Abstract
Chlorpyrifos is an organophosphate pesticide used around the world to protect food crops against insects and mites. Despite guidelines for chlorpyrifos usage, including precautions to protect beneficial insects, such as honeybees from spray drift, this pesticide has been detected in bees in various countries, indicating that exposure still occurs. Here, we examined chlorpyrifos levels in bees collected from 17 locations in Otago, New Zealand, and compared doses of this pesticide that cause sub-lethal effects on learning performance under laboratory conditions with amounts of chlorpyrifos detected in the bees in the field. The pesticide was detected at 17 % of the sites sampled and in 12 % of the colonies examined. Amounts detected ranged from 35 to 286 pg.bee−1, far below the LD50 of ~100 ng.bee−1. We detected no adverse effect of chlorpyrifos on aversive learning, but the formation and retrieval of appetitive olfactory memories was severely affected. Chlorpyrifos fed to bees in amounts several orders of magnitude lower than the LD50, and also lower than levels detected in bees, was found to slow appetitive learning and reduce the specificity of memory recall. As learning and memory play a central role in the behavioral ecology and communication of foraging bees, chlorpyrifos, even in sublethal doses, may threaten the success and survival of this important insect pollinator.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There is a growing body of evidence that sublethal doses of pesticides impact the behavioral and chemical ecology of pollinators, such as the honeybee, Apis mellifera. Chlorpyrifos (O, O-diethyl O-3,5,6-trichloro-2-pyridinyl phosphorothioate) is an organophosphate pesticide used to protect food crops against insects and mites (Solomon et al. 2014). It has a moderate persistence in the environment, and its various dissipation pathways, including volatilization, can result in regional and long-range atmospheric transport (Mackay et al. 2014). While it is authorized for use in nearly 100 countries, concerns over its toxicity have led to it being banned in some countries, including Denmark, Finland, Latvia, Lithuania, Sweden and Yemen (Watts 2013). However, in the European Union, the USA, and New Zealand, risks associated with its use have recently been reassessed, and its usage re-authorized, with minor restrictions aimed at reducing the risk of this pesticide to humans (EFSA 2014; EPA 2015; NZEPA 2013).
In New Zealand, chlorpyrifos recently has been detected in non-sprayed areas, including in air samples (Lavin et al. 2012), pine needles (Lavin and Hageman 2013), and stream sediments (Shahpoury et al. 2013). The high potential for chlorpyrifos to volatilize (Davie-Martin et al. 2013) and disperse by air, as well as its common use, explain why this pesticide has been found ubiquitously in the environment, even in olives and lemons from organic farms in New Zealand (MPI 2012). As chlorpyrifos has a relatively high toxicity compared to other pesticides in use (Johnson et al. 2010; Sanchez-Bayo and Goka 2014), its detection in a wide range of environmental samples is of concern, especially as a growing number of reports indicate that pesticides may be contributing to the worldwide decline in honey bee populations (Blacquière et al. 2012; Gill et al. 2012; Kessler et al. 2015; Rundlöf et al. 2015; van der Sluijs et al. 2013).
Honey bees (Apis mellifera L.) are particularly prone to pesticide exposure because of their extensive use as pollinators in agricultural areas. Foraging honey bees can complete up to fifty flights a day (Perry et al. 2015), covering an area of up to seven square kilometers (Celli and Maccagnani 2003), increasing the potential for contact with pesticides. Foragers that collect water, nectar, pollen, or propolis contaminated with pesticide residues expose the entire colony to these chemicals (Johnson et al. 2010). Sub-lethal effects of pesticides on beneficial insects, such as honey bees, have received a great deal of attention (Blacquière et al. 2012; Decourtye et al. 2005; El-Hassani et al. 2008; Herbert et al. 2014; Weick and Thorn 2002; Williamson and Wright 2013; Yang et al. 2008). Many of these chemicals induce motor or even cognitive impairments in insects (e.g., Balbuena et al. 2015; Feltham et al. 2014; Gill et al. 2012; Henry et al. 2012; Yang et al. 2008). Cognitive deficits reduce a bee’s ability to learn and remember the characteristics of rewarding flowers, and to communicate the whereabouts of food sources to nestmates (Dobson 2006; Dötterl and Vereecken 2010; Reinhard and Srinivasan 2009; Stanley et al. 2015; Wright and Schiestl 2009). Volatile blends produced by plants also may be affected by environmental pollutants; thus, further jeopardizing recognition by bees (Lusebrink et al. 2015).
Chlorpyrifos has been detected in bees in North America (Mullin et al. 2010), Uruguay (Pareja et al. 2011), France (Lambert et al. 2013), and Egypt (Al-Naggar et al. 2015). Here, we show that this pesticide also is in bees in New Zealand. Further, we compare levels of chlorpyrifos that impact bee behavior in a laboratory setting to levels in forager bees in the field and show that learning performance by bees can be severely compromised by exposure to this insecticide, even by amounts ingested that are several orders of magnitude lower than the LD50, and lower than amounts detected in bees collected from the field.
Methods and Materials
Sampling of Bees for Chemical Analysis
Honey bees were collected from 17 sites in the Otago region of the South Island of New Zealand. The sites were spread throughout the region, with some sites ~250 km apart. To avoid potential contamination of samples, the glass vials used to collect bees from the field were baked for 4 h at 400 °C, and subsequently rinsed, along with their Teflon-lined caps, with acetone, ethyl acetate, and hexane. At each sampling site, field blanks were collected by exposing an empty sample vial to the environment during honey bee sampling. Sampling occurred during December 2014 and January 2015 (i.e., austral summer). Air temperatures at collection sites at the time of sampling ranged from 23 to 34 °C. Sampling sites were selected to represent a range of land types and agricultural activities, and included dry grasslands (4 sites), a river flat (1 site), a cherry orchard (1 site), brassica fields (5 sites), lucerne fields (3 sites), dairy farms (3 sites), and paddocks for grazing sheep and cattle (2 sites). Colonies were chosen at random from an apiary located at each site, and three colonies were sampled at each location. Some, but not all, of the colonies sampled had been treated for Varroa mites with Bayverol or Apivar (this information was not available for every colony). A clean glass vial (40 ml) was placed close to a hive entrance in order to collect foragers (50–60 bees per vial) as they departed on foraging trips. We chose to sample forager bees because they are directly exposed to the environment during foraging. To reduce the likelihood of contamination from food, water, or propolis collected by foragers, bees returning to the colony were not included. Vials of live bees were placed immediately on ice in a cooler and later transferred to a freezer at −20 °C until analysis.
Extraction and Quantification of Chlorpyrifos in Honey Bees
Chlorpyrifos was extracted from honey bees using a matrix solid-phase dispersion method, based on that previously described by Morzycka (2002). Briefly, bees were thawed, and samples (ca. 0.5 g of bees, 4–7 individuals collected from each hive) were analyzed, for a total of 51 samples. Bees were transferred to a porcelain mortar containing 1.5 g of Florisil (60/100 mesh, Restek pesticide grade, LECO Australia). The bee/Florisil mixture was spiked with 15 μl of 3 ng.μl−1 of chlorpyrifos-D10 (Dr. Ehrenstorfer GmbH), which was used as a surrogate standard to account for analyte loss during sample workup. The mixture was homogenized with a porcelain pestle. A glass column (300 × 10 mm i.d.) then was packed from bottom to top with a plug of glass wool, 1.0 g of anhydrous sodium sulfate, 2.0 g of silica gel 60 (200–400 mesh, 40–60 μm), the bee/Florisil homogenate, and 1.5 g of anhydrous sodium sulfate. Elution of chlorpyrifos was performed by gravity flow using 15 ml each of hexane, 9:1 hexane: diethyl ether, 8:2 hexane: diethyl ether, and 7:3 hexane: ethyl acetate. Each solvent also was used to wash the mortar and pestle and the rinsate added to the column. The fractions were collected together and reduced to 1 ml under nitrogen flow and filtered (0.45 μm hydrophobic polytetrafluoroethylene) to remove particulates. All solvents were of analytical grade, while solid-phase materials were freshly activated by baking for 4 h at 400 °C before use.
