Abstract
Giant silk moths (Lepidoptera: Saturniidae) typically are not well represented as larvae or adults in community level inventories of Lepidoptera, and as a result, little is known about their population dynamics. Furthermore, in recent years, many species of silk moths appear to have experienced population declines. Volatile sex pheromones are powerful sampling tools that can be used in operational conservation and monitoring programs for insects. Here, we describe the identification of the sex attractant pheromone of a giant silk moth, the luna moth Actias luna. Coupled gas chromatography-electroantennographic detection and gas chromatography-mass spectrometric analyses of extracts from pheromone glands of female luna moths supported the identification of (6E,11Z)-6,11-octadecadienal (E6,Z11–18:Ald), (6E)-6-octadecenal (E6–18:Ald), and (11Z)-11-octadecenal (Z11–18:Ald) as the compounds in extracts that elicited responses from antennae of male moths. These identifications were confirmed by synthesis, followed by testing of blends of the synthetic compounds in field trials in Ontario, Canada, and Kentucky, USA. Male moths were attracted to synthetic E6,Z11–18:Ald as a single component. Attraction appeared to be enhanced by addition of E6–18:Ald but not Z11–18:Ald, suggesting that the luna moth pheromone consists of a blend of E6,Z11–18:Ald and E6–18:Ald.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Giant silk moths (Lepidoptera: Saturniidae) are large, charismatic moths and as a result are well known to both collectors and the general public. Consequently, they are attractive targets for conservation efforts (e.g., the Spanish moon moth Graellsia isabellae; Millar et al. 2010). In northeastern North America there have been reports of wide-scale declines of populations of many species of giant silk moths (Boettner et al. 2000; Ferguson 1971; Kellog et al. 2003; Schweitzer 1988; Tuskes et al. 1996). The extent of and reason for these declines is a source of continuing debate, and may include habitat loss, light pollution disrupting normal behaviors, non-target effects of insecticides, and generalist classical biological control agents such as the tachinid wasp Compsilura concinnata, originally introduced to control gypsy moth, attacking luna moth and other nontarget species (Boettner et al. 2000; Holden 1992; Johnson et al. 1995; Kellog et al. 2003; Schweitzer 1988; Tuskes et al. 1996).
The luna moth, Actias luna (L.), is among the species of giant silk moths reported to be affected by one or more of these factors (Kellog et al. 2003; Peigler 2001; Schweitzer 1988). This pale lime-green Nearctic species is one of the largest moths in North America with a wingspan of up to 135 mm. It occurs in the US east of the Great Plains to the Atlantic coast, in the south from Texas to Florida, and in Canada from Ontario to Nova Scotia (Bouseman and Sternburg 2002). Its larvae are moderately polyphagous, feeding on trees from at least eight families (Baker 1972; Tietz 1972) and its habitat includes urban and natural forests. Nutritional and enzymatic studies suggest that host plant use by the luna moth is more specialized at the individual or population than species level (Lindroth 1989). It also is noteworthy that whereas northern populations are univoltine, southern populations may have as many as three generations per year (Tuskes et al. 1996).
One of the challenges to characterizing the extent and cause(s) of the reported declines in populations of the giant silk moths is that larvae and adults often are poorly represented in forest community inventories (e.g., Butler and Stazanc 2000a, 2000b; Le Corff et al. 2000; Marquis and Passoa 1989; Thomas and Thomas 1994; Thomas 1996; Wagner et al. 1996), and as a result it is difficult to estimate population trends. Pheromone-baited traps are widely used to detect and sample pest insect populations because of their high degree of specificity and the strong attraction of the target species to the pheromone lure. These same attributes make pheromone lures ideal tools for sampling cryptic, rare, or endangered species, as shown by several recent studies with beetles (e.g., Larsson and Svensson 2009) and moths (e.g., Millar et al. 2010; Gago et al. 2013). The objectives of this study were to identify, synthesize, and bioassay the female-produced sex pheromone of the luna moth, so that it could be developed as a tool for assessing population sizes, voltinism, and ranges of this iconic species.
