC), where L. canus, L. californicus and L. infuscatus co-occur, a randomly-selected subsample of approximately 50 beetles each was taken from each treatment from randomly selected replicates (four in 2019; seven in 2020). These beetles were identified to species, using an identification key developed for economic Limonius species in western Canada and the Pacific Northwest states (Frank Etzler, unpublished). In treatments with fewer than 50 beetles in total (e.g., unbaited controls), all beetles were identified. While (nearly) all beetles from Kelowna were identified as either L. canus or L. infuscatus (2019: 400/400; 2020: 988/990), relative ratios of these species differed between years and among treatments. For both the 2019 and 2020 studies, the calculated mean proportions were used to estimate the number of beetles of these two species captured per treatment.
Trap catch data were analyzed per species on either actual beetle numbers (Granum), or interpolated beetle numbers (Kelowna), using generalized linear models with a negative binomial distribution and a log link function (Proc GENMOD, SAS 9.2, SAS Institute, Cary, NC, USA), including both treatment and replicate as factors. Pair-wise treatment comparisons were made with log-odds ratios using the ESTIMATE function.
Results
Chemical Studies 2018
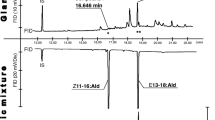
GC-EAD analyses of collections of headspace volatiles from female L. canus and L. californicus on a DB-5 column (Fig. 1), and on DB-23 and DB-210 columns, revealed modest responses from male antennae to aliphatic carboxylic acids (pentanoic, hexanoic, heptanoic, octanoic, nonanoic, decanoic) and a very strong response to an unknown candidate pheromone component (CPC). This CPC eluted between nonanoic and decanoic acid, but was below detection threshold of the GC-FID and GC-MS. The retention indices (RI) of the CPC (Table 1 A), and its RI inter-column differentials, all indicated an acid functionality. That the CPC was likely a carboxylic acid was also suggested by its poor chromatography, resulting in unusually wide antennal responses and a rather slow recovery phase (Fig. 1).
Representative responses of a gas chromatographic flame ionization detector (FID) and an electroantennographic detector (EAD: antenna of a male Limonius canus and male L. californicus) to headspace volatile collections from conspecific females on a DB-5 column: (1) hexanoic acid, (2) heptanoic acid, (3) octanoic acid, (4) nonanoic acid and (5) the candidate pheromone component (CPC). Note that the very strong antennal response to the CPC is elicited by a compound other than the visible peak in the FID trace
The exceedingly low amount of the CPC in volatile collections precluded identification based on spectrometric data and necessitated structural elucidation of the CPC through a combination of micro-analytical reactions and comparison of the retention indices and electrophysiological activities of model compounds.
The BSTFA derivative of the CPC was still below GC detection threshold. Hydrogenation of an aliquot of the collection of volatiles and analysis by GC-EAD elicited the same antennal response pattern as seen before hydrogenation. We therefore concluded that the CPC was a saturated molecule or that any double bond was in a “hindered” position remaining unaffected by hydrogenation.
Because the CPC eluted between nonanoic and decanoic acid on each of three GC columns, we hypothesized that it was a methyl branched nonanoic acid, and we synthesized nonanoic acids with a methyl branch in all possible positions (C2, C3, C4, C5, C6, C7 or C8) (Supplementary Information). Although none of these had retention indices matching those of the CPC (Table 1 B), we subjected these acids to GC-EAD analyses to determine the branch position(s) which elicited the strongest antennal responses. As these methyl branched acids had similar retention times in GC-EAD analyses, they were injected singly and consecutively in split mode and chromatographed isothermally, re-randomizing the order of injection in each of three replicates. In these GC-EAD analyses with antennae of male L. canus, 5-methylnonanoic acid, closely followed by 4-methylnonanoic acid, elicited the strongest antennal responses (Fig. 2), suggesting that the CPC may have a methyl branch at C5. However, the CPC and 5-methylnonanoic acid had different retention indices (Table 1 A B).