Quantification and analyte identification were performed by gas chromatography/mass spectrometry (GC/MS) using an Agilent 6890 N Network interfaced with an Agilent 5975 N Inert XL. One microliter of sample was injected via an autosampler in the pulsed-spitless mode. The inlet temperature was 325 °C and the oven temperature was ramped from 90 to 170 °C at 15 °C.min−1, to 210 °C at 1 °C.min−1, to 320 °C at 5 °C.min−1, and then held for 10 min. A Thermo Scientific TraceGOLD TG-5MS GC column (0.25 mm i.d., 0.25 μm film thickness) was used in conjunction with a 5-m guard column (Restek, deactivated nonpolar). The MSD was operated in negative chemical ionization mode, using methane as the reagent gas, and in the selected ion monitoring mode. For unlabeled chlorpyrifos, m/z 312.9 was monitored for quantification (with m/z 211.9 and 314.9 also monitored for qualitative identification). For the standard chlorpyrifos-D10, m/z 322.0 was monitored for quantification (and m/z 211.9, and 324.0 for qualitative identification). The source and quadrupole temperatures were 150 °C.
Chlorpyrifos concentrations in bee extracts were quantified using a ten-point calibration curve ranging from 0.1 to 250 pg.μl−1, constructed using the ratios of the areas of the m/z 312.9/322.0. A standard containing 25 pg.μl−1 chlorpyrifos was run after every five samples as a quality control measure to verify method performance. The sample-specific method detection limit, as determined by US Environmental Protection Agency method 8280A, was 150 pg.g−1 or 15 pg.bee−1. Concentrations in pg.g−1 were converted to pg.bee−1 by multiplying by the actual mass of bees and dividing by the number of bees used in each specific sample. Spike and recovery experiments were conducted to validate the analytical method. In these experiments, 15 μl of 1500 pg.μl−1 chlorpyrifos standard were added to bees (~0.5 g per sample) from a site in which chlorpyrifos was not detected. These samples were analyzed in the same manner as field samples, except that the surrogate standard was added to the final extract. The average recovery of spiked chlorpyrifos was 90 % (N = 3, 11 % relative standard deviation). Method blanks were analyzed by the same method as field samples, except that in their case 2 g of acid-washed baked sand were homogenized with Florisil rather than ~0.5 g of bees. A larger mass of sand was used, because its greater density meant more was needed to give the same volume as that used for bee samples. To quantify chlorpyrifos in the field blanks, vials were rinsed with the elution solvents, which were reduced to 1 ml and analyzed by GC/MS. Chlorpyrifos was not detected in method or field blanks.
Sampling and Preparation of Bees for Behavioral Experiments
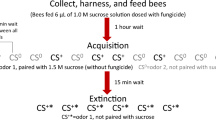
Honey bees used for behavioral experiments were collected every morning at around 9.00 am (during summer) or 10.30 am (during winter) from 3 hives (2 hives in winter, 1 hive in summer) located at the Department of Zoology, University of Otago. Bees were collected at the hive entrance, as described above, so we assumed that these bees were also departing foragers. The bees were transported in ventilated glass vials and cooled on ice until immobile (about 3–5 min). They then were harnessed individually in tubes that allowed free movements of the mouthparts and antennae. The animals were fed shortly thereafter with 20 μl of 50 % (w/w) sucrose solution. Six to seven hours later, bees were fed a second time, either with chlorpyrifos or with vehicle (sucrose solution containing solvent, see below) alone and left in a dark humid compartment until the next day. Although chlorpyrifos is reported to be present at higher levels in pollen than in nectar (Cutler et al. 2014; Mullin et al. 2010), we chose to deliver pesticide added to sucrose solution, rather than in pollen, because this allowed us to control more precisely both the dosage and the route of exposure. At the conclusion of the behavioral experiments, samples of control bees and bees fed chlorpyrifos were analyzed for chlorpyrifos content, as described above.
Chlorpyrifos Solutions
Bees were given a range of field-realistic chlorpyrifos doses based initially on previously published results (e.g., Mullin et al. 2010; Williamson et al. 2013). However, because our initial set of experiments conducted with winter bees suggested that learning behavior might be affected by dosages considerably lower than expected, we increased the range of dosages tested in an attempt to identify a pesticide concentration that had no observable effect on learning performance. Chlorpyrifos (Fluka, 99.9 % purity) was dissolved in DMSO to obtain a 1 mg.ml−1 stock solution and then diluted in 50 % sucrose in order to obtain five concentrations: 500, 50, 5, 0.5, and 0.05 pg.μl−1. The control group was fed sucrose solution containing DMSO at a concentration equivalent to the maximum level ingested by bees treated with chlorpyrifos (0.2 μl.ml−1). Previous studies demonstrated that DMSO, used as solvent for insecticides, is not toxic to bees (Williamson and Wright 2013; Yang et al. 2008). Each bee consumed 10 μl of feeding solution (DMSO and sucrose, with or without chlorpyrifos). The total amounts of chlorpyrifos ingested were 5000, 500, 50, 5, or 0.5 pg. Controls consumed no pesticide. The feeding solution was delivered orally using a micropipette, 21 hr before the behavioral tests. Honey bee mortality was assessed throughout the treatment period. Honey bees were recorded as being dead when there was no evidence of movement of the abdomen, or the antennae.
Preparation of Odors for Conditioning and Memory Tests
Shortly before conditioning, olfactory stimuli were prepared by placing 5 μl of pure odorant (Sigma Aldrich) on a 1 cm2 piece of filter paper inserted in a 20 ml plastic syringe that was used to deliver odor-filled air to the antennae.
Appetitive Olfactory Conditioning
We compared the appetitive learning performance of bees fed chlorpyrifos with that of bees fed sucrose containing 0.5 % DMSO (Controls). First, the proboscis extension reflex was tested in all bees by stimulating the antennae with 50 % sucrose solution (the unconditioned stimulus, US); any bee that failed to respond reflexively at that time, or during conditioning, was discarded. Olfactory appetitive conditioning was performed according to a standard protocol (Matsumoto et al. 2012), using 1-nonanol as the conditioned (reinforced) stimulus (CS). Each bee was placed in the learning arena 15 sec before the start of each conditioning trial. During each trial, the CS was presented to the bee for 4 sec. The US (50 % sucrose) was presented 3 sec after odor onset and was delivered first to the antennae to elicit proboscis extension and then to the proboscis. Bees were allowed to lick the sucrose solution for 3 sec. Each bee received four or five paired CS-US presentations (i.e., conditioning trials) with a 10 min interval between each trial. We did not use more conditioning trials as recommended by De Stefano et al. (2014) because preliminary data showed a decline in learning rate after the fourth or fifth trial. To avoid odor contamination, conditioning trials were performed in front of an air exhaust system. Bees that showed learning extended their proboscis in response to an odor before the reward was delivered (conditioned proboscis extension response, PER). Learning performance was the percentage of bees in a group that displayed the conditioned PER. At least 60 bees were tested in each group.