Materials and Methods
Insects
Insects used in initial studies were purchased as pupae in December 2008 from Tom Hupf (Atlantic Co., NJ, USA), who had reared them on sweetgum, Liquidambar spp. Additional insects, also reared on Liquidambar spp., were collected from Micanopy, Alachua Co., and Gulf Hammock, Levy Co., FL, USA by ATD, and were shipped to UC Riverside in May, 2009. Two final shipments of pupae in May, 2014 were reared from eggs laid by females collected by Pierre Bolduc near Lac-Drolet and Lac-Mégantic in southern Quebec. The resulting larvae were reared on cut foliage of Betula papyrifera and B. populifolia. Pupae from all populations were shipped to the quarantine facility at the University of California, Riverside (USDA-APHIS permit #P526P-08-02,964).
The first shipment of pupae from New Jersey were refrigerated after arrival in December until mid-February, and adults began to emerge 2 wk after the pupae were brought to room temp. None of the subsequent shipments of pupae were refrigerated because they all occurred during the spring/summer months. Upon receipt, pupae were separated by sex and placed into 15 cm tall by 14 cm diam screen cages made from 6.3 mm mesh hardware cloth with a 150 mm Petri plate bottom. Paper toweling was placed on the bottom of the cage to absorb moisture, with another moistened towel on top, and the cage was covered with an unzipped 4 L Ziplock bag to retain humidity. Pupae were held in an environmentally controlled room at ca. 22 °C, 50 % RH and on a 16:8 h L:D light cycle using fluorescent lighting. The scotophase of the room lighting was reversed, with lights off around 9:30 AM, and females began calling ~4 h later. Calling females were sampled from 4 to 7 h after onset of scotophase. Adults emerged during the photophase and so prior to lights off, emerging males were placed individually in glassine envelopes in the refrigerator in a 4 L Ziplock bag with a damp towel until needed for experiments. For all males, the genitalia were removed before they were taken out of the quarantine facility (a requirement for the permit) for use in coupled GC-EAD experiments. Emerging females were held individually in their screen cages until used for pheromone collections.
A colony of luna moths for use in field bioassays was started in Lexington, KY, USA from eggs laid by a single female collected near Bloomington, IN, USA in May 2014. Her offspring were reared on leaves of black walnut (Juglans nigra). Adult females that originated from these eggs were mated to their siblings or to males that they attracted in the Berea College Forest, Berea, KY, USA (GPS 37°32′39″N, 84°13′43’). The offspring of these matings were reared on sweetgum leaves (Liquidambar styraciflua), which proved to be superior to black walnut as a host because the leaves remained hydrated for a longer period. The resulting pupae were held in their cocoons under ambient conditions until early May 2015, at which time the pupae were removed from their cocoons and separated by sex. Adults emerged into screened cages. On emergence, females were transferred to individual 15 cm diam screen cages. Females were used in trapping experiments when they were 1–5 d-old, preferentially choosing the youngest available females as lures.
Solid Phase Micro-Extraction (SPME) of Sex Pheromone Glands
Methods were the same as those reported for Graellsia isabellae (Millar et al. 2010). Briefly, females were observed during the scotophase by using a flashlight with a red filter, choosing only actively calling females for sample collection. Thus, a calling female was removed from her cage and her abdomen was firmly squeezed to evert the sex pheromone gland. A solid phase micro-extraction fiber (SPME, 100 μm PDMS, Supelco, Bellefonte, PA, USA) was taped to the benchtop such that the fiber overhung the edge with the terminal 5–7 mm of the fiber being exposed. The sex pheromone gland (the scale-free area on the penultimate segment of the abdomen) was wiped over the exposed fiber. SPME wipe samples were prepared from both single and multiple females for use in coupled GC-EAD or GC-MS analyses. Sampled females typically were returned to their cages, where they usually ceased calling and began depositing unfertilized eggs, or were placed in glassine envelopes and refrigerated for reuse.
Solvent Extraction of Sex Pheromone Glands
A total of 7 solvent rinses of excised sex pheromone glands from calling females were made. Two of the extracts were from moths reared from the New Jersey pupae, with the remaining 5 being from the Canadian pupae. Glands were removed from 4 to 7 h into the scotophase, and extracts contained from 2 to 4 gland rinses. Glands were excised and extracted in pentane for ~1 min as previously described (Millar et al. 2010). Extracts then were transferred to clean vials and stored in a freezer at −20 °C until used in experiments.