Representative responses of a gas chromatographic flame ionization detector (FID) and an electroantennographic detector (EAD: antenna of a male L. canus or male L. infuscatus) to synthetic standards of octanoic acid (3) and methyl branched nonanoic acids with the branch at C3 (5), C6 (6), C4 (7), C7 (8), C8 (9), C2 (10) and C5 (11) on a DB-5 column operated at 120 °C isothermal with consecutive injections (5 ng each) at 0.4- to 0.5-min intervals. At the time of this analysis, all the males were thought to be L. canus but it was learned later that some males may possibly have been L. infuscatus
Therefore, it was considered that the CPC might have a double bond in a hindered position, which had failed to hydrogenate in the earlier attempt, and 5-methylnonenoic acids with E/Z double bonds in C2, C3, C4, C5 and C6 positions were synthesized (Supplementary Information; Table 1 C). Because none of these 5-methylnonenoic acids had the GC retention characteristics of the CPC (Table 1 A C), ethyl branched octanoic acids, instead of methyl branched nonanoic acids, were synthesized (Supplementary Information; Table 1 D). Disappointingly, 3-, 4-, and 5-ethyloctanoic acid all eluted too early relative to the CPC, particularly on the polar DB-23 column (Table 1 D). To increase the retention times on the polar columns, 5-ethyloctenoic acids with E/Z double bonds in C4, C5, or C6 were synthesized (Supplementary Information; Table 1 E). (E)-5-Ethyloct-4-enoic acid and the CPC had identical retention times on each of three GC columns (DB-5, DB-210, DB-23) (Table 1 A E), and elicited similar antennal responses, but equivalent analyses on a critically important fourth GC column (FFAP) could not be completed in time due to a lack of beetles prior to the onset of the 2019 field season.
Field Trapping Experiments 2019
During field Experiments 1 and 2 in 2019, large numbers of L. canus and L. infuscatus (Kelowna, BC) and L. californicus (Granum, AB) were caught in traps baited with lures containing 4 mg or 40 mg of (E)-5-ethyloct-4-enoic acid but not in unbaited control traps (L. canus: χ2 = 172.77, P < 0.0001, χ2 = 155.59, P < 0.0001, respectively; L. infuscatus: χ2 = 74.70, P < 0.0001, χ2 = 74.54, P < 0.0001, respectively; L. californicus: χ2 = 541.88, P < 0.0001, χ2 = 535.42, P < 0.0001, respectively; Fig. 3).
A Field captures of male Limonius spp. and male L. californicus in Experiments 1 and 2 in traps unbaited or baited with (E)-5-ethyloct-4-enoic acid at 4 or 40 mg per lure. B With co-occurrence of L. canus and L. infuscatus in Experiment 1, interpolated trap catch data for the two species are shown based on proportions derived from subsamples. For each data set, bars with a different letter indicate statistically different results (P < 0.05)
In Experiment 1 near Kelowna BC, the mean (SE) proportions of L. canus and L. infuscatus for the 4-mg lure of (E)-5-ethyloct-4-enoic acid, 0.680 (0.058) and 0.320 (0.058), respectively, and the 40-mg lure, 0.609 (0.059) and 0.391 (0.059), respectively, differed significantly (ANOVA: F = 66.36, df = 1,3, P = 0.0039) after analysis of transformed proportions with the control treatment omitted due to low counts.
Chemical Studies 2019–2020
During the 2019 field season, new beetles were obtained. Comparative GC-EAD analyses on the FFAP column showed a slight retention time mismatch between the CPC and synthetic (E)-5-ethyloct-4-enoic acid (Table 1), indicating that this compound was not the major pheromone component of L. canus and L. californicus. Nevertheless, the significant attractiveness of this compound in field experiments suggested that its structure closely resembled that of the CPC.
In GC-EAD analyses of 3-, 4- and 5-ethyloctanoic acids, antennal responses were obtained not only to 4- and 5-ethyloctanoic acids but also to a synthetic impurity (SI in Fig. 4) which occurred below GC detection threshold. GC-MS analyses of synthetic 4- and 5-ethyloctanoic acids at high concentration indicated that SI was one of six (E/Z)-4-ethyloct-4-enoic acids that remained in trace quantities in the synthetic 4-ethyloctanoic acid due to incomplete hydrogenation in the final synthetic step, i.e. with the double bond at C3, C4, or in the ethyl branch (Scheme 1). This synthetic sample was treated with dimethyl disulfide (DMDS) which typically reacts with double bonds to give methylsulfide adducts indicating the positions of double bonds in GC-MS analysis (Dunkelblum et al. 1985). Here, treatment of these unsaturated acids with iodine and the electrophile DMDS resulted in lactonization, generating (methylthio)dihydrofuranones characterized by molecular ions at m/z 216. However, these still revealed the double bond positions at C3, C4 and in the ethyl branch (Fig. 5). Following syntheses of (E/Z)-4-ethyloct-4-enoic acid, (E/Z)-4-ethyloct-3-enoic acid and (E/Z)-4-ethylidene octanoic acid (Scheme 2), GC analyses showed that only (E)-4-ethyloct-4-enoic acid had retention characteristics consistent with those of the CPC on each of four GC columns (DB-5, DB-23, DB-210, FFAP) (Table 1 A F). This was confirmed by stereoselective synthesis of (E)-4-ethyloct-4-enoic acid, with the mass spectrum shown in Fig. 6.