One-Hour Appetitive Memory Recall
Memory retention was assessed 1 hr after the last conditioning trial. In addition to the CS, 1-nonanol, bees were exposed to 2-hexanol and also to nonanal to determine whether or not they were responding selectively to the CS. The two additional odors were chosen according to their degree of structural similarity with the CS; nonanal was expected to be perceived by bees similarly to 1-nonanol, whereas 2-hexanol was expected to be perceived differently (Guerrieri et al. 2005). Each bee was placed in the learning arena and, 15 sec later, the bee was presented with the three odors in a randomized order, with an inter-stimulus interval of 10 min, in absence of any reinforcement. At the completion of these tests, the bee was stimulated with sucrose to determine whether the reflexive response to sucrose was still intact. Any bee that failed to display the proboscis extension reflex was discarded.
Sucrose Responsiveness
As appetitive learning performance can be affected by sucrose responsiveness, tests were carried out to determine whether chlorpyrifos affected a bee’s motivation for sucrose. Sucrose responsiveness of harnessed summer bees, collected from the same hive as the bees used for conditioning, was assessed by stimulating each bee’s antennae successively with solutions containing 0.1, 0.3, 1, 3, 10, and 30 % sucrose, each interspersed with water stimulations to avoid sensitization (Scheiner et al. 2003). Typically, a bee that extends the proboscis to a given concentration of sucrose will respond also when tested with sucrose at higher concentrations. If motor function were impaired (e.g., abnormal feeding activity) or if individuals did not answer to a subsequent 50 % sucrose stimulation, they were discarded. Control bees (N = 52) were compared with bees fed 5000 pg chlorpyrifos (N = 51).
Aversive Olfactory Conditioning
We also investigated the effect of chlorpyrifos on aversive learning and 1 hr memory recall in summer bees. We maintained and fed the bees in tube holders as described above and transferred them into the holders used for aversive conditioning only 2–3 hr before the experiment, because survival tends to be lower in these holders than in the tubes. Bees were placed on ice until motionless, and then fixed individually on a metallic holder so that the bee formed a bridge between two brass plates used to deliver electric shock stimuli to the bee. Conductive gel (Ultrasound transmission gel, Parker laboratories) was used to ensure a good contact between the bee and the plates. A strap was used to immobilize the thorax. Bees were fed with 50 % sucrose ad libitum after harnessing, and left at least 2 hr under bright light (necessary to see the sting extensions) until the conditioning phase.
To examine aversive learning, a differential conditioning paradigm was used (Vergoz et al. 2007). One odor, eugenol, was reinforced with electric shock (CS+), which elicited reflexive sting extension, while a second odor (2-hexanol) was not reinforced (CS-). The first odor presented to the bees during conditioning was randomized. Bees were placed in the setup 20 sec before either the CS+, or the CS- was presented to them for 5 s. Presentations of the CS+ were paired with an electric shock (7.5 V) applied for 2 sec so that the two stimuli ended simultaneously. Each bee received 6 paired CS+/ electric shock and 6 CS- presentations delivered in a pseudo randomized order, with a 10-min interval between each of the 12 conditioning trials. To avoid odor contamination, conditioning trials were performed in front of an air exhaust system. During conditioning, bees that showed learning extended their sting in response to the CS+ before the shock was delivered (conditioned sting extension response, SER), and did not respond when the CS- was presented. The percentage of bees showing sting extension during odor presentation, prior to the reinforcement, is used as a measure of learning. Bees failing to respond to the electric shock with sting extension were discarded. A minimum of 51 bees per group was used.
One-Hour Aversive Memory Recall
Memory retention was assessed 1 hr after the last aversive conditioning trial. Bees were placed in the setup again and presented 20 sec later with each of the test odors, eugenol (CS+) and 2-hexanol (CS-), in a randomized order and in the absence of any reinforcement. After the 1-hr memory test, each bee was stimulated with electric shock to ensure that it displayed reflexive sting extension. Any bee that failed to respond was discarded.
Shock Responsiveness
As a further control, we also investigated whether chlorpyrifos altered the bees’ sensitivity to electric shock. Summer bees harnessed on their back, as for aversive conditioning, were submitted to a series of 2 sec electric shocks of increasing voltage: 0.25, 0.5, 1, 2, 4, and 8 V, each interspersed with sham treatments in which bees were placed in the setup but no electric shock delivered. An interval of 2 min was used between shock or sham stimuli (Roussel et al. 2009). Typically, a bee that extends the sting to a given voltage will also do so for higher voltages. A voltmeter was used to check the conductivity of the bee in the harness in case of no response; the non-responders were discarded. Controls (N = 57) were compared with bees fed 5000 pg chlorpyrifos (N = 58).
Statistical Analysis
The responses of each bee were scored as binary responses (PER or SER was scored as 1, no response as 0). Data were analyzed with generalized linear mixed effects modeling (GLMM) using the R package lme4 (Bates et al. 2012), using the binomial error structure with the logit-link function, a method recommended by Jaeger (2008) for the analysis of categorical data. GLMMs enable comparison of the slopes of the response curves in different treatment groups, with treatment and trial number as fixed factors. Several models (with or without interactions between factors) were tested and the best was selected using AIC. Bee identity always was used as a random factor. For learning experiments, the fixed-factor trial was rearranged such that the last trial was set to zero, thus becoming the intercept of the model. In this way, model coefficients represent the difference in slopes between a reference group (usually the control) and a given treatment. Results are presented as coefficients ± standard error.
Appetitive learning was analyzed with GLMM to investigate the fixed effects of trial and chlorpyrifos treatment on responses. No interaction was included, as it did not improve the model. Aversive learning data were also analyzed using GLMM to investigate the fixed effects of trial and treatment (combining chlorpyrifos and CS identity) on responses. Interaction between trial and treatment was included (reflecting both differences in learning speed in response to CS identity and pesticide treatment), as it improved the model. We included a random effect structure taking into account the responses of each individual bee across the trials in combination with the CS identity (CS+ or CS-).
Appetitive memory tests also were analyzed using GLMM, using odor identity and treatment as fixed factors. Interaction between these two factors was included as it greatly improved the model. The same approach could not be applied to aversive memory data; because of the very low number of responses to the CS-, the model did not converge. Instead we compared the responses of chlorpyrifos-treated bees and control bees to CS+ and CS- independently, using the function brglm in the package brglm (Kosmidis 2007) to minimize the bias caused by the lack of, or low number of, responses in one group (as this breaks some assumptions of the model). McNemar Chi-square tests were used to determine whether, in each group, responses to CS+ differed from responses to CS-.
Sucrose responsiveness also was analyzed with GLMM, using concentration and treatment as fixed factors, as well as their interaction. Bee identities, as well as session identity (sucrose or water), were included as random factors. Shock responsiveness was analyzed as described above for appetitive learning. Responses to placement were not included because they prevented the model from converging due to the very low proportion of responses.
We used Chi-square tests to compare the mortality of chlorpyrifos-treated bees and control bees. Calculations were performed using R (R Core Team 2013). Comparisons in which P < 0.05 were considered significant.