Gas Chromatography-Electroantennographic Detection (GC-EAD)
GC-EAD analyses were performed using DB-5 (New Jersey and Florida samples), DB-17 (Canadian samples), and DB-Wax (all 3 samples) columns mounted in a Hewlett-Packard 5890 GC (now Agilent, Avondale PA, USA). All columns were 30 m × 0.25 mm i.d., 0.25 μm film thickness (J&W Scientific, Folsom CA, USA), and helium was used for both the carrier and makeup gas. Temperature programs were 100 °C for 1 min, 10 °C per min to 275 °C for DB-5, 100 °C for 1 min, 10 °C per min to 275 °C or 100 °C for 1 min, 10 °C per min to 190 °C for 15 min, then 10 °C per min to 280 °C for DB-17, and 100 °C for 1 min, 10 °C per min to 250 °C for DB-Wax. All columns used a final hold time of 20 to 60 min to elute less volatile components of extracts. Loaded SPME fibers were desorbed in splitless mode for 60 s prior to starting the run (injector temperature 250 °C). Solvent extracts of pheromone glands (1 μl aliquots) also were analyzed in splitless mode. The effluent from the columns was split using an ‘X’ cross, with helium makeup gas being added through the fourth arm at 3 ml/min, and half of the sample going to the FID detector and the other half to the EAD. The portion directed to the EAD was diluted in a humidified air stream prior to exposure to the antenna. Males used in GC-EAD analyses were removed from quarantine after the genitalia had been removed, allowed to warm to room temperature, and then an antenna was removed by pulling it off at the scape. The terminal rami were removed with a razor blade, and the tip was excised to ensure good contact with the saline solution (7.5 g NaCl, 0.21 g CaCl2, 0.35 g KCl, and 0.20 g NaHCO3 in 1 l Milli-Q purified water) in the glass electrodes, which were fitted with an internal gold wire for connection to the amplifier. A single antennal preparation was used for as many as four runs. Kovat’s indices were calculated for unknowns and standards relative to blends of straight-chain hydrocarbons.
Coupled Gas Chromatography-Mass Spectrometry
GC-MS analyses were carried out with an Agilent 6890 N GC interfaced to an Agilent 5975C mass selective detector, with electron impact ionization (70 eV). The GC was fitted with a DB-5 column (30 m × 0.25 mm ID, 0.25 μm film thickness), programmed from 50 °C/1 min, 10 °C/min to 280 °C, hold for 20 min, with injector and transfer line temperatures of 280 °C. Loaded SPME fibers were desorbed in the injector for 30 s in splitless mode, then the run was started. Solvent extracts and solutions of standards also were injected in splitless mode, with the purge valve opening after 30 s.
Synthesis of Authentic Standards
The syntheses of authentic standards of (11Z)-11-octadecenal (Z11–18:Ald), (6E)-6-octadecenal (E6–18:Ald), and (6E,11Z)-6,11-octadecadienal (E6,Z11–18:Ald) are shown in Fig. 1, with experimental details reported in the online Supporting Information.
Synthesis of (11Z)-11-octadecenal, (6E)-6-octadecenal, and (6E,11Z)-6,11-octadecadienal. a Dihydropyran, PTSA; b Lithium acetylide-ethylenediamine complex, NaI, DMSO; c BuLi, hexyl iodide, THF, 50 °C; d PTSA, MeOH, e P-2 nickel, ethylenediamine, H2, EtOH; f PDC, CH2Cl2; g LiAlH4, THF; h Mesyl chloride, Et3N, CH2Cl2, then LiBr, acetone; i Cp2ZrHCl, THF, NMP; j LiBr, Pd(acac)2, (Z)-4-undecenyl bromide 16
Field Trials
Lures used in field trials were prepared from rubber septa (5 mm diam; Thomas Scientific, Swedesboro, NJ, USA) that were extracted twice with hexane and allowed to dry, loaded as described below for each experiment, and stored in a − 20 °C freezer until needed. In experiment 1, carried out from June 28 – July 6 2014 at Caribou Lake, Ontario, Canada (~13 km from the town of Desbarats ON), control septa loaded with 300 μl hexane were compared to septa loaded with 300 μg of E6,Z11–18:Ald in 300 μl hexane. This dose was arbitrarily chosen for this and subsequent experiments based on the limited amount of synthetic pheromone available. Moths were captured in cube-shaped traps 51 cm on a side constructed of a wood frame covered with fiberglass screen, similar to those used in trapping Callosamia promethea (Gago et al. 2013). Each vertical side of the trap was fitted with an inward-pointing screen cone that narrowed from a 25 to a 12 cm diam opening inside the trap to limit escape. A total of 4 traps were hung from paper birch trees (Betula papyrifera), and the openings for the traps were ca. 1.5 m above ground level. Treatment and control traps were separated by at least 200 m. Traps were checked daily. Each replicate lasted one night (because there were 4 traps, there were two replicates per night × 5 nights =10 total replicates). The next day the lures were rotated one trap position, and were replaced at 2 d intervals.