Representative responses of a gas chromatographic flame ionization detector (FID) and an electroantennographic detector (EAD: antenna of a male Limonius canus or L. infuscatus) to synthetic standards of octanoic acid (3; reference compound), and ethyl branched octanoic acids with the branch at C3 (12), C4 (13), and C5 (14) on a DB-5 column operated at 120 °C isothermal with consecutive injections (5 ng each) at 0.4- to 0.5-min intervals. Note the strong antennal response to a synthetic impurity (SI) below GC detection threshold in 4-ethyloctanoic acid. At the time of this analysis, all the males were thought to be L. canus but it was learned later that some males may possibly have been L. infuscatus
Gas chromatograms of (a) incompletely hydrogenated 4-ethyloctanoic acid containing six 4-ethyloctenoic acid impurities with E/Z double bonds inferred to be in C3, C4 or the ethyl branch, based on the synthetic route for its preparation (Scheme 1), and (b-d) stereo-selectively synthesized (3Z/E)-4-ethyloct-3-enoic acid (3), (4Z/E)-4-ethyloct-4-enoic acid (4), and (E/Z)-4-ethylidene octanoic acid (5), respectively. The right column shows the mass spectra of the corresponding dihydrofuranones formed on treatment of the acids (3–5) with dimethyldisulfide. The fragmentation patterns reveal the double bond positions; m/z 74 is likely a fragmentation with a rearrangement rather than a simple cleavage
During 2018 and 2019 analyses, all males were thought to be L. canus but it was learned later that some males may possibly have been L. infuscatus. Electrophysiological responses of L. canus males to the sex pheromone component (E)-4-ethyloct-4-enoic acid produced by L. canus females were demonstrated in 2020 with a separate group of male and female beetles confirmed to be L. canus.
Field Trapping Experiments 2020
In 2020-field experiments, traps baited with (E)-4-ethyloct-4-enoic acid captured 9-times more male L. californicus (Granum, AB), 6.5-times more male L. infuscatus (Kelowna, BC) and 2.3-times fewer male L. canus (Kelowna, BC) than traps baited with (E)-5-ethyloct-4-enoic acid (L. californicus: χ2 = 1069.4, P < 0.0001; L. infuscatus: χ2 = 70.76, P < 0.0001; L. canus: χ2 = 15.16, P < 0.0001; Fig. 7).
A Field captures of male Limonius spp. and male L. californicus in Experiments 3 and 4 in traps unbaited or baited with (1) (E)-4-ethyloct-4-enoic acid (4 mg), (2) (E)-5-ethyloct-4-enoic acid (4 mg), and (3) (E)-4-ethyloct-4-enoic acid (2 mg) and (E)-5-ethyloct-4-enoic acid (2 mg). B With co-occurrence of L. canus and L. infuscatus in Experiment 3, interpolated trap catch data for the two species are shown based on proportions derived from subsamples. For each data set, bars with a different letter indicate statistically different results (P < 0.05)
In contrast to 2019, L. infuscatus was the predominant species captured in Experiment 3 near Kelowna in 2020, but the relative proportion of the two species again differed significantly among treatments (L. canus: F = 10.84, df = 2,12, P = 0.002; L. infuscatus: F = 9.80, df = 2,12, P = 0.003). Significantly more L. canus, mean: 0.271 (SE: 0.088), and fewer L. infuscatus, 0.729 (0.088), were captured in treatment 3 (T3), containing (E)-5-ethyloct-4-enoic acid, than in either T2, 0.024 (0.009) and 0.970 (0.009), respectively, or T4, 0.064 (0.034) and 0.936 (0.034), respectively, whereas the relative proportions of L. canus and L. infuscatus in T2 and T4 were similar.