Results
Chlorpyrifos in Honey Bees from the Field
Chlorpyrifos was detected in bees collected from 3 of the 17 (17 %) sites sampled, and in 6 of 51 (12 %) hives examined. However, at only 1 site (site #8, Table 1) was chlorpyrifos detected in bees sampled from all three examined colonies. Overall, the amounts of chlorpyrifos detected ranged from 35 to 286 pg.bee−1 (Table 1). Two of the three sites that tested positive for chlorpyrifos were in proximity to crop fields or orchards, but the third site had no obvious agricultural activity nearby. Chlorpyrifos was not detected in any of the field blanks, in control bees used for the behavioral experiments, or in bees that had been fed with up to 5000 pg of chlorpyrifos prior to examining their olfactory learning and memory.
Mortality
As bees used in the behavioral analysis were kept for more than 24 hr in harness, mortality levels were high, even in the control group (39 %). However, at the dosages used in this study, we found no evidence of an increase in mortality as a result of treatment with chlorpyrifos; on the contrary, at the highest dose tested, mortality was lower than in controls (χ 2 = 20.59, df = 1, P < 0.001).
Effects of Chlorpyrifos on Appetitive Learning and One-hour Memory Recall
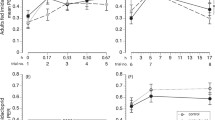
The effects of two concentrations of chlorpyrifos were tested first on the appetitive learning abilities of forager bees collected in winter. A majority of bees tested learned quickly to associate the conditioned odor with a sucrose reward over the four conditioning trials (5.17 ± 0.46, P < 0.001, Fig. 1a). However, fewer of the bees that had ingested 50 or 500 pg of chlorpyrifos learned than did the controls, and chlorpyrifos-treated bees appeared to learn at a slower rate than controls (50 pg: −0.90 ± 0.38, P < 0.05; 500 pg: −1.00 ± 0.39, P < 0.05). The learning curves for the two groups treated with chlorpyrifos were indistinguishable (50 vs. 500 pg: −0.10 ± 0.40, P = 0.80). When tested 1 hr later, both control bees and bees fed with chlorpyrifos responded greater to 1-nonanol, the CS, than to either nonanal, the similar odor (control: −5.03 ± 0.96, P < 0.001, 50 pg: −4.20 ± 0.96, P < 0.001, 500 pg: −2.89 ± 0.69, P < 0.001), or to 2-hexanol, the dissimilar odor (control: −7.76 ± 1.05, P < 0.001, 50 pg: −6.31 ± 0.97, P < 0.001, 500 pg: −4.54 ± 0.68, P < 0.001) (Fig. 1b). Chlorpyrifos did not affect the percentage of bees responding to the CS (control vs. 50 pg: 0.23 ± 1.24, P = 0.85, control vs. 500 pg: −0.44 ± 1.11, P = 0.68), and while it tended to increase responses to the odor similar to the CS, the increase was not significant (control vs. 50 pg: 1.31 ± 0.73, P = 0.07, control vs. 500 pg: 1.20 ± 0.74, P = 0.10). However, both doses increased the percentage of bees responding to the dissimilar odor, 2-hexanol (control vs. 50 pg: 1.94 ± 0.68, P < 0.01, control vs. 500 pg: 1.50 ± 0.69, P < 0.05). Classifying bees according to their individual responses (Fig. 1c) revealed that 48 % of winter control bees responded only to the CS, compared to 36 % of the chlorpyrifos-fed bees. A fairly high proportion of both treated and control winter bees generalized to a similar odor (24 to 26 %), and 17 to 26 % responded to all three odors. However, in the experiments conducted during the winter, the distribution of bees in each category was not affected by chlorpyrifos (χ 2 = 2.17, df = 6, P = 0.90).
In winter bees, chlorpyrifos impairs appetitive learning and increases odor generalization in the 1-hr memory test a. Acquisition curves show changes in the percentages of winter bees displaying the conditioned proboscis extension response (PER) over four successive conditioning trials. Inset: Letters (a, b) indicate significant differences between groups (P < 0.05): bees that ingested 50 or 500 pg of chlorpyrifos differed from controls but not from each other. The number of bees in each group is indicated in brackets. b. One-hour memory recall. Response levels do not differ (non significant, NS) for the CS+ (1-nonanol) and the similar odor (nonanal) but differ significantly in chlorpyrifos-fed bees compared to controls for 2-hexanol, the different odor (P < 0.05). c. Distribution of bees according to their individual responses during the memory test. No differences between control and chlorpyrifos-fed bees were detected
To investigate further the effects of chlorpyrifos on learning performance and 1 hr memory, we replicated this experiment in summer and tested a larger range of dosages of chlorpyrifos to determine the threshold dosage for behavioral effects. Overall, the probability of controls and chlorpyrifos-treated summer bees responding to the conditioned stimulus increased across trials (1.87 ± 0.12, P < 0.001, Fig. 2a). However, not all groups performed equally well. Differences in learning performance were detected between bees fed with chlorpyrifos (greys, Fig. 2a) and controls (black, Fig. 2a). The highest dose (5000 pg) impaired learning the most. The acquisition curve for bees in this group differed from controls (−1.84 ± 0.37, P < 0.001) and from all other groups (P < 0.01). Of the two doses that induced learning deficits in winter bees (see Fig. 1), only the lower dose (50 pg) had detrimental effects on memory acquisition in summer bees (−0.83 ± 0.32, P < 0.01); the intermediate dose (500 pg) did not (−0.32 ± 0.32, P = 0.31).
In summer bees, chlorpyrifos impairs appetitive learning and increases generalization in the 1-hr memory test a. Acquisition curves show changes in the percentages of bees displaying the conditioned proboscis extension response (PER) over five successive conditioning trials. Inset: Letters (a, b, c) indicate significant differences between groups (P < 0.05). Groups that share a letter are not significantly different. The number of bees in each group is indicated in brackets. b. One-hour memory recall. Stars indicate statistically significant differences between chlorpyrifos-fed bees and controls (P < 0.05). c. Distribution of bees according to their individual responses during the memory test reveals that doses greater than 0.5 pg significantly altered the individual responses to the different odors, in particular reducing the number of bees having specific responses (CS only) and increasing the number of bees responding to all odors. Stars indicate statistically significant differences compared to control (P < 0.05)
One hour memory recall also was affected by chlorpyrifos ingestion (Fig. 2b). Control bees responded more to the CS (1-nonanol) than to the similar odor, nonanal (−3.78 ± 0.64, P < 0.001), and showed the lowest level of response to 2-hexanol, the odor chosen for its lack of similarity to the CS (−5.93 ± 0.76, P < 0.001). In contrast, most groups of chlorpyrifos-treated bees failed to differentiate clearly between these three odors. For example, bees that ingested 5000 pg of chlorpyrifos responded as much to 2-hexanol as they did to the CS, 1-nonanol (−0.94 ± 0.55, P = 0.09), while bees that ingested 5 pg of chlorpyrifos had lower responses to the CS than did the control bees (−1.81 ± 0.79, P < 0.05), but their responses to 2-hexanol were higher (2.72 ± 0.80, P < 0.01). Chlorpyrifos ingestion increased the proportion of bees responding to 2-hexanol in a dose-dependent manner (Fig. 2c). Groups of bees that ingested 5 pg or more of chlorpyrifos responded more to this odor than did the control group (P < 0.05 in all cases). By examining the responses of individuals during the 1 hr memory test (Fig. 2c), one can see that chlorpyrifos had profound effects on the selectivity of responses to the conditioned stimulus (χ 2 = 69.9, df = 15, P < 0.001). Fewer of the bees that ingested 5 pg or more of chlorpyrifos responded to the CS alone than did the controls (controls: 41 %; 5000 pg: 6 %), and more chlorpyrifos-fed bees than control bees showed odor generalization and extended their proboscis to all three of the odors tested (P < 0.01 in all pairwise comparisons to the control group). These two experiments revealed a pronounced negative effect on memory specificity of chlorpyrifos at very low doses.