In experiment 2, conducted 11–19 May 2015, eleven replicates were run at the Berea College Forest site. Traps baited with a rubber septum loaded with 300 μg of E6,Z11–18:Ald in 100 μl hexane were compared to traps baited with one live caged virgin female. Moths were captured in 53 × 53 × 53 cm cube-shaped traps constructed of PVC pipe covered with fiberglass screen. Each vertical side of the trap had an inward-pointing cone as described above. Traps were hung in pairs, with traps separated by at least 30 m within a block, and blocks were separated by at least 100 m. Traps were suspended from sweetgum trees at a height of 1 to 1.5 m above ground level. Each replicate was in position for 1–2 nights. Trap positions were switched within a pair after each replicate. Lures and females were replaced after 1–2 nights.
In experiment 3, deployed May 15–19 2015 at the Berea College site, rubber septa loaded with 300 μg of E6,Z11–18:Ald in 100 μl hexane were compared to septa loaded with 300 μg of E6,Z11–18:Ald, 7.5 μg Z11–18:Ald, 7.5 μg E6–18:Ald, and 210 μg 18:Ald in 100 μl hexane. Lures were deployed in cube traps as described above, and were separated by at least 30 m within a block, with blocks separated by at least 100 m. Traps were suspended from sweetgum trees at a height of 1–1.5 m above ground level. Trap position within a block was re-randomized with each replicate. Six replicates of traps baited with septa containing E6,Z11–18:Ald or the blend of all four compounds were run at the same location, along with four control traps. Each replicate was in position for 1–2 nights, and lures were used for 1–3 nights before being replaced.
Experiment 4, deployed at the Caribou Lake site from June 12–27 2015, had the following treatments: septa loaded with: 1) 300 μg of E6,Z11–18:Ald in 300 μl hexane; 2) 300 μg of E6,Z11–18:Ald + 75 μg of E6–18:Ald in 300 μl hexane; 3) 300 μg of E6,Z11–18:Ald + 75 μg of Z11–18:Ald in 300 μl hexane; and 4) 300 μg of E6,Z11–18:Ald, 75 μg of E6–18:Ald, and 75 μg of Z11–18:Ald in 300 μl hexane. A total of 4 traps (as above) were hung from paper birch trees, with the trap openings ca. 1.5 m above ground level. Traps were separated by at least 200 m. Collections were made daily June 12–16 and June 18, 19, and 24–27, 2015. Each replicate lasted one night, and the next day the lures were rotated one trap position. One set of septa were used June 12–16 and another June 18, 19, and 24–27, 2015. Each morning, after the moths were collected from traps, the septa were placed in labelled glass vials and placed in a freezer (on site) until the next evening trapping period when they were returned to the traps.
Statistics Total catches of A. luna for experiments 1 and 4 were analyzed using a blocked multi-response permutation procedure (MRBP; McCune et al. 2002). MRBP has the benefit that assumptions regarding the distribution of dependent variables are relaxed. All analyses were conducted with PC-ORD 6.0 (MjM Software Design, Gleneden Beach OR, USA) using Euclidean distances to construct the distance matrix, and blocks were aligned before analysis (McCune et al. 2002). The multiplicity effect was controlled using step-up FDR (Benjamini and Hochberg 1995; Garcia 2004). Experiments 2 and 3 were analyzed using a paired t-test with the null hypothesis that there was no difference between treatments (Analytical Software 2013).
Results and Discussion
Identification of Possible Pheromone Components
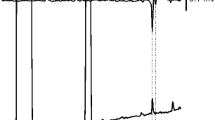
GC-EAD analyses of SPME wipe samples of the pheromone glands of calling female moths on a medium polarity DB-17 column showed one major response (Fig. 2, peak B), and a small response to a compound eluting slightly earlier (Fig. 2, peak A). Additional SPME wipe samples were analyzed by GC-MS on a nonpolar DB-5 column, to provide additional retention time information. These analyses located a compound with a mass spectrum characterized by a small molecular ion at m/z 264, and a small fragment at m/z 246 from loss of water, suggesting at least one oxygen in the compound (Fig. S2). The molecular ion suggested a molecular formula of C18H32O, possibly corresponding to a triene alcohol or a diene aldehyde. The relatively low retention index of 1991 on the DB-5 column argued in favor of the dienal, and further suggested that the two C = C bonds were not conjugated. A second compound, eluting shortly after the first on the nonpolar DB-5 column, with a retention index of 2004, a molecular ion at m/z 266 and a fragment from loss of water at m/z 248 was tentatively identified as a C18 monoenal (C18H34O) by reasonable matches with database spectra. A third compound, with retention index 2024 on the nonpolar DB-5 column, was positively identified as octadecanal by matching its retention time and mass spectrum with those of an authentic standard.