Captures of male L. canus and male L. infuscatus in traps baited with (E)-4-ethyloct-4-enoic acid alone and in binary combination with (E)-5-ethyloct-4-enoic acid were dissimilar, indicating an interaction between the two acids (L. canus: χ2 = 11.50, P = 0.0007; L. infuscatus: χ2 = 54.27, P < 0.0001).
Discussion
Our field data support laboratory data that (E)-4-ethyloct-4-enoic acid – ‘limoniic acid’ – is the major pheromone component of L. californicus. The data also show that limoniic acid is a pheromone component of L. canus even though its analogue, (E)-5-ethyloct-4-enoic acid, was two-fold more attractive in this particular experiment. These unexpected results are reminiscent of findings reported in a recent study with the click beetle Cardiophorus edwardsi, where traps baited with a synthetic pheromone mimic captured more males than pheromone-baited traps (Serrano et al. 2020). Experiments currently under way in other locations across North America should help determine the relative attractiveness of limoniic acid and its analogue to various populations of L. canus.
Various carboxylic acids in headspace collections from L. canus and L. californicus elicited responses from male antennae (Fig. 1) but emission of these acids by both males and females made it unlikely that they had a functional role as sex pheromone components. With limoniic acid now shown to be a pheromone component of L. californicus and L. canus, and a sex attractant for L. infuscatus (production of limoniic acid by female L. infuscatus is yet to be demonstrated), the question remains whether spatial and/or temporal separation of Limonius congeners suffice to maintain species-specificity of sexual communication. There are numerous local populations of Limonius spp. across North America, with geno- and phenotypic characteristics likely shaped by physical and biological factors of the local environment. If we accept the premise that the presence of congeners is a biological factor (Linn and Roelofs 1995), then there is selection pressure to improve the signal-to-noise ratio of communication channels in “crowded” communities (Baker 1985; Cardé and Baker 1984). Analyzing the sex pheromone of various Limonius species, tracking their seasonal appearance, and studying the diel periodicity of their sexual communication in communities with single or multiple congeners would reveal not only whether limoniic acid is a signature pheromone component of Limonius spp., but also whether any type of competition-induced reproductive character displacement sensu Butlin (1987) may have caused signal divergence (e.g., contrasting pheromone or time of signaling) to avoid heterospecific matings, as implied for populations of the nun moth, Lymantria monacha, in the Czech Republic and Japan (Gries et al. 2001).
The structure of limoniic acid contrasts with the acetate/ester functionality of most sex pheromones identified in elaterids to date, mainly in the genera Agriotes, Elater and Melanotus (Tóth 2013). Both the low amount of limoniic acid in headspace collections from females and its poor chromatography during chromatographic analyses might explain why this pheromone has eluded structural elucidation for such a long time. Our data provide impetus and incentive to pursue pheromone identification in other elaterid genera that have no immediately obvious, or readily identifiable, sex pheromones.
Increasing knowledge of the compositions of elaterid sex pheromones provides new opportunities to explore potential roles of synthetic sex pheromones for integrated management of click beetle populations (Traugott et al. 2015). Captures of click beetles in pheromone-baited traps would aid in the detection of target species and help predict crop damage, if trap captures of adult beetles were to correlate locally with numbers of soil-dwelling wireworm larvae (Furlan et al. 2020; Vernon and van Herk 2013). Synthetic sex pheromones could also be developed for mass trapping adult beetles (Vernon et al. 2014) or for luring them to micro-locations, where they get infected by, and subsequently spread, lethal microbial pathogens (Leung 2018), for example.
Data Availability
Data are reported in the manuscript and in the electronic Supplementary Information.