Effect of Chlorpyrifos on Sucrose Responsiveness
To determine whether the observed effects on learning and memory performance were due to a change in motivational state, we assessed the effects of chlorpyrifos on sucrose responsiveness. Independent groups of bees were fed with different doses of chlorpyrifos and compared to controls for responsiveness to water and increasing concentrations of sucrose. Only the highest dose of chlorpyrifos (5000 pg per bee) affected sucrose and water responsiveness. Bees fed 5000 pg chlorpyrifos were less likely than controls to respond to sucrose stimulations (Fig. 3, −2.99 ± 0.79, P < 0.001), but this was not the case for bees that had ingested either 500 pg (−1.01 ± 0.79, P = 0.20) or 0.5 pg of the pesticide (−0.49 ± 0.77, P = 0.52). Interestingly, the same was also true for responses to water, which were lower in bees fed 5000 pg than in the controls (−1.86 ± 0.76, P < 0.05). Responses to water were not affected by treatments with either, 0.5 or 500 pg of pesticide (P > 0.5 in both cases).
Chlorpyrifos at high dose decreases responsiveness to sucrose. Percentage of bees extending their proboscis to increasing sucrose concentrations (solid lines) and interspersed water stimulations (dashed lines). Inset: Letters (a, b, c, d) indicate significant differences between groups and treatments (P < 0.05). In controls and bees fed with 0.5 or 500 pg of chlorpyrifos, responses to sucrose (a) were similar and higher than responses to water (c). Only bees fed with the highest chlorpyrifos dose tested (5000 pg) displayed reduced responsiveness to sucrose (b) and water stimulation (d) compared to control bees (a and b, respectively). The number of bees in each group is indicated in brackets
Aversive Learning and 1 hr Memory Recall
Control bees increased their responses to the negatively reinforced odor (CS+) across the first four conditioning trials (0.66 ± 0.24, P < 0.01, Fig. 4a), but the level of responses dropped slightly on the last conditioning trial and, as a result, the change in response levels overall, was not significant (0.28 ± 0.15, P = 0.06). However, the control bees responded more to the reinforced odor (eugenol) than to 2-hexanol, the odor that was not reinforced (−1.91 ± 0.84, P < 0.05), indicating that these bees are able to differentiate between these two odors. Chlorpyrifos-treated bees showed clearer learning, increasing their responses to CS+ over the conditioning (0.43 ± 0.15, P < 0.01), and responding more to CS+ than to CS- (−5.17 ± 1.34, P < 0.001). Importantly, chlorpyrifos did not affect the level of response to either CS+ (0.86 ± 0.76, P = 0.25) or CS- (−2.41 ± 1.60, P = 0.13) compared to controls, although chlorpyrifos-fed bees appeared to differentiate between the two odors sooner than controls. No differences were observed in the 1 hr memory retention test between the level of responses displayed by control and chlorpyrifos-fed bees (Fig. 4b); most bees responded to CS+, and the percentage of bees responding to CS+ and to CS- was similar in both groups (0.12 ± 0.44, P = 0.78 and −0.34 ± 0.86, P = 0.70, respectively). Both control and chlorpyrifos-fed bees responded more to CS+ than to CS- (McNemar χ 2 = 9.1, df = 1, P < 0.01 for controls, χ 2 = 11.1, df = 1, P < 0.001 for chlorpyrifos).
Chlorpyrifos does not affect aversive learning, 1-hr memory recall or responsiveness to electric shock a. Acquisition curves show changes in the percentages of bees displaying the conditioned sting extension response (SER) over six successive conditioning trials in response to the reinforced odor (CS+) and to the non-reinforced odor (CS-). Inset: Letters (a, b) indicate significant differences between groups and treatments (P < 0.05). Responses were higher for the CS+ (a) than for the CS- (b) in both groups (P < 0.05). Groups that share a letter are not significantly different. The number of bees in each group is indicated in brackets. b. One-hour memory recall. No differences were detected between groups for each specific odor, but each group responded more to the CS+ than the CS-. c. Percentage of bees extending their sting to electric shock (solid lines) or to placement alone (dashed lines). No difference was detected between controls and bees fed with 5000 pg of chlorpyrifos, and hence these groups share the same letter (a). The number of bees in each group is indicated in brackets
Effect of Chlorpyrifos on Shock Responsiveness
We also tested if chlorpyrifos affected the responsiveness to the unconditioned stimulus used for aversive conditioning by measuring the bees’ sensitivity to electric shocks of increasing intensity (Fig. 4c). The percentage of controls and chlorpyrifos-fed bees showing sting extension rose as the voltage applied increased, but the response curves of the two groups did not differ (−0.28 ± 0.77, P = 0.71). Responses to placement (with no shock) could not be analyzed due to the very low numbers of responses.
Discussion
This study shows that feeding bees with chlorpyrifos has profound effects on the specificity of appetitive olfactory-mediated memory that could potentially have negative impacts on foraging and pollination by honey bees. Indeed, treated bees were less likely to respond specifically to an odor that was previously rewarded, especially during the summer. A forager returning to the hive usually will transfer food scents during trophallaxis and the waggle dance, with attending honey bees learning these odors (Farina et al. 2005, 2006; Gil and de Marco 2005; von Frisch 1993). Consequently, honey bees rely on olfactory memory to target flowers (Friesen 1973; Reinhard and Srinivasan 2009; Wenner et al. 1969). Thus, a decrease in specificity induced by pesticide exposure would affect foraging efficiency. For example, in a recent study examining effects of pesticide on pollination by bees, Stanley et al. (2015) found that bumblebees exposed to the neonicotinoid thiamethoxam were 2–3 times more likely to switch among different food sources. This is consistent with the reduction in memory specificity observed in the present investigation in honey bees exposed to the organophosphate chlorpyrifos. Chlorpyrifos also was found to slow the acquisition of appetitive olfactory memories, an effect reported for a variety of pesticides (e.g., Decourtye et al. 2004a, b; Weick and Thorn 2002; Williamson and Wright 2013). In bumblebees, variations in learning speed have been shown to correlate directly with foraging performance (Raine and Chittka 2008). Navigation also can be affected by pesticides (Balbuena et al. 2015; Fischer et al. 2014), but effects of chlorpyrifos on broader aspects of honeybee behavioral and chemical ecology, such as navigation and communication, have yet to be investigated.
This study identified the threshold dose for sub-lethal effects of chlorpyrifos on appetitive learning as 50 pg of chlorpyrifos ingested per bee. To place this value in perspective, we first compare it to the LD50 for oral toxicity of chlorpyrifos, which is in the range of hundreds of nanograms per bee (Cutler et al. 2014; Mullin et al. 2010), depending on the formulation. If the reference LD50 for pure chlorpyrifos is taken to be ~100 ng.bee−1 (Stevenson 1978), the results from the current study indicate that sub-lethal effects on appetitive learning occur at doses ~2000 times lower than the LD50 (~20,000 lower for memory in summer). For further perspective, we compare the threshold doses for sub-lethal effects to the amounts of chlorpyrifos measured in bees collected from the field (35–286 pg.bee−1) and find a clear overlap, indicating that the threshold dose has an environmental relevance. It is important to note that this overlap was observed even though our study was not designed to maximize detection frequency or determine the highest likely chlorpyrifos concentrations in bees, since sites were selected from a variety of agricultural and non-agricultural lands, and collection was not correlated with known spray events.