Representative coupled gas chromatography-electroantennogram analysis on a 30 m × 0.25 mm id × 0.25 μm film, medium polarity DB-17 column (temperature program 100 °C for 1 min, 10 °C to 280 °C) of a SPME wipe sample of the extruded pheromone gland of two female Actias luna moths. Upper trace is the gas chromatogram, lower inverted trace is the male A. luna antennal response. Peak A = E6–18:Ald and Z11–18:Ald, peak B = E6,Z11–18:Ald. Note that the elution order of peaks A and B is reversed in comparison to that on a nonpolar DB-5 column
To identify the positions of the double bonds in the suspected dienal and monoenal, an aliquot of pheromone gland extract was derivatized with dimethyl disulfide. GC-MS analysis of the resulting extract showed an adduct with a molecular ion at m/z 360 (12 %) from addition of two thiomethyl groups across a single double bond, and two large fragments at m/z 145 (100 %) and 215 (95 %), indicating that the double bond in the parent monoenal was in either the 6 or 11 position. The DMDS derivative of a Z11–18:Ald standard matched the retention time and ion ratios of the derivatized compound in the insect extract, whereas the retention time of the adduct from E11–18:Ald was markedly longer on a DB-5 column, eliminating it from consideration.
Because the DMDS adducts of 6–18:Ald and 11–18:Ald give the same major cleavage fragments at m/z 145 and 215, and because the retention times of E6–18:Ald and Z11–18:Ald were within a couple of seconds of each other, although that of Z6–18:Ald was slightly shorter so it could be excluded, we could not yet be certain whether the monoenal peak in the insect extract corresponded to E6–18:Ald or to Z11–18:Ald. Examination of the mass spectra of the pure standards showed that the ratio of the m/z 95 and 98 ions was different between the two compounds, with 95 being slightly more abundant than 98 in Z11–18:Ald (Fig. S3), whereas in E6–18:Ald, the 98 ion was markedly larger than the 95 ion (Fig. S4). There were also differences in the ratios of the ions at m/z 180, 222, 248, and 266. Careful re-examination of the monoenal peak in the insect extracts showed that the main peak had a mass spectrum that matched that of Z11–16:Ald. However, the peak had a small but consistent shoulder on its trailing edge, the mass spectrum of which showed the 98 ion larger than the 95 ion. Re-examination of the DMDS-derivatized sample also showed a small peak with a molecular ion of m/z 360 and fragment ions of 145 and 215, eluting a few seconds after the peak from DMDS-derivatized Z11–18:Ald. Thus, the monoenal peak appears to consist primarily of Z11–18:Ald, with a lesser amount of E6–18:Ald.
The adduct from the putative dienal was found to have a molecular ion at m/z 390, with a base peak at m/z 197 and significant fragments at m/z 145 and 245. In sum, these ions correspond to the DMDS adduct from a 6,11-dienal, which forms a six-membered tetrahydrothiopyran ring with the sulfur bridging carbons 7 and 11, and with MeS groups on carbons 6 and 12. The m/z 245 ion arises from cleavage between carbons 6 and 7, or carbons 11 and 12, with the charge retained on the portions containing the tetrahydrothiopyran ring, whereas the m/z 145 ion results from the charge being retained on either one of the alkyl chains being cleaved from the ring. The m/z 197 ion results from loss of methanethiol from either of the m/z 245 ions. Given that the two monoenals in the extract had (6E)- and (11Z)-geometries, respectively, it seemed likely that the dienal was E6,Z11–18:Ald. This was confirmed by matching the retention times of an authentic standard on three GC columns of different polarity (DB-5, DB-17, and DB-Wax) with those of the insect-produced compound, by matching the mass spectra of the standard and the insect-produced compound, and by matching the retention times and mass spectra of the corresponding DMDS adducts of the insect-produced compound and the E6,Z11–18:Ald standard. Furthermore, the retention time of a standard of Z6,Z11–18:Ald was significantly shorter than that of the insect-produced dienal, eliminating it as a possibility. In sum, the evidence strongly supported the identification of the peak eliciting the largest antennal response as E6,Z11–18:Ald, a previously undescribed lepidopteran pheromone component.