References
Andrews KR, Gerritsen A, Rashed A, Crowder DW, Rondon SI, van Herk WG, Vernon R, Wanner KW, Wilson CM, New DD, Fagnan MW (2020) Wireworm (Coleoptera: Elateridae) genomic analysis reveals putative cryptic species, population structure, and adaptation to pest control. Commun Biol 3:1–13
Baker TC (1985) Insect mating and courtship behavior. In: Kerkut GA, Gilbert LI (eds) Comprehensive insect physiology. Biochemistry and Pharmacology. Pergamon Press, New York, pp 621–672
Butler LI, McDonough LM, Onsager JA, Landis BJ (1975) Sex pheromones of the Pacific coast wireworm, Limonius canus. Environ Entomol 4:229–230
Butlin RK (1987) Speciation by reinforcement. Trends Ecol Evol 2:8–13
Cardé RT, Baker TC (1984) Sexual communication in insects. In: Bell W, Cardé RT (eds) Chemical ecology of insects. Sinauer, Sunderland, Massachusetts, pp 355–386
Dunkelblum E, Tan SH, Silk PJ (1985) Double-bond location in monounsaturated fatty acids by dimethyldisulfide derivatization and mass spectrometry: application to analysis of fatty acids in pheromone glands of four Lepidoptera. J Chem Ecol 11:265–277
Etzler FE, Wanner KW, Morales-Rodriguez A, Ivie MA (2014) DNA barcoding to improve the species-level management of wireworms (Coleoptera: Elateridae). J Econ Entomol 107:1476–1485
Furlan L, Conterio B, Chiarini F, Benvegnu I, Tóth M (2020) The use of click beetle pheromone traps to optimize the risk assessment of wireworm (Coleoptera: Elateridae) maize damage. Sci Rep 10:8780. https://doi.org/10.1038/s41598-020-64347-z
Gries G, Schaefer PW, Gries R, Liška J, Gotoh T (2001) Reproductive character displacement in Lymantria monacha from northern Japan. J Chem Ecol 27:1163–1176
Gries R, Khaskin G, Gries G, Bennett RG, King GGS, Morewood P, Slessor KN, Morewood WD (2002) (Z,Z)-4,7-Tridecadien-(S)-2-yl acetate: sex pheromone of Douglas-fir cone gall midge, Contarinia oregonensis. J Chem Ecol 28:469–478
Jacobson M, Lilly CE, Harding C (1968) Sex attractant of sugar beet wireworm: identification and biological activity. Sci (Washington) 159:208–210
Lehman RS (1932) Experiments to determine the attractiveness of various aromatic compounds to adults of the wireworms Limonius (Pheletes) canus Lec. And Limonius (Pheletes) californicus Mann. J Econ Entomol 25:949–958
Leung JPS (2018) The response of Agriotes obscurus click beetles to pheromone and its impact on the acquisition of a fungal pathogen. MPM thesis. Simon Fraser University, Burnaby, BC, Canada
Lilly CE (1959) Response of males of Limonius californicus (Mann) to a sex attractant separable by paper chromatography. Can Entomol 91:145–146
Linn CE, Roelofs WL (1995) Pheromone communication in moths and its role in the speciation process. In: Lambert DM, Spencer HG (eds) Speciation and the recognition concept. John Hopkins University Press, Baltimore, Maryland, pp 263–300
Milosavljević I, Esser AD, Crowder DW (2016) Effects of environmental and agronomic factors on soil-dwelling pest communities in cereal crops. Agric Ecosyst Environ 225:192–198
Morales-Rodriguez A, O'Neill RP, Wanner KW (2014) A survey of wireworm (Coleoptera: Elateridae) species infesting cereal crops in Montana. Pan-Pac Entomol 90:116–125
Onsager JA, McDonough LM, George DA (1968) A sex pheromone in the Pacific coast wireworm. J Econ Entomol 61:691–693
Reddy GVP, Tangtrakulwanich K, Wu S, Miller JH, Ophus VL, Prewett J, Jaronski ST (2014) Evaluation of the effectiveness of entomopathogens for the management of wireworms (Coleoptera: Elateridae) on spring wheat. J Invertebr Pathol 120:43–49
Serrano JM, Zou Y, Millar JG (2020) Identification of a hyperactive pheromone analog in field tests of pheromone mimics for two click beetle species in the genus Cardiophorus (Coleoptera: Elateridae). Chemoecology 30:297–304. https://doi.org/10.1007/s00049-020-00319-z
Staddon BW, Everton IJ (1980) Haemolymph of milkweed bug Oncopeltus fasciatus (Heteroptera: Lygaeidae): inorganic constituents and amino acids. Comp Biochem Physiol 65A:371–374
Tóth M (2013) Pheromones and attractants of click beetles: an overview. J Pest Sci 86:3–17
Traugott M, Benefer C, Blackshaw RP, van Herk WG, Vernon RS (2015) Biology, ecology, and control of elaterid beetles in agricultural land. Annu Rev Entomol 60:313–334