While relatively low amounts of ingested chlorpyrifos caused sub-lethal effects on memory and learning in honey bees in the laboratory trials, more information is needed about chlorpyrifos accumulation and exposure in field bees to understand the full implications of the results. For example, our experimental setup (in which one acute oral dose of chlorpyrifos was delivered per bee) does not accurately reflect how bees are likely to be exposed to chlorpyrifos in the environment. In the field, bees contact pesticides during foraging via physical contact with treated plants, as well as consumption of contaminated pollen, nectar, or honey (Cutler et al. 2014; Sanchez-Bayo and Goka 2014). Sanchez-Bayo and Goka (2014) investigated accumulation of chlorpyrifos in honey bees by calculating T50 values, which correspond to the time it takes for an animal to reach the LD50 given the amount of nectar or pollen it ingests (or is in contact with) per day, the prevalence of contaminated food in the environment, and the quantity of the pesticide in the food. Based on data taken from studies in Europe, the Americas, and Asia, they calculated the T50 for chlorpyrifos for nectar foragers to be 2 days for contact exposure and 764 days for oral exposure, indicating that accumulation via contact occurs much more rapidly and is more lethal than that via ingestion. While this does not necessarily discount oral exposure as a stressor for honey bees, especially given that the T50 calculations were based on lethal and not sub-lethal doses, it does raise interesting questions about how exposure path affects toxicity.
An important consideration when comparing sub-lethal threshold doses determined in laboratory trials to amounts of chlorpyrifos measured in field bees is that pesticide concentrations in bees are controlled by multiple competing processes. For example, pesticide accumulation occurs via chronic exposure; however concentrations also decrease through metabolism and excretion. Thus, the detected levels may underestimate the cumulative exposure. Additionally, accumulation of chlorpyrifos in a bee’s body is probably the result of several different physiological processes depending on the exposure route. Further investigation of the degradation of pesticides and their excretion will improve this model. The results suggest that these processes are important because chlorpyrifos could not be detected in bees 24 hr after ingesting 5000 pg.
Evidence that sub-lethal effects of chlorpyrifos occur at much lower levels than its LD50 in honey bees raises challenging questions for regulators. Specifically, they will need to decide if sub-lethal effects are important when establishing pesticide use and application regulations. Currently, regulations in many countries focus only on the lethal effects of pesticides. For example, the Environmental Protection Authority of New Zealand (Application for the Reassessment of a Group of Hazardous Substances, 2013) stated that with respect to chlorpyrifos, “All risks to bees are considered negligible as bees are only expected to be killed when they are directly exposed to the spray solution.” It notes further, “Controls are assumed to be effective in restricting application to times when bees are not present”. This recommendation does not take into account evidence that exposure to plants, pollen, or nectar induces mortality for up to 7 days after chlorpyrifos is applied to a crop (Lunden et al. 1986, cited in Cutler et al. 2014), or that chlorpyrifos readily disperses into non-sprayed areas due its tendency to undergo repeated volatilization- atmospheric transport-deposition cycles. It is likely that the detection of chlorpyrifos in the bees in this study was due to its persistence and environmental dispersal, rather than breaches of application guidelines. The detection of chlorpyrifos in the honey bee colonies sampled in the Otago region of New Zealand mirrors reports showing that the pesticide is detectable also in air, water, and plant samples from non-sprayed areas of the country (Lavin et al. 2012; Lavin and Hageman 2013; Shahpoury et al. 2013).
Interestingly, despite negative effects on appetitive learning and memory, aversive learning using electric shock as the negative reinforcer was not impaired by chlorpyrifos. One potential explanation is that chlorpyrifos alters the responsiveness of bees to sucrose, which is not involved in aversive conditioning. Bees with low sucrose responsiveness generally learn at a slower rate, and also tend to perform less well in appetitive memory retention tests than bees with high sucrose responsiveness (Scheiner et al. 1999). However, the results indicate that this factor alone cannot explain fully the impacts of chlorpyrifos on appetitive learning. We found that acquisition rate was reduced by chlorpyrifos in several dosages, but only the highest dose reduced the responsiveness of bees to sucrose. Moreover, while reductions in sucrose responsiveness generally lower the percentage of bees responding to a conditioned odor in memory retention tests (Scheiner et al. 1999), this was not the case following treatments with chlorpyrifos.
One of the several metabolites of chlorpyrifos, chlorpyrifos oxon, is known to be particularly toxic to many invertebrates. However, the chlorpyrifos metabolism rate and the metabolites produced are known to vary among invertebrates (Racke 1993), and essentially no information about chlorpyrifos metabolism in honey bees exists. Nevertheless, chlorpyrifos oxon has been shown to be a potent inhibitor of acetylcholine esterase in bees (AChE, Williamson et al. 2013); it, therefore, has the potential to enhance responses to odors by increasing acetylcholine levels in synaptic cleft regions within olfactory pathways of the brain (Gauthier and Grünewald 2012). This may explain why acute treatments with AChE inhibitors, such as coumaphos (Williamson et al. 2013) and metrifonate (Shapira et al. 2001), enhance the rate of acquisition of appetitive olfactory memories in honey bees, and why AChE inhibitors lead to improvements in memory recall (Guez et al. 2010). However, prolonged exposure to AChE inhibitors leads to desensitization and a reduction in responses mediated via cholinergic pathways (Katz et al. 1997; Palmer et al. 2013; Pohanka 2011). The effects of chlorpyrifos observed in the present investigation are interesting because the pesticide reduces the percentage of bees responding to the conditioned stimulus during memory acquisition, but enhances their responsiveness to non-target odors at the time of memory recall. This is difficult to explain and suggests that the effects of this pesticide are likely to be complex and may involve changes at the peripheral level, as well as impacts on the functioning of the brain. This complexity might explain the non-linear effects of chlorpyrifos, and differences between the impact of this pesticide on summer and winter bees, both of which warrant further attention.
The absence of effects of chlorpyrifos on aversive learning performance in this study may assist our interpretation of the effects of the pesticide on appetitive learning. It appears from our analysis of aversive learning, for example, that the ability of bees to differentiate odors is not severely compromised by chlorpyrifos and, moreover, that inhibitory effects of chlorpyrifos on appetitive learning are unlikely to result from direct impairment of neural circuits involved in olfaction, as aversive learning under the same conditions remains intact. However, it is noted that a negative effect of a neonicotinoid on a honey bee’s ability to perform a natural form of avoidance learning has been described. Tan et al. (2014) trained bees to visit sucrose feeders and then tested their choices between two feeders, one safe and one with a predation risk. In contrast to the control bees they tested, imidacloprid-fed foragers failed to avoid the nectar feeder in which they encountered a hornet predator. While this experiment differs from ours in a number of respects, it suggests that under certain conditions, pesticides can also have a significant impact on avoidance learning.