Synthesis of E6,Z11–18:Ald, E6–18:Ald, and Z11–18:Ald
Z11–18:Ald was synthesized as shown in Fig. 1a. Thus, the alcohol of 10-chlorodecanol 1 was protected as the THP derivative 2, followed by NaI-catalyzed reaction with lithium acetylide-ethylenediamine complex to yield the terminal alkyne 3 (Sonnet and Heath 1980). Alkylation of the alkyne with hexyl iodide (Buck and Chong 2001), reduction of the alkyne to the Z-alkene with P2-nickel (Brown and Ahuja 1973), removal of the protecting group, and oxidation to the aldehyde 7 with pyridinium dichromate (PDC) in methylene chloride (Corey and Schmidt 1979) completed the synthesis. E6–18:Ald 10 was readily synthesized by reduction of commercially available (E)-6-octadecenoic acid 8 (petroselaidic acid) to the alcohol 9, followed by oxidation to the aldehyde with PDC (Fig. 1b).
The synthesis of E6,Z11–18:Ald 21 is shown in Fig. 1c. THP-protected 4-pentyn-1-ol 12 was alkylated with hexyl iodide, followed by deprotection and stereoselective reduction of the alkyne 14 to the Z-alkene, and conversion of the alcohol 15 to the corresponding bromide 16 via the mesylate. Bromide 16 then was coupled with organozirconium intermediate 18, with Pd(acac)2 catalysis (Wiskur et al. 2004). Acid-catalyzed removal of the THP protecting group and oxidation of the resulting alcohol 20 with PDC then completed the synthesis.
Field Trials
In experiment 1, a total of 45 male A. luna were captured, 44 in traps baited with 300 μg of E6,Z11–18:Ald [4.4 ± 0.9 (SEM)] and 1 in an unbaited control trap [0.1 ± 0.1 (SEM)]. In experiment 2, comparing attraction of males to 300 μg E6,Z11–18:Ald vs. virgin female moths, female-baited traps caught 5.6 ± 1.45 (SEM) males per replicate (62 total), which was not significantly different than the 3.5 ± 0.81 males caught per replicate (39 total) with the synthetic pheromone (paired t-test, T = 1.23, 10 df, P = 0.25). In experiment 3, testing possible effects of the monoenals and octadecanal, traps baited with 300 μg of E6,Z11–18:Ald as a single component captured 3.3 ± 0.71 males per replicate (20 total), whereas traps baited with a blend of the four aldehydes (300 μg of E6,Z11–18:Ald + 75 μg of E6–18:Ald + 75 μg of Z11–18:Ald + 210 μg of 18:Ald) caught 3.5 ± 2.01 males (21 total) (paired t-test, T = 0.07, 5 df, P = 0.94). The four control traps captured no males. In experiment 4, which further examined the possible effects of the minor components, traps baited with a blend of 300 μg of E6,Z11–18:Ald + 75 μg of E6–18:Ald + 75 μg of Z11–18:Ald captured 48 males (4.4 ± 1), significantly more than the 11 males caught in traps baited with 300 μg of E6,Z11–18:Ald + 75 μg of Z11–18:Ald (1.0 ± 0.3) and 17 males captured by traps baited with 300 μg of E6,Z11–18:Ald (1.5 ± 0.7), but not different from the 34 males captured by traps baited with 300 μg of E6,Z11–18:Ald + 75 μg of E6–18:Ald (3.1 ± 1.1) (Fig. 3). In sum, the analytical and bioassay data support E6,Z11–18:Ald being the major component of the pheromone, with at least one of the monoenals appearing to increase the activity. Furthermore, no other moth species were attracted, indicating that the pheromone was species-specific at the sites and dates where it was tested.