Van Den Dool H, Kratz PD (1963) A generalization of the retention index system including linear temperature programmed gas–liquid partition chromatography. J Chromatogr 2:463–471
van Herk WG, Vernon RS (2014) Click beetles and wireworms (Coleoptera: Elateridae) of Alberta, Saskatchewan, and Manitoba. In: Giberson DJ, Carcamo HA (eds) Arthropods of Canadian grasslands, vol. 4. Biological Survey of Canada, Ottawa, pp 87–117
van Herk WG, Labun TJ, Vernon RS (2018a) Efficacy of diamide, neonicotinoid, pyrethroid, and phenyl pyrazole insecticide seed treatments for controlling the sugar beet wireworm, Limonius californicus (Coleoptera: Elateridae), in spring wheat. J Entomol Soc Brit Columbia 115:86–100
van Herk WG, Vernon RS, Borden JH (2018b) A pheromone-baited pitfall trap for monitoring Agriotes spp. click beetles (Coleoptera: Elateridae) and other soil surface insects. J Entomol Soc BC 115:101–103
Vernon RS, van Herk WG (2013) Wireworms as pests of potato. In: Giordanengo P, Vincent C, Alyokhin A (eds) Insect pests of potato: global perspectives on biology and management. Academic Press, Elsevier, Amsterdam, pp 103–164
Vernon WG, Blackshaw RP, van Herk WG, Clodius M (2014) Mass trapping wild Agriotes obscurus and A. lineatus males with pheromone traps in a permanent grassland population reservoir. Agric For Entomol 16:227–239
Acknowledgements
We thank Jocelyn Millar for a most valuable suggestion regarding the interpredation of DMDS treatment results, Associate Editor David Hall for his meticulous review of the manuscript, Sharon Oliver for word processing and comments, Russ Alcock and Blaine Dunlop for permitting access to their farms, David Shack, Emmanuel Hung, and Sebastian Damin for assistance with processing beetles, Stephen Takács for help with graphical illustrations, and two anonymous reviewers for complimentary comments. The research was supported by an AAFC Canadian Agricultural Partnership Cluster Project (Developing IPM tools for wireworm management in Canada) administered by the Canadian Horticulture Council and supported by the Potato Growers of Alberta, the B.C. Potato Industry Development Committee, the B.C. Lower Mainland Horticultural Improvement Association, and by a Natural Sciences and Engineering Research Council of Canada (NSERC) – Industrial Research Chair to GG, with BASF Canada Inc. and Scotts Canada Ltd. as the industrial sponsors.
Funding
The research was supported by an AAFC Canadian Agricultural Partnership Cluster Project (Developing IPM tools for wireworm management in Canada) administered by the Canadian Horticulture Council and supported by the Potato Growers of Alberta, the B.C. Potato Industry Development Committee, the B.C. Lower Mainland Horticultural Improvement Association, and by a Natural Sciences and Engineering Research Council of Canada (NSERC) – Industrial Research Chair to GG, with BASF Canada Inc. and Scotts Canada Ltd. as the industrial sponsors.
Author information
Authors and Affiliations
Contributions
WvH & GG conceived the study; WvH and HC captured beetles for pheromone analyses; RG captured headspace odorants, and analyzed odorant extract as well as model compounds by GC-EAD and GC-MS; SA synthesized chemicals; WvH, HC, SM and EL ran field experiments; EL and WvH identified and determined the sex of beetles captured in traps; WvH analyzed capture data statistically; RG and GG wrote the first draft, and all authors reviewed and approved of the final draft.
Corresponding author
Ethics declarations
Conflict of Interest / Competing Interests
The authors declare no conflicts of interest.
Ethics Approval
Not applicable
Consent to Participate
Not applicable
Consent for Publication
All authors approved of the submission of the manuscript.
Code Availability
Not applicable
Additional information
Regine Gries and Gerhard Gries are dedicating this article to Prof. Dr. Dr. h.c. mult. Wittko Francke, in memoriam.
Supplementary Information
ESM 1
(PDF 3750 kb)
Rights and permissions
About this article
Cite this article
Gries, R., Alamsetti, S.K., van Herk, W.G. et al. Limoniic Acid - Major Component of the Sex Pheromones of the Click Beetles Limonius canus and L. californicus. J Chem Ecol 47, 123–133 (2021). https://doi.org/10.1007/s10886-020-01241-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-020-01241-y