The differences observed in this study between the susceptibility of aversive- and appetitive-learning to disruption by chlorpyrifos warrant further attention. Currently, we cannot rule out the possibility that they are a reflection of differences in the conditioning protocols used to examine appetitive and aversive learning. In the case of appetitive learning, an absolute conditioning paradigm was used in which the animal, during conditioning, was exposed only to the reinforced odor. In the case of aversive learning, a differential conditioning protocol was applied in which the animal was exposed to two odors, one of which was paired with the reinforcer, while the second odor received no reinforcement. In the latter case, the animal is likely to learn, not only which odor is paired with shock but, also, which odor is ‘safe’, an outcome that might be expected to enhance odor discrimination and reduce the likelihood of odor generalization. Aversive reinforcement might also increase a honey bee’s attention to task, and thus improve learning performance. Such a phenomenon has been shown in difficult visual tasks that can only be solved when wrong choices are punished with quinine and not when only rewarding the correct ones (Avarguès-Weber et al. 2010).
Many issues arise from the chlorpyrifos quantification results and the behavioral consequences of exposing bees to this pesticide. For instance, the influence of exposure route (e.g., contact vs. oral) on the sub-lethal effects of the insecticide, effects of acute vs. chronic toxicity, the accumulation and metabolism mechanisms, and the distribution among body tissues. All should be determined in order to address the true impact of this chemical on honey bee populations. In sum, there is a need for greater understanding of the impact of chlorpyrifos accumulation in bees.
References
Al-Naggar Y, Codling G, Vogt A, Naiem E, Mona M et al (2015) Organophosphorus insecticides in honey, pollen and bees (Apis mellifera L.) and their potential hazard to bee colonies in Egypt. Ecotoxicol Environ Saf 114:1–8
Avarguès-Weber A, de Brito Sanchez MG, Giurfa M, Dyer AG (2010) Aversive reinforcement improves visual discrimination learning in free-flying honeybees. PLoS One 5:e15370
Balbuena M, Tison L, Hahn M, Greggers U, Menzel R et al (2015) Effects of sub-lethal doses of glyphosate on honeybee navigation. J Exp Biol 218:2799–2805
Bates D, Maechler M, Bolker, B (2012) lme4: linear mixed-effects models using S4 classes. R package version 0.999999–0
Blacquière T, Smagghe G, Gestel C, Mommaerts V (2012) Neonicotinoids in bees: a review on concentrations, side-effects and risk assessment. Ecotoxicology 21:973–992
Celli G, Maccagnani B (2003) Honey bees as bioindicators of environmental pollution. Bull Insect 56:137–139
Cutler GC, Purdy J, Giesy JP, Solomon KR (2014) Risk to pollinators from the use of chlorpyrifos in the United States. Rev Environ Contam Toxicol 231:219–265
Davie-Martin CL, Hageman KJ, Chin Y-PP (2013) An improved screening tool for predicting volatilization of pesticides applied to soils. Environ Sci Technol 47:868–876
De Stefano LA, Stepanov II, Abramson CI (2014) The first order transfer function in the analysis of agrochemical data in honey bees (Apis mellifera L.): Proboscis extension reflex (PER) studies. Insects 5:167–198
Decourtye A, Armengaud C, Renou M, Devillers J, Cluzeau S et al (2004a) Imidacloprid impairs memory and brain metabolism in the honeybee (Apis mellifera L.). Pestic Biochem Physiol 78:83–92
Decourtye A, Devillers J, Cluzeau S, Charreton M, Pham-Delègue M-H (2004b) Effects of imidacloprid and deltamethrin on associative learning in honeybees under semi-field and laboratory conditions. Ecotoxicol Environ Saf 57:410–419
Decourtye A, Devillers J, Genecque E, Le Menach K, Budzinski H et al (2005) Comparative sublethal toxicity of nine pesticides on olfactory learning performances of the honeybee Apis mellifera. Arch Environ Contam Toxicol 48:242–250
Dobson HEM (2006) Relationship between floral fragrance composition and type of pollinator. In: Pichersky E, Dudareva N (eds.). Biology of floral scent. CRC Press 2006, pp 147–198
Dötterl S, Vereecken N (2010) The chemical ecology and evolution of bee–flower interactions: a review and perspectives. Can J Zool 88:668–697
EFSA (European Food Safety Authority) (2014) Conclusion on the peer review of the pesticide human health risk assessment of the active substance chlorpyrifos. EFSA J 12:3640
El-Hassani AK, Dacher M, Gary V, Lambin M, Gauthier M et al (2008) Effects of sublethal doses of acetamiprid and thiamethoxam on the behavior of the honeybee (Apis mellifera). Arch Environ Contam Toxicol 54:653–661. doi:10.1007/s00244-007-9071-8
EPA (Environmental Protection Agency) (2015) Chlorpyrifos: Revised human health risk assessment. Environmental Protection Agency, USA
Farina WM, Grüter C, Díaz PC (2005) Social learning of floral odours inside the honeybee hive. Proc Biol Sci 272:1923–1928
Farina W, Grüter C, Acosta L, Cabe S (2006) Honeybees learn floral odors while receiving nectar from foragers within the hive. Naturwissenschaften 94:55–60
Feltham H, Park K, Goulson D (2014) Field realistic doses of pesticide imidacloprid reduce bumblebee pollen foraging efficiency. Ecotoxicology 23:317–323
Fischer J, Müller T, Spatz A-K, Greggers U, Grünewald B et al (2014) Neonicotinoids interfere with specific components of navigation in honeybees. Plos One 9:e91364
Friesen LJ (1973) The search dynamics of recruited honey bee Apis mellifera. Biol Bull 144:107–131
Gauthier M, Grünewald B (2012) Neurtransmitter systems in the honeybee brain: Functions in learning and memory. In: Galizia CG, Eisenhardt D, Giurfa M (eds) Honeybee neurobiology and behavior. Springer Verlag, Heidelberg, pp 155–169
Gil M, de Marco R (2005) Olfactory learning by means of trophallaxis in Apis mellifera. J Exp Biol 208:671–680
Gill RJ, Ramos-Rodriguez O, Raine NE (2012) Combined pesticide exposure severely affects individual- and colony-level traits in bees. Nature 491:105–108
Guerrieri F, Schubert M, Sandoz J-CC, Giurfa M (2005) Perceptual and neural olfactory similarity in honeybees. PLoS Biol 3:e60
Guez D, Zhu H, Zhang S, Srinivasan M (2010) Enhanced cholinergic transmission promotes recall in honeybees. J Insect Physiol 56:1341–1348
Henry M, Béguin M, Requier F, Rollin O, Odoux J-F et al (2012) A common pesticide decreases foraging success and survival in honey bees. Science 336:348–350
Herbert L, Vazquez D, Arenas A, Farina W (2014) Effects of field-realistic doses of glyphosate on honeybee appetitive behaviour. J Exp Biol 217:3457–3464
Jaeger T (2008) Categorical data analysis: away from ANOVAs (transformation or not) and towards logit mixed models. J Mem Lang 59:434–446
Johnson R, Ellis M, Mullin C, Frazier M (2010) Pesticides and honey bee toxicity - USA. Apidologie 41:312–331
Katz EJ, Cortes VI, Eldefrawi ME (1997) Chlorpyrifos, parathion, and their oxons bind to and desensitize a nicotinic acetylcholine receptor: relevance to their toxicities. Toxicol Appl Pharmacol 146:227–236