Mean total captures (+SE) of Actias luna in traps baited with septa loaded with 300 μg of E6,Z11–18:Ald in 300 μl hexane, 300 μg of E6,Z11–18:Ald + 75 μg of E6–18:Ald in 300 μl hexane, 300 μg of E6,Z11–18:Ald + 75 μg of Z11–18:Ald in 300 μl hexane, or 300 μg of E6,Z11–18:Ald, 75 μg of E6–18:Ald, and 75 μg of Z11–18:Ald in 300 μl hexane (n = 110). There were eleven replicates per treatment. Means (+SE) with the same letter are not significantly different at P = 0.05
Relatively few pheromone components with an 18 carbon chain have been identified from the Lepidoptera, with most being reported from the Sessiidae. E6,Z11–18:Ald is the first 18 carbon pheromone identified from the family Saturniidae, and in fact, represents a novel lepidopteran pheromone structure. However, it clearly is a homolog of the pheromone components E6,Z11–16:Ald and E4,Z9–14:Ald known from the saturniid genera Antheraea and Saturnia, respectively (summarized in El-Sayed 2016). Similar to the biosynthesis of E6,Z11–16:Ald and analogs (Löfstedt et al. 2016; Wang et al. 2010), the two additional carbons on the terminal end of the chain of E6,Z11–18:Ald suggest that the pheromone is derived from the sequential actions of Δ6 and Δ11-desaturases on a saturated 18:Acyl intermediate, instead of a 16:Acyl substrate. Similarly, the E6–18:Ald and Z11–18:Ald minor components are likely generated by action of one or the other of the two desaturases on a saturated 18:Acyl intermediate.
In summary, the identification of the pheromone of this iconic species should provide a useful tool for delineating its range, and the possible expansion of its range due to climate change. It also may be possible to use the pheromone to obtain quantitative estimates of local populations, and to monitor the effects of habitat disruption and other human activities on population sizes. It further represents an interesting expansion of the few known 18-carbon pheromones in the Lepidoptera, demonstrating that the chain lengths of the so-called Type I aldehyde, acetate, and alcohol pheromones can be extended beyond 16 carbons to provide additional structural variation while using a limited number of substrates and biosynthetic enzymes, in the process providing unique signals to minimize interference in the pheromone communication channel.
References
Analytical Software (2013) Statistix 10 User’s Manual. Tallahassee, FL
Baker WL (1972) Eastern forest insects. USDA Forest Service, Miscellaneous publication no. 1175, Washington DC
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Royal Stat Soc B 57:289–300
Boettner GH, Elkinton JS, Boettner CJ (2000) Effects of a biological control introduction on three nontarget native species of saturniid moths. Conserv Biol 14:1798–1806
Bouseman JK, Sternburg JG (2002) A field guide to silkmoths of Illinois. Illinois Natural History Survey Manual 10. Illinois Dept. of Natural Resources,, Springfield IL, p. 97 pp
Brown CA, Ahuja VK (1973) Catalytic hydrogenation. VI. Reaction of sodium borohydride with nickel salts in ethanol solution. P-2 nickel, a highly convenient, new, selective hydrogenation catalyst with great sensitivity to substrate structure. J Org Chem 38:2226–2230
Buck M, Chong JM (2001) Alkylation of 1-alkynes in THF. Tetrahedron Lett 42:5825–5827
Butler L, Stazanc J (2000a) Occurrence of Lepidoptera on selected host trees in two central Appalachian national forests. Ann Entomol Soc Am 93:500–511
Butler L, Stazanc J (2000b) Macrolepidopteran larvae sampled by tree bands in temperate Mesic and xeric forests in eastern United States. Proc Entomol Soc Wash 102:188–197
Corey EJ, Schmidt G (1979) Useful procedures for the oxidation of alcohols involving pyridinium dichromate in aprotic media. Tetrahedron Lett 20:399–402
El-Sayed, AM (2016). The pherobase: Database of pheromones and semiochemicals. http://www.pherobase.com. Accessed June 1, 2016.