Kessler S, Tiedeken E, Simcock K, Derveau S, Mitchell J et al (2015) Bees prefer foods containing neonicotinoid pesticides. Nature 521:74–76
Kosmidis I (2007) brglm: bias reduction in binary-response GLMs
Lambert O, Piroux M, Puyo S, Thorin C, L’Hostis M et al (2013) Widespread occurrence of chemical residues in beehive matrices from apiaries located in different landscapes of western France. PLoS One 8:e67007
Lavin K, Hageman K (2013) Contributions of long-range and regional atmospheric transport on pesticide concentrations along a transect crossing a mountain divide. Environ Sci Technol 17:1390–1398
Lavin K, Hageman K, Marx S, Dillingham P, Kamber B (2012) Using trace elements in particulate matter to identify the sources of semivolatile organic contaminants in air at an alpine site. Environ Sci Technol 46:268–276
Lunden J, Mayer D, Johansen C, Shanks C, Eves J (1986) Effects of chlorpyrifos insecticide on pollinators. Am Bee J 126:441–444
Lusebrink I, Girling RD, Farthing E, Newman TA, Jackson CW et al (2015) The effects of diesel exhaust pollution on floral volatiles and the consequences for honey bee olfaction. J Chem Ecol 41:904–912
Mackay D, Giesy J, Solomon K (2014) Fate in the environment and long-range atmospheric transport of the organophosphorus insecticide, chlorpyrifos and its oxon. Rev Environ Contam Toxicol 231:35–76
Matsumoto Y, Menzel R, Sandoz J-CC, Giurfa M (2012) Revisiting olfactory classical conditioning of the proboscis extension response in honey bees: a step toward standardized procedures. J Neurosci Methods 211:159–167
Ministry for Primary Industries (2012) Food residue surveillance programme 2011–2012 quarterly report. ISBN No: 978-0-478-40047-2
Morzycka B (2002) Simple method for the determination of trace levels of pesticides in honeybees using matrix solid-phase dispersion and gas chromatography. J Chromatogr A 982:267–273
Mullin CA, Frazier M, Frazier JL, Ashcraft S, Simonds R et al (2010) High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health. PLoS One 5:e9754
NZEPA (New Zealand Environmental Protection Authority) (2013) Application for the reassessment of a group of hazardous substances under Section 63 of the Hazardous Substances and New Organisms Act 1996
Palmer MJ, Moffat C, Saranzewa N, Harvey J, Wright GA et al (2013) Cholinergic pesticides cause mushroom body neuronal inactivation in honeybees. Nat Commun 4:1634
Pareja L, Colazzo M, Pérez-Parada A, Niell S, Carrasco-Letelier L et al (2011) Detection of pesticides in active and depopulated beehives in Uruguay. Int J Environ Res Public Health 8:3844–3858
Perry C, Søvik E, Myerscough M, Barron A (2015) Rapid behavioral maturation accelerates failure of stressed honey bee colonies. Proc Natl Acad Sci U S A 112:3427–3432
Pohanka M (2011) Cholinesterases, a target of pharmacology and toxicology. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 155:219–223
R Core Team (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Racke KD (1993) Environmental fate of chlorpyrifos. Rev Environ Contam Toxicol 131:1–150
Raine N, Chittka L (2008) The correlation of learning speed and natural foraging success in bumble-bees. Proc R Soc B 275:803–808
Reinhard J, Srinivasan MV (2009) The role of scents in honey bee foraging and recruitment. In: Jarau S, Hrncir M (eds) Food exploitation by social insects: ecological, behavioral, and theoretical approaches 1. CRC Press/Taylor & Francis Group, Boca Raton, pp 65–182
Roussel E, Carcaud J, Sandoz J-C, Giurfa M (2009) Reappraising social insect behavior through aversive responsiveness and learning. PLoS One 4:e4197
Rundlöf M, Andersson G, Bommarco R, Fries I, Hederström V et al (2015) Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 521:77–80
Sanchez-Bayo F, Goka K (2014) Pesticide residues and bees—a risk assessment. PLoS One 9:e94482
Scheiner R, Erber J, Page RE (1999) Tactile learning and the individual evaluation of the reward in honey bees (Apis mellifera L.). J Comp Physiol A 185:1–10
Scheiner R, Barnert M, Erber J (2003) Variation in water and sucrose responsiveness during the foraging season affects proboscis extension learning in honey bees. Apidologie 34:67–72
Shahpoury P, Hageman K, Matthaei C, Magbanua F (2013) Chlorinated pesticides in stream sediments from organic, integrated and conventional farms. Environ Pollut 181:219–225
Shapira M, Thompson C, Soreq H, Robinson G (2001) Changes in neuronal acetylcholinesterase gene expression and division of labor in honey bee colonies. J Mol Neurosci 17:1–12
Solomon KR, Williams WM, Mackay D, Purdy J, Giddings JM et al (2014) Properties and uses of chlorpyrifos in the United States. Rev Environ Contam Toxicol 231:13–34
Stanley DA, Garratt MP, Wickens JB, Wickens VJ, Potts SG et al (2015) Neonicotinoid pesticide exposure impairs crop pollination services provided by bumblebees. Nature 528:548–550
Stevenson J (1978) The acute toxicity of unformolated pesticides to worker honey bees (Apis mellifera L.). Plant Pathol 27:38–40
Tan K, Chen W, Dong S, Liu X, Wang Y et al (2014) Imidacloprid alters foraging and decreases bee avoidance of predators. PLoS One 9:e102725
van der Sluijs J, Simon-Delso N, Goulson D, Maxim L, Bonmatin J-M et al (2013) Neonicotinoids, bee disorders and the sustainability of pollinator services. Curr Opin Environ Sustain 5:293–305
Vergoz V, Roussel E, Sandoz J-C, Giurfa M (2007) Aversive learning in honeybees revealed by the olfactory conditioning of the sting extension reflex. PLoS One 2:e288
von Frisch K (1993) The dance language and orientation of bees. Harvard University Press, Cambridge, MA
Watts M (2013) Chlorpyrifos. Pesticide action network Asia and the pacific. Penang, Malaysia
Weick J, Thorn R (2002) Effects of acute sublethal exposure to coumaphos or diazinon on acquisition and discrimination of odor stimuli in the honey bee (Hymenoptera: Apidae). J Econ Entomol 95:227–236
Wenner AM, Wells PH, Johnson DL (1969) Honey bee recruitment to food sources: olfaction or language? Science 164:84–86
Williamson SM, Wright GA (2013) Exposure to multiple cholinergic pesticides impairs olfactory learning and memory in honeybees. J Exp Biol 216:1799–1807
Williamson S, Moffat C, Gomersall M, Saranzewa N, Connolly C et al (2013) Exposure to acetylcholinesterase inhibitors alters the physiology and motor function of honeybees. Front Physiol 4:13
Wright GA, Schiestl FP (2009) The evolution of floral scent: the influence of olfactory learning by insect pollinators on the honest signalling of floral rewards. Funct Ecol 23:841–851
Yang E, Chuang Y, Chen Y, Chang L (2008) Abnormal foraging behavior induced by sublethal dosage of imidacloprid in the honey bee (Hymenoptera: Apidae). J Econ Entomol 107:1743–1748
Acknowledgments
Marsden Fund Grant UOO1207
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Urlacher, E., Monchanin, C., Rivière, C. et al. Measurements of Chlorpyrifos Levels in Forager Bees and Comparison with Levels that Disrupt Honey Bee Odor-Mediated Learning Under Laboratory Conditions. J Chem Ecol 42, 127–138 (2016). https://doi.org/10.1007/s10886-016-0672-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-016-0672-4