Ferguson, DC (1971) The moths of America north of Mexico: Fascicle 20.2 A Bombycoidea Saturniidae (Part). E.W. Classey Limited and R.B.D. Publications, London
Gago R, Allison JD, McElfresh JS, Haynes KF, McKenney J, Guerrero A, Millar JG (2013) A tetraene aldehyde as the major sex pheromone component of the promethea moth (Callosamia promethea (Drury. J Chem Ecol 39:1263–1272
Garcia LV (2004) Escaping the Bonferonni iron claw in ecological studies. Oikos 105:657–663
Holden C (1992) Thousands of insects “enroll” at Yale. Science 256:313
Johnson KS, Scriber JM, Nitao JK, Smitley DR (1995) Toxicity of Bacillus thuringiensis var kurstaki to three nontarget Lepidoptera in field studies. Environ Entomol 24:288–297
Kellog SK, Fink LS, Brower LP (2003) Parasitism of native Luna moths, Actias luna (L.) (Lepidoptera: Saturniidae) by the introduced Compsilura concinnata (Meigen) (Diptera: Tachinidae) in Central Virginia, and their hyperparasitism by trigonalid wasps (hymenoptera: Trigonalidae. Environ Entomol 32:1019–1027
Larsson MC, Svensson GP (2009) Pheromone monitoring of rare and threatened insects: Exploiting a pheromone-kairomone system to estimate prey and predator abundance. Conserv Biol 23:1516–1525
Le Corff J, Marquis RJ, Whitfield JB (2000) Temporal and spatial variation in a parasitoid community associated with the herbivores that feed on Missouri Quercus. Environ Entomol 29:181–194
Lindroth RL (1989) Chemical ecology of the Luna moth: effects of host plant on detoxification enzyme activity. J Chem Ecol 15:2019–2029
Löfstedt C, Wahlberg N, Millar JG (2016) Evolutionary patterns of pheromone diversity in Lepidoptera, Chapter 4. In: Allison JD, Cardé RT (eds) Pheromone communication in moths. University of California Press, Berkeley
Marquis RJ, Passoa S (1989) Seasonal diversity and abundance of the herbivore fauna of striped maple Acer pensylvanicum L. (Aceraceae) in western Virginia. Am Midland Nat 122:313–320
McCune B, Grace JB, Urban DL (2002) Analysis of ecological communities. MjM Software Design, Gleneden Beach
Millar JG, McElfresh JS, Romero C, Vila M, Mari-Mena N, Lopez-Vaamonde C (2010) Identification of the sex pheromone of a protected species, the Spanish moon moth Graellsia isabellae. J Chem Ecol 36:923–932
Peigler RS (2001) We now know what happened to our biggest moths. News Lepidop Soc 43:30
Schweitzer DF (1988) Status of Saturniidae in the northeastern USA: A quick review. News Lepidop Soc 1:4–5
Sonnet PE, Heath RR (1980) Stereospecific synthesis of (Z,Z)-11,13-hexadecadienal, a female sex pheromone of the navel orangeworm, Amyelois transitella (Lepidoptera: Pyralidae. J Chem Ecol 6:221–228
Thomas AW (1996) Light-trap catches of moths within and above the canopy of a northeastern forest. J Lepidop Soc 50:21–45
Thomas AW, Thomas GM (1994) Sampling strategies for estimating moth species diversity using a light trap in a northeastern softwood forest. J Lepidop Soc 48:85–105
Tietz, HM (1972) An index to the described life histories, early stages and hosts of the macrolepidoptera of the continental United States and Canada. A. C. Allyn, Sarasota FL, USA
Tuskes PM, Tuttle JP, Collins MM (1996) The wild silk moths of North America. Cornell University, Ithaca NY, USA
Wagner DL, Peacock JW, Carter JL, Talley SE (1996) Field assessment of Bacillus thuringiensis on non-target Lepidoptera. Environ Entomol 25:1444–1454
Wang HL, Liénard MA, Zhao CH, Wang CZ, Löfstedt C (2010) Neofunctionalization in an ancestral insect desaturase lineage led to rare ∆6 pheromone signals in the Chinese tussah silkworm. Insect Biochem Molec. Biol 40:742–751
Wiskur SL, Korte A, GC F (2004) Cross-couplings of alkyl electrophiles under “ligandless” conditions: Negishi reactions of organozirconium reagents. J Am Chem Soc 126:82–83
Acknowledgments
Nick Boyonoski and Scott Bessin provided technical assistance, Dr. Martha Lutz reared the first generation of luna moths in Kentucky, and Dr. Eric Chapman helped find suitable field locations in Kentucky. Clint Patterson, Berea College Forester, provided us with access to the Berea College Forest. The experiments conducted in Kentucky were supported by the Kentucky Agricultural Experiment Station (National Institute of Food and Agriculture, U.S. Department of Agriculture, Hatch Project KY008066 1003549), the experiments in Ontario were supported by Natural Resources Canada, and JGM acknowledges support from Hatch Project CA-R*ENT-5181-H.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Millar, J.G., Haynes, K.F., Dossey, A.T. et al. Sex Attractant Pheromone of the Luna Moth, Actias luna (Linnaeus). J Chem Ecol 42, 869–876 (2016). https://doi.org/10.1007/s10886-016-0751-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-016-0751-6